Abstract
Heart failure is accompanied by severely impaired β-adrenergic receptor (βAR) function, which includes loss of βAR density and functional uncoupling of remaining receptors. An important mechanism for the rapid desensitization of βAR function is agonist-stimulated receptor phosphorylation by the βAR kinase (βARK1), an enzyme known to be elevated in failing human heart tissue. To investigate whether alterations in βAR function contribute to the development of myocardial failure, transgenic mice with cardiac-restricted overexpression of either a peptide inhibitor of βARK1 or the β2AR were mated into a genetic model of murine heart failure (MLP−/−). In vivo cardiac function was assessed by echocardiography and cardiac catheterization. Both MLP−/− and MLP−/−/β2AR mice had enlarged left ventricular (LV) chambers with significantly reduced fractional shortening and mean velocity of circumferential fiber shortening. In contrast, MLP−/−/βARKct mice had normal LV chamber size and function. Basal LV contractility in the MLP−/−/βARKct mice, as measured by LV dP/dtmax, was increased significantly compared with the MLP−/− mice but less than controls. Importantly, heightened βAR desensitization in the MLP−/− mice, measured in vivo (responsiveness to isoproterenol) and in vitro (isoproterenol-stimulated membrane adenylyl cyclase activity), was completely reversed with overexpression of the βARK1 inhibitor. We report here the striking finding that overexpression of this inhibitor prevents the development of cardiomyopathy in this murine model of heart failure. These findings implicate abnormal βAR-G protein coupling in the pathogenesis of the failing heart and point the way toward development of agents to inhibit βARK1 as a novel mode of therapy.
One of the most important mechanisms for rapidly regulating β-adrenergic receptor (βAR) function is agonist-stimulated receptor phosphorylation by G protein-coupled receptor kinases (GRKs) resulting in decreased sensitivity to further catecholamine stimulation (1, 2). βARK1 is a member of the multigene GRK family that regulates a wide variety of receptors that couple to heterotrimeric G proteins (1, 2). Desensitization of agonist-occupied receptors by the cytosolic βAR kinase (βARK1) requires a membrane-targeting event before its activation and receptor phosphorylation, which is mediated by a direct physical interaction between residues within the carboxyl terminus of βARK1 and the dissociated membrane-anchored βγ subunits of G proteins (Gβγ) (3, 4).
Heart failure is a disease characterized by left ventricular (LV) dysfunction associated with a complex of symptoms that relate to inadequate perfusion of tissues and pulmonary congestion. Although the fundamental molecular abnormality that causes this progressive deterioration in cardiac function is unknown, one of the leading candidates is abnormal βAR signaling. Chronic human heart failure is characterized by severely attenuated βAR signaling, resulting from diminished receptor number and impaired receptor function (5), leading to inotropic subsensitivity and impaired exercise tolerance (6). Increased levels of βARK1, found in failing human heart tissue, have been postulated to account for impaired receptor function in response to agonist stimulation (7, 8). In animal models of heart disease, prominent βAR desensitization recently has been shown to be associated with heightened levels of βARK1 (9, 10). Our findings in transgenic mice that overexpress different members of the GRK family demonstrate how the up-regulation of βARK1 in the diseased heart could markedly alter βAR function through receptor desensitization (9, 11, 12). Importantly, inhibition of myocardial βARK1 activity because of cardiac-targeted expression of a peptide inhibitor of βARK1 (βARKct) led to enhanced contractility demonstrating the critical role of βARK1 in normal heart function (11). The βARKct is composed of the last 195 aa of βARK1, which contains the binding site for Gβγ and competes with endogenous βARK1 for Gβγ membrane translocation and activation (11).
A limitation in addressing mechanistic pathways in the setting of heart failure has been the lack of a well characterized model of murine heart failure that has fidelity to the human condition (13). Recently, a genetic model of murine-dilated cardiomyopathy has been described that involves ablation of a muscle-restricted gene that encodes the muscle LIM protein (MLP−/−) (14). Our goal was to determine whether abnormalities in βAR signaling in the failing heart play a causative role in the progressive deterioration in cardiac function and whether reversal of these alterations can lead to improvement of cardiac function. To accomplish this, our strategy was to mate transgenic mice with cardiac-targeted overexpression of either the βARKct (11) or the β2AR (15) into the MLP−/− model of heart failure.
METHODS
Experimental Animals.
MLP−/− mice were mated with transgenic mice with cardiac-targeted overexpression of the βARKct (11). F1 pups generated from an MLP−/− × βARKct(t/t) homozygote cross were mated to create the MLP+/−/βARKct(t/0) double heterozygote (where t represents the presence of a transgene). F2 offspring generated from a MLP−/− × MLP+/−/βARKct(t/0) double heterozygote mating generated mice that were MLP−/− and MLP−/−/βARKct(t/0) with the remainder of the pups heterozygous for MLP with and without the βARKct transgene. F3 offspring were generated by back-crossing a MLP−/−/βARKct(t/0) mouse into the MLP−/− parent to generate MLP−/− and MLP−/−/βARKct(t/0) littermates. A similar strategy was used to generate MLP−/−/β2AR(t/0) gene-targeted mice. The genotype of the various gene-targeted crosses was determined by PCR on genomic DNA isolated from tail biopsies as described previously (11, 14, 15). Expression of the βARKct peptide in hearts of MLP−/−/βARKct mice was confirmed by immunoblotting using a polyclonal antibody directed against the C terminus of βARK1. The animals in this study were handled according to approved protocols and the animal welfare regulations of the University of North Carolina at Chapel Hill, Duke University, and the University of California at San Diego.
