Abstract
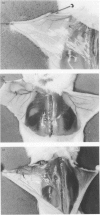
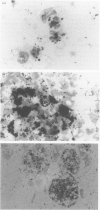
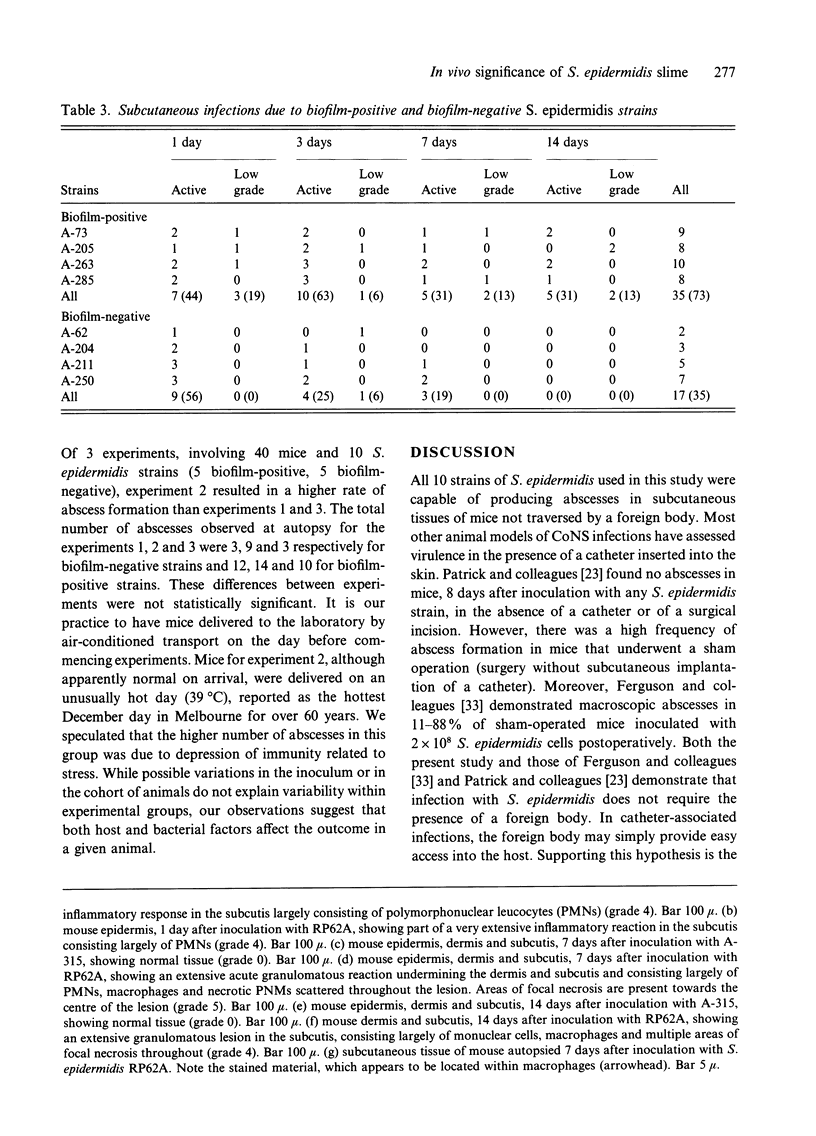
The ability to produce large quantities of biofilm on solid surfaces in vitro is believed to distinguish potentially pathogenic strains of Staphylococcus epidermidis from commensals. Biofilm consists of staphylococcal cells encased in a matrix of extracellular polysaccharide (also referred to as slime), firmly adherent to each other and to the underlying surface structure. The association of slime with colonization of catheter surfaces in vivo has been examined extensively. Less attention has been paid to the contribution of slime to infections that occur in the absence of an inserted device. In a mouse model of subcutaneous infection without an implanted device 10 S. epidermidis strains (5 slime-positive, 5 slime-negative) produced abscesses; thus a foreign body is not essential for the expression of virulence by S. epidermidis. Biofilm-positive strains produced significantly more abscesses, that persisted longer than biofilm-negative strains. In these chronic infections, large numbers of staphylococci were associated with macrophages and viable staphylococci were cultured from specimens of pus collected at autopsy. Thus slime or components of slime appear to delay the clearance of S. epidermidis from host tissues, possibly by interfering with intracellular killing mechanisms. However, differences in the capacity to produce abscesses, within both the slime-positive and slime-negative groups, indicate that other factors also contribute to the virulence of S. epidermidis.
