Abstract
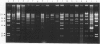
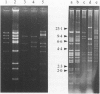
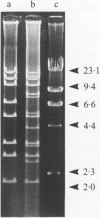
A total of 279 Vibrio anguillarum strains were serotyped and examined for plasmid content. Plasmids were subjected to digestion with restriction enzymes. Most strains belonged to serogroup O1 (39%) and O2 (16%). In total 164 strains (53%) carried plasmids. Of the O1 and O2 isolates, 92% and 30%, respectively, carried one or more plasmids. Restriction fragment length polymorphism (RFLP) analysis of plasmid DNA indicated that plasmids belonged to several groups. Each group seemed to be restricted to a single O-serovar. The largest group was the pJM1-like plasmids among most serovar O1 strains. Most of these plasmids were about 67 kb like the pJM1 plasmid, but various derivatives ranged from 26-77 kb. RFLP studies of the 67 kb plasmids revealed 17 different restriction patterns. Some patterns were dominant among European strains whereas others were dominant among North American strains. The results confirmed the applicability of O-serotyping together with plasmid profile and restriction analysis of plasmids for typing of V. anguillarum. They also indicated that plasmids among strains which belonged to the traditional fish pathogenic serogroups, O1 and O2, showed more homology than did strains from most other serogroups, that were usually non-pathogenic, environmental bacteria.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Actis L. A., Potter S. A., Crosa J. H. Iron-regulated outer membrane protein OM2 of Vibrio anguillarum is encoded by virulence plasmid pJM1. J Bacteriol. 1985 Feb;161(2):736–742. doi: 10.1128/jb.161.2.736-742.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki T., Egusa S., Arai T. Detection of R factors in naturally occurring Vibrio anguillarum strains. Antimicrob Agents Chemother. 1974 Nov;6(5):534–538. doi: 10.1128/aac.6.5.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosa J. H. A plasmid associated with virulence in the marine fish pathogen Vibrio anguillarum specifies an iron-sequestering system. Nature. 1980 Apr 10;284(5756):566–568. doi: 10.1038/284566a0. [DOI] [PubMed] [Google Scholar]
- Crosa J. H., Schiewe M. H., Falkow S. Evidence for plasmid contribution to the virulence of fish pathogen Vibrio anguillarum. Infect Immun. 1977 Nov;18(2):509–513. doi: 10.1128/iai.18.2.509-513.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson L. S., Oliver J. D. Plasmid carriage in Vibrio vulnificus and other lactose-fermenting marine vibrios. Appl Environ Microbiol. 1986 Jul;52(1):211–213. doi: 10.1128/aem.52.1.211-213.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedges R. W., Vialard J. L., Pearson N. J., O'Grady F. R plasmids from Asian strains of Vibrio cholerae. Antimicrob Agents Chemother. 1977 Apr;11(4):585–588. doi: 10.1128/aac.11.4.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hintermann G., Fischer H. M., Crameri R., Hütter R. Simple procedure for distinguishing CCC, OC, and L forms of plasmid DNA by agarose gel electrophoresis. Plasmid. 1981 May;5(3):371–373. doi: 10.1016/0147-619x(81)90012-3. [DOI] [PubMed] [Google Scholar]
- Kado C. I., Liu S. T. Rapid procedure for detection and isolation of large and small plasmids. J Bacteriol. 1981 Mar;145(3):1365–1373. doi: 10.1128/jb.145.3.1365-1373.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen J. L., Olsen J. E. Occurrence of plasmids in Danish isolates of Vibrio anguillarum serovars O1 and O2 and association of plasmids with phenotypic characteristics. Appl Environ Microbiol. 1991 Aug;57(8):2158–2163. doi: 10.1128/aem.57.8.2158-2163.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macrina F. L., Kopecko D. J., Jones K. R., Ayers D. J., McCowen S. M. A multiple plasmid-containing Escherichia coli strain: convenient source of size reference plasmid molecules. Plasmid. 1978 Jun;1(3):417–420. doi: 10.1016/0147-619x(78)90056-2. [DOI] [PubMed] [Google Scholar]