Transthoracic Echocardiography.
Echocardiography was performed in anesthetized mice (2.5% avertin, 14 μl/g intraperitoneally) by using an Apogee CX echocardiograph (Interspec-ATL, Bothell, WA) as described previously (16). Echocardiography was performed in nonlittermate control MLP+/+ mice by using a different anesthesia (mixture of 100 mg/kg ketamine and 5 mg/kg xylazine, i.p.), which is associated with a lower heart rate and therefore mean velocity of circumferential fiber shortening (mean Vcf) (16).
Cardiac Catheterization.
Hemodynamic evaluation in intact mice was performed as described previously (9). Mice were anesthetized with a mixture of ketamine (100 mg/kg) and xylazine (2.5 mg/kg) and, after endotracheal intubation, were connected to a rodent ventilator. After bilateral vagotomy, the left carotid artery was cannulated with a flame-stretched PE-50 catheter connected to a modified P-50 Statham transducer (17). A 1.4 French (0.46 mm) high-fidelity micromanometer catheter (Millar Instruments, Houston, TX) was inserted into the right carotid and advanced retrograde into the LV. Hemodynamic measurements were recorded at baseline and 45–60 sec after injection of incremental doses of isoproterenol (9).
Adenylyl Cyclase Activity and βAR Binding.
Adenylyl cyclase activity and βAR binding were performed from myocardial sarcolemmal membranes (9, 11). For cyclase activity, membranes (30–40 μg protein) were incubated for 15 min at 37°C with [α -32P]ATP under basal conditions or indicated agonists and cAMP was quantified (9, 11). Total β AR density was determined by incubating 25 μg of sarcolemmal membranes with a saturating concentration of [125I]cyanopindolol and 20 μM alprenolol to define nonspecific binding (9, 11).
Immunoblotting.
Immunodetection of myocardial levels of βARK1 was performed on cytosolic extracts after immunoprecipitation by using a monoclonal βARK1/2 antibody as described previously (9, 11, 18). The ≈80-kDa βARK1 protein was visualized with the mAb raised against an epitope within the carboxyl terminus of βARK1 and chemiluminescent detection of anti-mouse IgG conjugated with horseradish peroxidase (ECL, Amersham). GRK activity was measured in myocardial membranes by using rhodopsin-enriched rod outer segment membranes as an in vitro substrate and [γ-32P]ATP as described previously (9, 11, 18). [32P] incorporation into rhodopsin was quantified by using a using a Molecular Dynamics PhosphorImager (9, 11, 18).
Histological Analysis of Gene-Targeted Mouse Hearts.
Unfixed myocardial cryosections were obtained and histological staining of myocytes was done with Masson’s trichrome by standard protocols (14).
Statistical Analysis.
Data were expressed as mean ± SEM. To test for statistical differences in echocardiographic data between all groups, a one-factor ANOVA was performed accompanied by a Newman–Keuls post hoc analysis when appropriate. Hemodynamic data were analyzed with a repeated-measures ANOVA, and post hoc analysis with regard to differences in mean values between the groups at a specific dose was conducted with a Newman–Keuls test. Cyclase activity was analyzed with a repeated-measures ANOVA. βAR density and NaF stimulation were determined with a one-factor ANOVA. A Student’s t test was used to test differences in the level of myocardial βARK1.
RESULTS
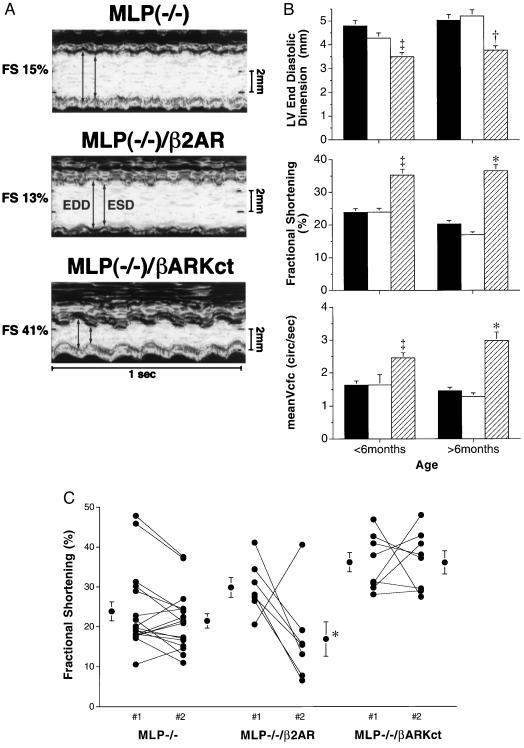
To investigate whether abnormalities in βAR signaling are responsible for the progressive deterioration in cardiac function in the failing heart, we used a strategy whereby transgenic mice with cardiac-targeted overexpression of either the βARKct (11) or the β2AR (15) were mated into the MLP−/− model of heart failure. In vivo cardiac function in these novel, dual gene-targeted mice was assessed by echocardiography. MLP−/− mice have enlarged cardiac chambers with depressed function as shown by the increase in LV end-diastolic and end-systolic dimension associated with a reduction in fractional shortening (FS) and mean Vcf (Table 1 and Fig. 1A). Cardiac overexpression of the β2AR in the MLP-deficient background did not influence the development of the dilated cardiomyopathic phenotype, because cardiac chambers remained enlarged whereas FS and mean Vcf continued to be depressed compared with MLP−/− littermates (Table 1 and Fig. 1A). In striking contrast, cardiac-targeted βARK1 inhibition through overexpression of the βARKct prevented the deterioration in cardiac function (Table 1 and Fig. 1A). In MLP−/−/βARKct mice compared with age-matched MLP−/− littermates, LV end-diastolic and end-systolic dimensions were significantly smaller, whereas FS and mean Vcf were significantly higher, indicating preserved cardiac function (Table 1 and Fig. 1A).