Full text
PDF


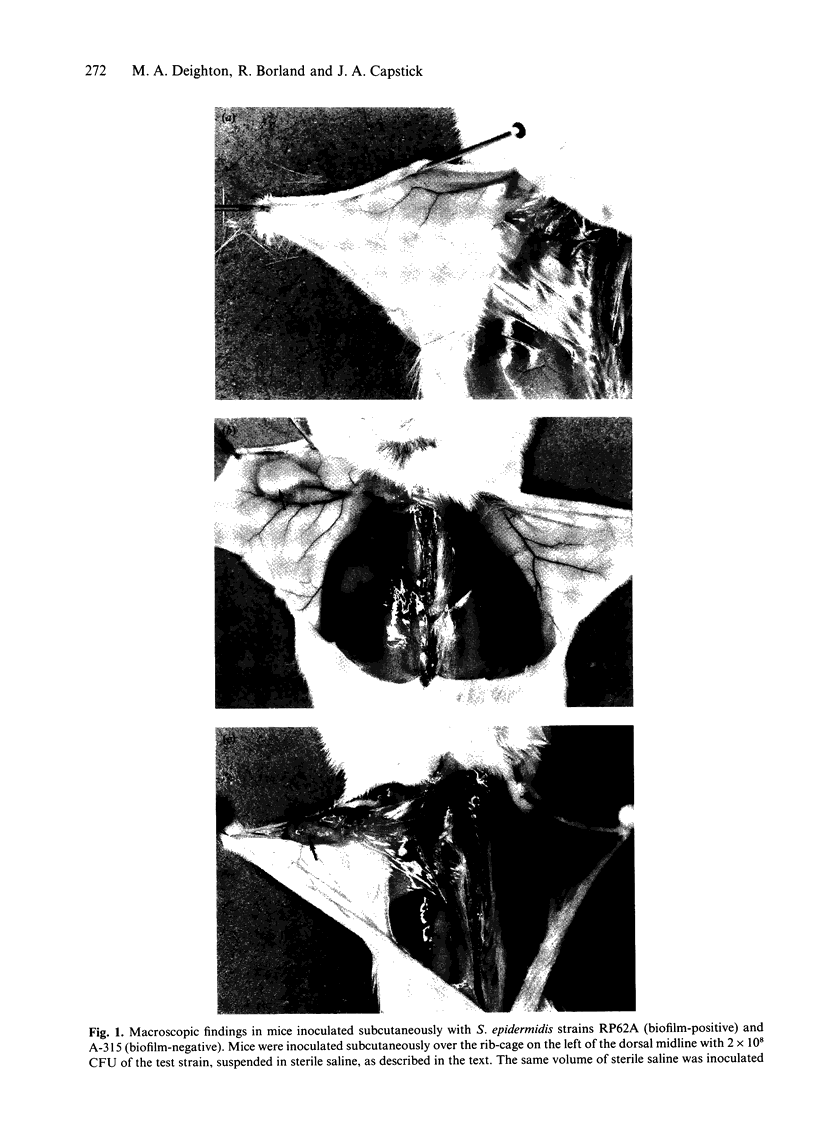

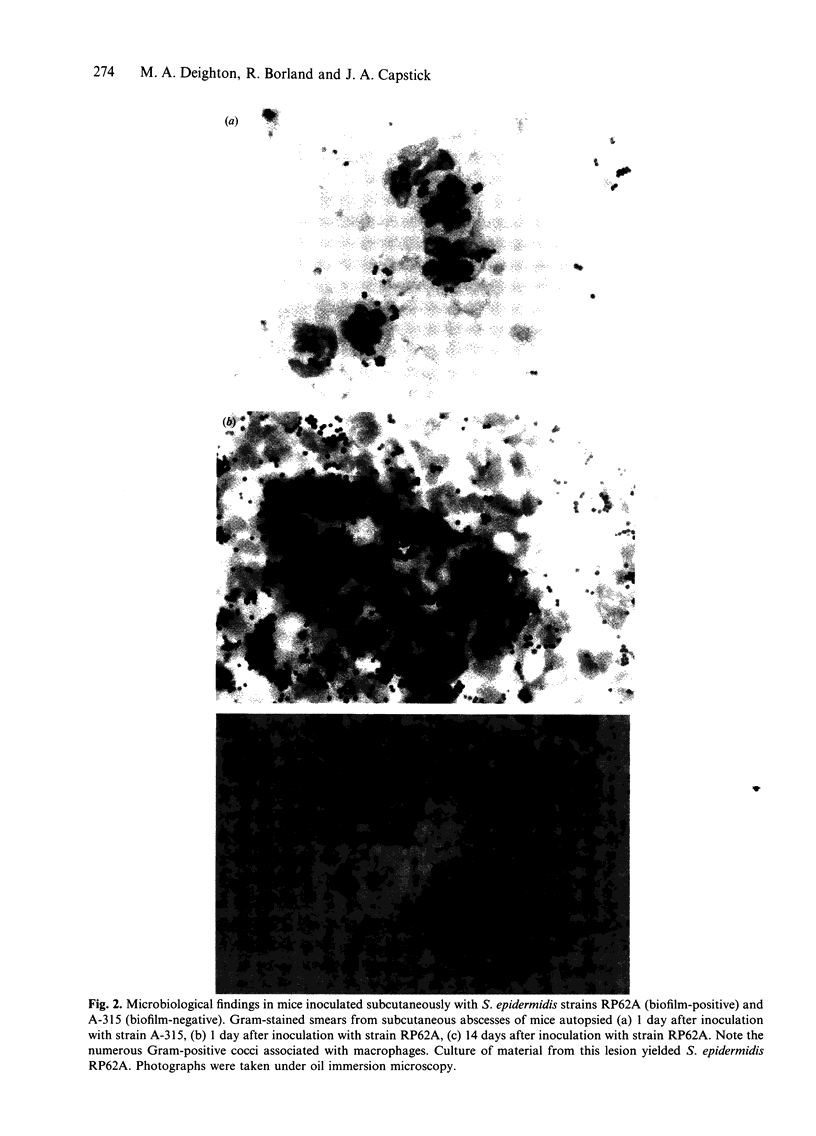

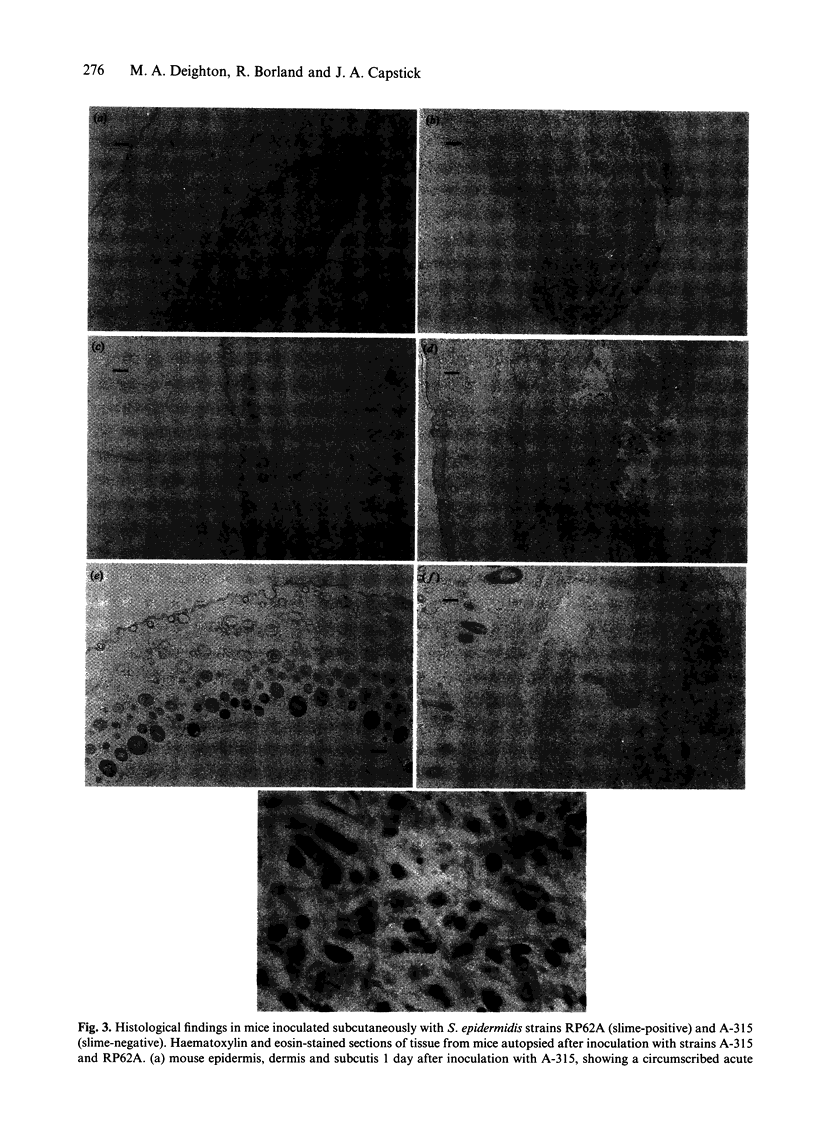



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barth E., Myrvik Q. M., Wagner W., Gristina A. G. In vitro and in vivo comparative colonization of Staphylococcus aureus and Staphylococcus epidermidis on orthopaedic implant materials. Biomaterials. 1989 Jul;10(5):325–328. doi: 10.1016/0142-9612(89)90073-2. [DOI] [PubMed] [Google Scholar]
- Bayston R., Rodgers J. Production of extra-cellular slime by Staphylococcus epidermidis during stationary phase of growth: its association with adherence to implantable devices. J Clin Pathol. 1990 Oct;43(10):866–870. doi: 10.1136/jcp.43.10.866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen G. D., Baddour L. M., Madison B. M., Parisi J. T., Abraham S. N., Hasty D. L., Lowrance J. H., Josephs J. A., Simpson W. A. Colonial morphology of staphylococci on Memphis agar: phase variation of slime production, resistance to beta-lactam antibiotics, and virulence. J Infect Dis. 1990 Jun;161(6):1153–1169. doi: 10.1093/infdis/161.6.1153. [DOI] [PubMed] [Google Scholar]