- Mitoma Y., Aoki T., Crosa J. H. Phylogenetic relationships among Vibrio anguillarum plasmids. Plasmid. 1984 Nov;12(3):143–148. doi: 10.1016/0147-619x(84)90038-6. [DOI] [PubMed] [Google Scholar]
- Olsen J. E. An improved method for rapid isolation of plasmid DNA from wild-type gram-negative bacteria for plasmid restriction profile analysis. Lett Appl Microbiol. 1990 May;10(5):209–212. doi: 10.1111/j.1472-765x.1990.tb01335.x. [DOI] [PubMed] [Google Scholar]
- Olsen J. E., Larsen J. L. Restriction fragment length polymorphism of the Vibrio anguillarum serovar O1 virulence plasmid. Appl Environ Microbiol. 1990 Oct;56(10):3130–3132. doi: 10.1128/aem.56.10.3130-3132.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen J. E., Larsen J. L. Ribotypes and plasmid contents of Vibrio anguillarum strains in relation to serovar. Appl Environ Microbiol. 1993 Nov;59(11):3863–3870. doi: 10.1128/aem.59.11.3863-3870.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen K., Larsen J. L. Evidence for the existence of distinct populations of Vibrio anguillarum serogroup O1 based on plasmid contents and ribotypes. Appl Environ Microbiol. 1995 Jun;61(6):2292–2296. doi: 10.1128/aem.61.6.2292-2296.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sizemore R. K., Colwell R. R. Plasmids carried by antibiotic-resistant marine bacteria. Antimicrob Agents Chemother. 1977 Sep;12(3):373–382. doi: 10.1128/aac.12.3.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skov M. N., Pedersen K., Larsen J. L. Comparison of Pulsed-Field Gel Electrophoresis, Ribotyping, and Plasmid Profiling for Typing of Vibrio anguillarum Serovar O1. Appl Environ Microbiol. 1995 Apr;61(4):1540–1545. doi: 10.1128/aem.61.4.1540-1545.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Søorum H., Poppe T. T., Olsvik O. Plasmids in Vibrio salmonicida isolated from salmonids with hemorrhagic syndrome (Hitra disease). J Clin Microbiol. 1988 Sep;26(9):1679–1683. doi: 10.1128/jcm.26.9.1679-1683.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sørensen U. B., Larsen J. L. Serotyping of Vibrio anguillarum. Appl Environ Microbiol. 1986 Mar;51(3):593–597. doi: 10.1128/aem.51.3.593-597.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Threlfall E. J., Rowe B., Ferguson J. L., Ward L. R. Characterization of plasmids conferring resistance to gentamicin and apramycin in strains of Salmonella typhimurium phage type 204c isolated in Britain. J Hyg (Lond) 1986 Dec;97(3):419–426. doi: 10.1017/s0022172400063609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiainen T., Pedersen K., Larsen J. L. Ribotyping and plasmid profiling of Vibrio anguillarum serovar O2 and Vibrio ordalii. J Appl Bacteriol. 1995 Oct;79(4):384–392. doi: 10.1111/j.1365-2672.1995.tb03152.x. [DOI] [PubMed] [Google Scholar]
- Toranzo A. E., Barja J. L., Colwell R. R., Hetrick F. M. Characterization of plasmids in bacterial fish pathogen. Infect Immun. 1983 Jan;39(1):184–192. doi: 10.1128/iai.39.1.184-192.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toranzo A. E., Barja J. L., Potter S. A., Colwell R. R., Hetrick F. M., Crosa J. H. Molecular factors associated with virulence of marine vibrios isolated from striped bass in Chesapeake Bay. Infect Immun. 1983 Mar;39(3):1220–1227. doi: 10.1128/iai.39.3.1220-1227.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiik R., Hoff K. A., Andersen K., Daae F. L. Relationships between plasmids and phenotypes of presumptive strains of Vibrio anguillarum isolated from different fish species. Appl Environ Microbiol. 1989 Apr;55(4):826–831. doi: 10.1128/aem.55.4.826-831.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willingham A. L., Johansen M. V., Vennervald B. J., Christensen N. O., Nansen P. Experimental infection of Danish Landrace/Yorkshire crossbred pigs with Schistosoma japonicum from the People's Republic of China. Acta Vet Scand. 1994;35(4):395–400. doi: 10.1186/BF03548314. [DOI] [PMC free article] [PubMed] [Google Scholar]