Table 1.
Transgene overexpression in MLP-deficient mice: In vivo echocardiographic assessment
| MLP−/−a, n = 12 | MLP−/−/β2AR, n = 9 | MLP−/−b, n = 7 | MLP−/−/βARKct, n = 9 | MLP+/+, n = 16 | |
|---|---|---|---|---|---|
| LVEDD, mm | 4.86 ± 0.29 | 4.78 ± 0.27 | 4.89 ± 0.24 | 3.74 ± 0.22† | 3.94 ± 0.09† |
| LVESD, mm | 3.84 ± 0.30 | 3.94 ± 0.31 | 3.97 ± 0.28 | 2.43 ± 0.22* | 2.47 ± 0.08* |
| FS, % | 22.3 ± 2.3 | 18.5 ± 3.6 | 19.3 ± 2.2 | 35.8 ± 2.4* | 37.4 ± 1.2* |
| SEPth, mm | 0.67 ± 0.04 | 0.56 ± 0.05 | 0.56 ± 0.03 | 0.59 ± 0.04 | 0.67 ± 0.02 |
| PWth, mm | 0.66 ± 0.04 | 0.58 ± 0.05 | 0.58 ± 0.04 | 0.58 ± 0.05 | 0.68 ± 0.02 |
| HR, beats/min | 437 ± 28 | 517 ± 44 | 483 ± 36 | 474 ± 28 | 258 ± 12‡ |
| mean Vcf, circ/s | 4.06 ± 0.35 | 3.74 ± 0.65 | 4.11 ± 0.57 | 7.73 ± 0.81* | 4.64 ± 0.20‡ |
| mean Vcfc, circ/s | 1.51 ± 0.14 | 1.32 ± 0.21 | 1.48 ± 0.22 | 2.88 ± 0.25* | 2.24 ± 0.12*‡ |
| BW, g | 36.3 ± 3.0 | 34.2 ± 2.5 | 33.6 ± 1.7 | 29.9 ± 1.8 | 33.8 ± 1.8 |
| Mean age at echo, months | 7.3 ± 0.8 | 5.1 ± 0.7 | 5.3 ± 0.1 | 6.9 ± 0.7 | 3.4 ± 0.1 |
Analysis of in vivo cardiac size and function by echocardiography in gene-targeted mice. Mating of transgenic mice with cardiac overexpression of the β2AR or a a βARKct into the MLP-deficient background resulted in mice homozygous for the MLP gene ablation and heterozygous for either the β2AR (MLP−/−/β2AR) or the βARKct (MLP−/−/βARKct), MLP−/−
and MLP−/−b are littermates for the β2AR and βARKct cross, respectively. MLP+/+ are adult nonlittermate wild-type mice of the same genetic background that were used as controls. LVEDD, left ventricular end diastolic dimension; LVESD, left venrtricular end systolic dimension; HR, heart rate; FS, fractional shortening calculated as (LVEDD − LVESD)/LVEDD × 100; SEPth, septal wall thickness; PWth, posterior wall thickness; mean Vcf, mean velocity of circumferential fiber shortening; mean Vcfc, heart rate-corrected mean Vcf; BW, body weight. *P < 0.0005,
P < 0.01, MLP−/−/βARKct and MLP+/+ vs. either MLP−/−b or MLP−/−a;
P < 0.01, MLP(+/+) vs. MLP−/−/βARKct. The MLP−/−/βARKct and MLP−/−a mice were significantly older than the other groups. (P < 0.05). MLP+/+ mice underwent echocardiography with a different anesthesia, accounting for the slower heart rate and therefore a lower mean Vcf (16). However, for the echocardiographic parameters measured, no significant differences in any of the other variables were found between the MLP−/−/β2AR and their MLP−/−a littermates, or between the MLP−/−a and MLP−/−b littermate groups. Therefore, for subsequent analysis of echocardiographic data, the MLR−/− groups were pooled.
Figure 1.
Analysis of cardiac function by echocardiography. (A) Transthoracic M-mode echocardiographic tracings in a MLP−/− (Top), MLP−/−/β2AR (Middle), and a MLP−/−/βARKct mouse (Bottom). Left ventricular dimensions are indicated by the double-sided arrows. EDD, end diastolic dimension; ESD, end systolic dimension. Both the MLP−/− and MLP−/−/β2AR mice have chamber dilatation with reduced wall motion indicating depressed cardiac function, whereas chamber size and cardiac function are normal in the MLP−/−/βARKct mouse. (B) Echocardiographic findings in mice under 6 months of age: MLP−/− (solid bar, mean age 4.1 ± 0.4 months, n = 18), MLP−/−/β2AR (open bar, mean age 4.3 ± 0.4 months, n = 8), and MLP−/−/βARKct (hatched bar, mean age 4.7 ± 0.7 months, n = 9); and more than 6 months of age: MLP−/− (solid bar, mean age 7.0 ± 0.5 months, n = 17), MLP−/−/β2AR (open bar, mean age 7.3 ± 0.7 months, n = 3), and MLP−/−/βARKct (hatched bar, mean age 6.9 ± 0.77 months, n = 8). Data represent serial echocardiograms in the same mouse at different ages except if the mouse died during the interval between studies. ∗, P < 0.005; †, P < 0.01; ‡, P < 0.05 MLP−/−/βARKct vs. MLP−/− and MLP−/−/β2AR, one-factor ANOVA. (C) Change in cardiac function over a 2.5- to 3-month period in the three groups of gene-targeted mice. #1, early study (mean age, 3.9 months); #2, later study (mean age, 6.4 months). For comparison, normal values obtained in MLP+/+ mice are shown in Table 1.
To determine whether changes in ventricular function were time-dependent, serial echocardiography was performed in the three groups of gene-targeted mice. MLP−/− mice and MLP−/−/β2AR under 6 months of age have enlarged LV chambers with depressed cardiac function (reduced FS and mean Vcfc) (Fig. 1B). In contrast, MLP−/−/βARKct mice under 6 months of age have normal LV chamber size and function (Fig. 1B). This beneficial effect of the βARKct on cardiac function persisted as the mice grew older, whereas little change and even some deterioration occurred in the MLP−/− and MLP−/−/β2AR mice (Fig. 1B). Individual data points are plotted for FS to determine whether a serial change in cardiac function occurred over a 3-month period (Fig. 1C). Whereas MLP−/− animals had reduced FS that did not change over time, the presence of additional cardiac β2ARs (MLP−/−/β2AR) resulted in deterioration of FS in the majority of mice. In contrast, FS was preserved in the MLP−/−/βARKct mice and remained in the normal range over the 3-month study interval (Fig. 1C).
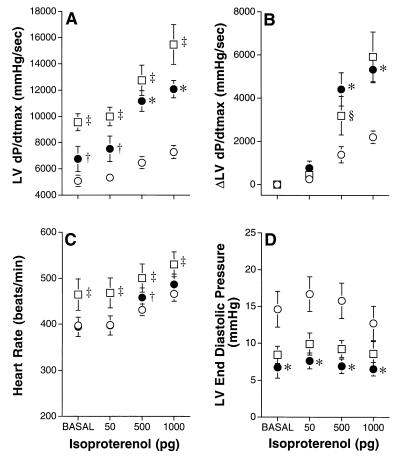
To determine whether overexpression of the βARKct can reverse the marked βAR desensitization associated with the MLP−/− mice, we performed cardiac catheterization in intact anesthetized mice. LV contractility (assessed by LV dP/dtmax) at baseline in the MLP−/−/βARKct mice was modestly but significantly increased compared with the MLP−/−, but was less than nonlittermate wild-type MLP+/+ control mice (Fig. 2A). However, the abnormal response to isoproterenol stimulation, characteristic of the MLP−/− animals, was reversed completely with βARKct overexpression (Fig. 2 A and B). This response to isoproterenol was essentially identical to that in the wild-type MLP+/+ mice and is consistent with a mechanism of βARK1 inhibition that preserves normal βAR-G protein coupling (Fig. 2 A and B). Similarly, the increase in heart rate from baseline in response to βAR stimulation was preserved in the MLP−/−/βARKct mice although basal heart rate was lower than in wild-type mice (Fig. 2C). Furthermore, the markedly elevated LV end diastolic pressure in the MLP−/− mice (an indication of the severe impairment of cardiac function in these animals) was normalized by chronic βARKct expression (Fig. 2D). The minimal first derivative of LV pressure, LV dP/dtmin, also was enhanced in the MLP−/−/βARKct compared with the MLP−/− mice at baseline (−3,964 ± 420 vs. −2,634 ± 140, mmHg/sec, P < 0.005) but was significantly less than that in wild-type mice (−7,411 ± 986, mmHg/s, P < 0.0005). This relationship persisted with isoproterenol administration (data not shown). Baseline LV systolic pressure was similar in the MLP−/−/βARKct animals compared with the MLP−/− mice (90 ± 4 vs. 96 ± 4 mmHg), but was lower than that in the MLP wild-type mice (128 ± 10 mmHg). Hemodynamic analysis could not be performed in the MLP−/−/β2AR mice because survival was considerably shortened and did not allow for an adequate number of animals.
Figure 2.
In vivo assessment of β-AR responsiveness. Cardiac catheterization was performed in intact, anesthetized mice. Parameters are shown at baseline and after progressive infusion of isoproterenol in MLP−/− (○), n = 15, MLP−/−/βARKct (•), n = 7, and wild-type MLP+/+ (□), n = 6, mice. (A) Maximal first derivative of LV pressure, LV dP/dtmax. (B) The difference from baseline for LV dP/dtmax, ΔLV dP/dtmax. (C) Heart rate. (D) LV end diastolic pressure. ∗, P < 0.0005; †, P < 0.01 MLP−/−/βARKct vs. MLP−/−; ‡, P < 0.005; §, P < 0.05 wild-type MLP+/+ vs. MLP−/−/βARKct. A significant between-group main effect in response to isoproterenol was found for LV dP/dtmax, P < 0.00001 (A); heart rate, P = 0.05 (C); and LV end diastolic pressure, P < 0.05 (D). The pattern of change between groups was statistically significantly for LV dP/dtmax, P < 0.00001 (A) and ΔLV dP/dtmax, P < 0.00001 (B).
βAR density and adenylyl cyclase activity were determined in cardiac membranes prepared from the various gene-targeted lines. βAR density was reduced significantly by 54% in the MLP−/− compared with wild-type hearts and was associated with marked attenuation of isoproterenol-stimulated adenylyl cyclase activity, indicating severe impairment of βAR coupling (Table 2). Although βAR density also was reduced in MLP−/−/βARKct compared with wild-type mice, isoproterenol-stimulated adenylyl cyclase activity was enhanced significantly and intermediate between wild-type and MLP-deficient mice (Table 2). Importantly, fold increase in membrane cyclase activity with isoproterenol stimulation in the MLP−/−/βARKct hearts was 2.8 vs. 2.6 in the wild-type hearts and 1.7 in the MLP−/− hearts, indicating restitution toward normal G protein coupling. These biochemical findings are completely concordant with the in vivo data (Fig. 2) and indicate that the functional uncoupling of βARs seen in the failing MLP−/− hearts can be prevented with chronic overexpression in vivo of the βARKct. This is also consistent with the LV weight data obtained after terminal hemodynamic study where a significant decrease in LV mass in the MLP−/−/βARKct hearts (n = 8) compared with MLP−/− hearts (n = 15) was observed (LV weight to tibia length ratio, 6.5 ± 0.3 vs. 7.8 ± 0.3 mg/mm, P < 0.02). LV mass in the MLP−/−/βARKct hearts was slightly, but not significantly, higher than that in the control hearts (MLP+/+, 5.5 ± 0.3 mg/mm, n = 6).
Table 2.
βAR signaling characteristics
| βAR density, fmol/mg membrane protein | Adenylyl cyclase activty, % NaF stimulation
|
||
|---|---|---|---|
| Basal | ISO, 10−4 M | ||
| MLP−/− | 16.6 ± 1.9, | 2.7 ± 0.1, | 4.5 ± 0.7, |
| n = 11 | n = 5 | n = 5 | |
| MLP−/−/βARKct | 23.0 ± 5.4, | 2.8 ± 0.7, | 7.8 ± 1.5†‡, |
| n = 6 | n = 5 | n = 5 | |
| MLP+/+ | 36.0 ± 4.3*, | 5.2 ± 0.6§, | 13.4 ± 0.7†, |
| n = 6 | n = 5 | n = 5 | |
NaF values in pmol per min per mg: MLP−/−, 33.0 ± 2.8; MLP−/−/βARKct, 32.8 ± 1.6; MLP+/+, 49.7 ± 4.8 (P < 0.02, MLP+/+ vs. MLP−/−/βARKct and MLP−/−). *P < 0.02, MLP+/+ vs. MLP−/−/βARKct and MLP−/−;
P < 0.005, ISO vs. basal for MLP−/−/βARKct and MLP+/+;
P < 0.02, ISO MLP−/−/βARKct vs. ISO MLP+/+ and ISO MLP−/−;
P = 0.07 basal MLP+/+ vs. basal MLP−/−/βARKct and basal MLP−/−. ISO, isoproterenol.
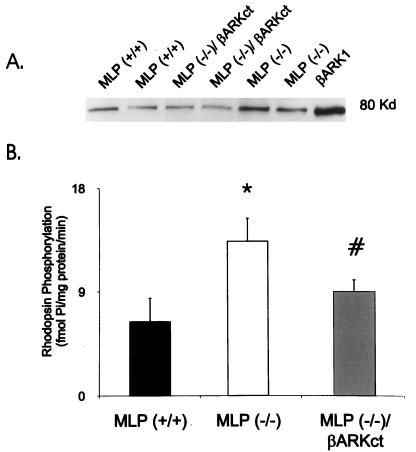
Because βARK1 levels and activity are enhanced in human heart failure, we sought to determine whether myocardial βARK1 levels are increased in the MLP−/− mouse, which could contribute to the marked βAR uncoupling observed. Myocardial βARK1 levels were assessed by immunoprecipitation of soluble heart extracts. Cytosolic βARK1 levels were ≈2-fold higher in the hearts of MLP−/− animals compared with MLP+/+ animals (Fig. 3A). Furthermore, we measured enzyme activity in extracts from myocardial membranes, because this is where βARK1 exerts its regulatory actions. We found membrane GRK activity to be significantly increased and parallel to the protein data (Fig. 3B). Immunoblotting for a second GRK found in the heart, GRK5, revealed no difference in expression between the different lines of mice (data not shown), indicating that the increase in membrane kinase activity can be accounted for by translocated βARK1 to the membrane. Taken together, these data show that chronic myocardial expression of the βARKct eliminates development of the severe heart failure phenotype and prevents the up-regulation of βARK1 as demonstrated by both protein immunoblotting and GRK activity (Fig. 3 A and B).
Figure 3.
Assessment of myocardial βARK1 levels and activity. (A) βARK1 protein levels of myocardial extracts were determined by protein immunoblotting. Shown is a representative experiment with two hearts from each gene-targeted mouse. Similar results were obtained in two more hearts in each group. βARK1 protein levels were ≈2-fold higher in the MLP−/− hearts compared with MLP+/+ hearts (P < 0.05) whereas myocardial βARK1 levels in the MLP−/−/βARKct hearts were not significantly different than wild-type hearts. (B) Rhodopsin-enriched rod outer segment membranes were used as a substrate for membrane myocardial GRK activity and 32P incorporation was quantified from dried gels. The data represent a sample size of four to six hearts in each group. ∗, P < 0.05 vs. MLP+/+; #, P < 0.03 vs. MLP−/−.
The deleterious effects of β2AR overexpression in the MLP−/− mice is demonstrated further by the marked adverse effect on survival. Cumulative survival probability for MLP−/−/β2AR mice (n = 11) was shortened significantly such that over a 9-month follow-up period the probability of survival was approximately 12% compared with 80% for the MLP−/− mice (n = 19; P < 0.0001, Mantel–Haenszel χ2 statistic, MLP−/−/β2AR vs. MLP−/−). Over the same time period only 1 of 10 MLP−/−/βARKct mice died, which occurred at 7 months of age, and did not differ significantly from the MLP−/− mice. These data clearly demonstrate that enhancing βAR signaling through marked overexpression of β2ARs in this murine model of myocardial failure has deleterious consequences that are accompanied by persistent cardiac chamber enlargement, progressive deterioration in cardiac function, and diminished survival. In contrast, reversal of βAR desensitization through chronic overexpression of a βARK1 inhibitor essentially prevents the development of heart failure in MLP−/− mice.
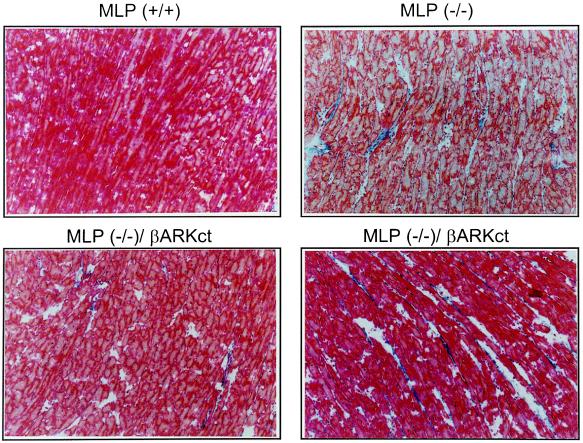
We also examined the hearts of these gene-targeted mice histologically and found that the characteristic fibrosis found in the MLP−/− hearts (14) did not seem to lessen because of the presence of the βARKct (Fig. 4). This is not surprising and suggests that the histopathological changes in the MLP−/− hearts are a result of the absence of the cytoskeletal MLP protein and are not a result of the dilated cardiomyopathy per se.
Figure 4.
Histological analysis of gene-targeted mouse hearts. Representative sections stained with Masson’s trichrome are shown from MLP+/+ and MLP−/− hearts and two MLP−/−/βARKct hearts.
DISCUSSION
The above echocardiographic and hemodynamic findings suggest that GRK-mediated βAR desensitization plays a causative role in the development of heart failure in this animal model. Moreover, they indicate the potential for novel therapeutic strategies that aim to modulate the activity level of myocardial βARK1 during the course of the cardiomyopathic process. We have chosen to use a genetic-based model of dilated cardiomyopathy through targeted disruption of the muscle LIM protein gene, which closely resembles the phenotype of human dilated cardiomyopathy (14). The MLP is a conserved positive regulator of myogenic differentiation, and recent findings suggest that it may act as a molecular adapter to promote protein assembly along the actin-based cytoskeleton. Hearts from MLP-deficient mice are characterized by marked disruption of cardiomyocyte architecture (14). Human heart failure also is a disease of cardiac muscle characterized by alterations in cardiomyocyte shape (19), cytoskeletal abnormalities (20), and βAR signaling (5), which supports our use of the MLP-deficient mouse as a model system to study underlying mechanisms in this disease.
Although abnormalities in βAR signaling have been postulated to promote cardiac dysfunction in the failing heart, other hypotheses have been suggested, which include altered Ca2+ availability (21), impaired ability of the L-type Ca2+ channel to activate sarcoplasmic reticulum Ca2+ release (22), and abnormalities in myocyte cytoskeleton such as microtubular polymerization (23). Although the precise mode of action by which overexpression of the βARKct provides this salutary effect is not clear, it is unlikely to be a result of a nonspecific effect of transgene overexpression because cardiac overexpression of a β2AR transgene in the MLP-deficient background had no effect on the heart failure phenotype. The strikingly opposite phenotypes observed with overexpression of the β2AR and overexpression of the βARKct suggests that marked chronic enhancement of βAR signaling by markedly increasing receptor number is not sufficient to prevent deterioration in cardiac function. In contrast, reversal of β1AR desensitization through overexpression of a βARK1 inhibitor acts to restore normal G protein coupling of the endogenous uncoupled β1ARs. The lack of a beneficial effect with β2AR transgene overexpression may in part be related to the extraordinarily high levels of receptor overexpression in those mice (15) or perhaps a result of different signaling properties of the β2AR compared with the β1AR (24). Furthermore, β2AR overexpression in this model leads to constant maximal signaling (15), whereas βARKct overexpression preserves myocardial βAR responsiveness to endogenous catecholamine stimulation (11). The deleterious effect of chronic βAR stimulation in the MLP−/−/β2AR mice is consistent with the experience from clinical studies using oral inotropic agents in severe heart failure (25). Our data suggest that restoring normal control of β1AR signaling by inhibiting enhanced desensitization is an important mechanism to prevent the progressive deterioration in cardiac function in this model of heart failure. Interestingly, it has been shown recently that treatment with the βAR antagonist, carvedilol, can improve survival in human heart failure (26). Although the mechanism(s) is not known, an intriguing basis for this beneficial effect of βAR antagonism may be the reduction of desensitization through lowering of βARK1 levels (ref. 10; G.I., E. D. Tomhave, R.J.L., and W.J.K., unpublished results).
Because the βARKct peptide inhibits βARK1 activity via Gβγ sequestration, we cannot definitively exclude the possibility that inhibition of other Gβγ-dependent pathways contributes to the improved cardiac status of the MLP−/−/βARKct animals. However, several lines of evidence argue strongly for inhibition of βARK1 activity being the primary mechanism. First, the MLP−/−/βARKct mice have increased βAR-coupling efficiency demonstrated by the increased isoproterenol-stimulated adenylyl cyclase activity and in vivo cardiac function, which clearly suggests inhibition of desensitization, just as would be expected for βARK1 inhibition. Second, overexpression of the βARKct does not prevent the development of cardiac hypertrophy in response to pressure overload but reverses βAR desensitization (9), suggesting that other signaling pathways critical for the hypertrophic phenotype are unaffected by the βARKct. Third, mice heterozygous for the βARK1 gene deletion, which possess 50% less βARK1 enzyme compared with wild-type animals, have a cardiac phenotype of enhanced contractility and sensitivity to βAR agonists (H.A.R., S. A. Akhter, D.-J.C., M. Jaber, R.J.L., M. G. Caron, and W.J.K. unpublished results) essentially identical to the βARKct-overexpressing animals (11). Finally, when the βARKct transgenic mice were crossed into the βARK1 heterozygous knockout background, this led to a further enhancement of cardiac function and isoproterenol responsiveness, which was associated with a stepwise decrement in Gβγ-dependent βARK1 activity (H.A.R., S. A. Akhter, D.-J.C., M. Jaber, R.J.L., M. G. Caron, and W.J.K. unpublished results).
Other known actions of Gβγ (27, 28) such as activation of the Ik.ach channel, adenylyl cyclase, PLCβ1–3, and MAP kinase appear not to be relevant in this situation. The Ik.ach channel is located in atrial and not ventricular tissue and would not be expected to directly alter contractility (27, 29). The isoforms of adenylyl cyclase that are regulated by Gβγ (I, II, IV) are not found in the heart (28). PLCβ is not activated by myocardial βARs (27). Finally, in vivo activation of MAP kinase does not affect contractility (30). Taken together, these data strongly suggest that the striking effects of the βARKct indeed are mediated primarily through βARK1 inhibition and restored βAR coupling and not through effects on other Gβγ-dependent pathways.
We do not, however, currently know whether the beneficial effect of βARK1 inhibition also is mediated in part through reduction of desensitization of G protein-coupled receptors other than βARs such as, for example, the angiotensin (18) or endothelin receptor. However, if this were the case, an opposite result would be expected. Inhibition of βARK1 by the βARKct would increase signaling through these pathways, whereas previous studies have demonstrated the marked benefit of blocking both angiotensin (31) and endothelin (32) signaling in heart failure.
The evidence we present here demonstrates the critical role that chronic βAR desensitization plays in the development of heart failure and the importance of preserving normal βAR coupling. That the inhibition of a single molecule (βARK1) can have such a dramatic effect on a cardiac phenotype caused by a structural abnormality (MLP deficiency) is surprising and indicates that βARK1 inhibition may offer a novel therapeutic target in heart failure with the potential to have significant impact on this disease.
Acknowledgments
We thank Dr. Lan Mao for her expertise in microsurgical techniques in the mouse, Ms. Nancy Dalton for expert technical assistance with mouse echocardiography, Julie Sheridan for help in setting up the cross-breeding, and Eric D. Tomhave for help with the biochemistry. This work was supported in part by National Institutes of Health Grants HL 56687 (H.A.R.), HL16037 (R.J.L.), HL46345 (K.R.C.), and HL53773 (J.R.).
ABBREVIATIONS
- LV
left ventricular
- βAR
β-adrenergic receptor
- βARK1
βAR kinase
- GRK
G protein-coupled receptor kinase
- MLP
muscle LIM protein
References
- 1.Inglese J, Freedman N J, Koch W J, Lefkowitz R J. J Biol Chem. 1993;268:23735–23738. [PubMed] [Google Scholar]
- 2.Lefkowitz R J. Cell. 1993;74:409–412. doi: 10.1016/0092-8674(93)80042-d. [DOI] [PubMed] [Google Scholar]
- 3.Pitcher J A, Inglese J, Higgins J B, Arriza J L, Casey P J, Kim C, Benovic J L, Kwatra M M, Caron M G, Lefkowitz R J. Science. 1992;257:1264–1267. doi: 10.1126/science.1325672. [DOI] [PubMed] [Google Scholar]
- 4.Koch W J, Inglese J, Stone W C, Lefkowitz R J. J Biol Chem. 1993;268:8256–8260. [PubMed] [Google Scholar]
- 5.Bristow M R, Ginsburg R, Minobe W, Cubicciotti R S, Sageman W S, Lurie K, Billingham M E, Harrison D C, Stinson E B. N Engl J Med. 1982;307:205–211. doi: 10.1056/NEJM198207223070401. [DOI] [PubMed] [Google Scholar]
- 6.Colucci W S, Denniss A R, Leatherman G F, Quigg R J, Ludmer P L, Marsh J D, Gauthier D F. J Clin Invest. 1988;81:1103–1110. doi: 10.1172/JCI113423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ungerer M, Bohm M, Elce J S, Erdmann E, Lohse M J. Circulation. 1993;87:454–463. doi: 10.1161/01.cir.87.2.454. [DOI] [PubMed] [Google Scholar]
- 8.Lohse M J, Krasel C, Winstel R, Mayor F., Jr Kidney Int. 1996;49:1047–1052. doi: 10.1038/ki.1996.153. [DOI] [PubMed] [Google Scholar]
- 9.Choi D J, Koch W J, Hunter J J, Rockman H A. J Biol Chem. 1997;272:17223–17229. doi: 10.1074/jbc.272.27.17223. [DOI] [PubMed] [Google Scholar]
- 10.Ping P, Gelzer-Bell R, Roth D A, Kiel D, Insel P A, Hammond H K. J Clin Invest. 1995;95:1271–1280. doi: 10.1172/JCI117777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koch W J, Rockman H A, Samama P, Hamilton R A, Bond R A, Milano C A, Lefkowitz R J. Science. 1995;268:1350–1353. doi: 10.1126/science.7761854. [DOI] [PubMed] [Google Scholar]
- 12.Rockman, H. A., Koch, W. J., Milano, C. A. & Lefkowitz, R. J. (1996) J. Mol. Med. . [DOI] [PubMed]
- 13.Chien K R. J Clin Invest. 1996;97:901–909. doi: 10.1172/JCI118512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arber S, Hunter J J, Ross J, Jr, Hongo M, Sansig G, Borg J, Perriard J-C, Chien K R, Caroni P. Cell. 1997;88:393–403. doi: 10.1016/s0092-8674(00)81878-4. [DOI] [PubMed] [Google Scholar]
- 15.Milano C A, Allen L F, Rockman H A, Dolber P C, McMinn T R, Chien K R, Johnson T D, Bond R A, Lefkowitz R J. Science. 1994;264:582–586. doi: 10.1126/science.8160017. [DOI] [PubMed] [Google Scholar]
- 16.Tanaka N, Dalton N, Mao L, Rockman H A, Peterson K L, Gottshall K R, Hunter J J, Chien K R, Ross J., Jr Circulation. 1996;94:1109–1117. doi: 10.1161/01.cir.94.5.1109. [DOI] [PubMed] [Google Scholar]
- 17.Rockman H A, Ross R S, Harris A N, Knowlton K U, Steinhelper M E, Field L J, Ross J, Jr, Chien K R. Proc Natl Acad Sci USA. 1991;88:8277–8281. doi: 10.1073/pnas.88.18.8277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rockman H A, Choi D J, Rahman N U, Akhter S A, Lefkowitz R J, Koch W J. Proc Natl Acad Sci USA. 1996;93:9954–9959. doi: 10.1073/pnas.93.18.9954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gerdes A M, Kellerman S E, Moore J A, Muffly K E, Clark L C, Reaves P Y, Malec K B, McKeown P P, Schocken D D. Circulation. 1992;86:426–430. doi: 10.1161/01.cir.86.2.426. [DOI] [PubMed] [Google Scholar]
- 20.Schaper J, Froede R, Hein S, Buck A, Hashizume H, Speiser B, Friedl A, Bleese N. Circulation. 1991;83:504–514. doi: 10.1161/01.cir.83.2.504. [DOI] [PubMed] [Google Scholar]
- 21.Gwathmey J K, Copelas L, MacKinnon R, Schoen F J, Feldman M D, Grossman W, Morgan J P. Circ Res. 1987;61:70–76. doi: 10.1161/01.res.61.1.70. [DOI] [PubMed] [Google Scholar]
- 22.Gomez A M, Valdivia H H, Cheng H, Lederer M R, Santana L F, Cannell M B, McCune S A, Altschuld R A, Lederer W J. Science. 1997;276:800–806. doi: 10.1126/science.276.5313.800. [DOI] [PubMed] [Google Scholar]
- 23.Tsutsui H, Ishihara K, Cooper G, IV. Science. 1993;260:682–687. doi: 10.1126/science.8097594. [DOI] [PubMed] [Google Scholar]
- 24.Xiao R P, Hohl C, Altschuld R, Jones L, Livingston B, Ziman B, Tantini B, Lakatta E G. J Biol Chem. 1994;269:19151–19156. [PubMed] [Google Scholar]
- 25.Packer M, Carver J R, Rodeheffer R J, Ivanhoe R J, DiBianco R, Zeldis S M, Hendrix G H, Bommer W J, Elkayam U, Kukin M L, et al. N Engl J Med. 1991;325:1468–1475. doi: 10.1056/NEJM199111213252103. [DOI] [PubMed] [Google Scholar]
- 26.Packer M, Bristow M R, Cohn J N, Colucci W S, Fowler M B, Gilbert E M, Shusterman N H. N Engl J Med. 1996;334:1349–1355. doi: 10.1056/NEJM199605233342101. [DOI] [PubMed] [Google Scholar]
- 27.Clapham D E, Neer E J. Nature (London) 1993;365:403–406. doi: 10.1038/365403a0. [DOI] [PubMed] [Google Scholar]
- 28.Tang W J, Gilman A G. Cell. 1992;70:869–872. doi: 10.1016/0092-8674(92)90236-6. [DOI] [PubMed] [Google Scholar]
- 29.Nair L A, Inglese J, Stoffel R, Koch W J, Lefkowitz R J, Kwatra M M, Grant A O. Circ Res. 1995;76:832–838. doi: 10.1161/01.res.76.5.832. [DOI] [PubMed] [Google Scholar]
- 30.Hunter J J, Tanaka N, Rockman H A, Ross J, Jr, Chien K R. J Biol Chem. 1995;270:23173–23178. doi: 10.1074/jbc.270.39.23173. [DOI] [PubMed] [Google Scholar]
- 31.Pitt B, Segal R, Martinez F A, Meurers G, Cowley A J, Thomas I, Deedwania P C, Ney D E, Snavely D B, Chang P I. Lancet. 1997;349:747–752. doi: 10.1016/s0140-6736(97)01187-2. [DOI] [PubMed] [Google Scholar]
- 32.Sakai S, Miyauchi T, Kobayashi M, Yamaguchi I, Goto K, Sugishita Y. Nature (London) 1996;384:353–355. doi: 10.1038/384353a0. [DOI] [PubMed] [Google Scholar]