- Christensen G. D., Baddour L. M., Simpson W. A. Phenotypic variation of Staphylococcus epidermidis slime production in vitro and in vivo. Infect Immun. 1987 Dec;55(12):2870–2877. doi: 10.1128/iai.55.12.2870-2877.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen G. D., Barker L. P., Mawhinney T. P., Baddour L. M., Simpson W. A. Identification of an antigenic marker of slime production for Staphylococcus epidermidis. Infect Immun. 1990 Sep;58(9):2906–2911. doi: 10.1128/iai.58.9.2906-2911.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen G. D., Parisi J. T., Bisno A. L., Simpson W. A., Beachey E. H. Characterization of clinically significant strains of coagulase-negative staphylococci. J Clin Microbiol. 1983 Aug;18(2):258–269. doi: 10.1128/jcm.18.2.258-269.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen G. D., Simpson W. A., Bisno A. L., Beachey E. H. Adherence of slime-producing strains of Staphylococcus epidermidis to smooth surfaces. Infect Immun. 1982 Jul;37(1):318–326. doi: 10.1128/iai.37.1.318-326.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen G. D., Simpson W. A., Bisno A. L., Beachey E. H. Experimental foreign body infections in mice challenged with slime-producing Staphylococcus epidermidis. Infect Immun. 1983 Apr;40(1):407–410. doi: 10.1128/iai.40.1.407-410.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen G. D. The confusing and tenacious coagulase-negative staphylococci. Adv Intern Med. 1987;32:177–192. [PubMed] [Google Scholar]
- Cooper G. L., Schiller A. L., Hopkins C. C. Possible role of capillary action in pathogenesis of experimental catheter-associated dermal tunnel infections. J Clin Microbiol. 1988 Jan;26(1):8–12. doi: 10.1128/jcm.26.1.8-12.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deighton M. A., Capstick J., Borland R. A study of phenotypic variation of Staphylococcus epidermidis using Congo red agar. Epidemiol Infect. 1992 Dec;109(3):423–432. doi: 10.1017/s095026880005041x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deighton M., Borland R. Regulation of slime production in Staphylococcus epidermidis by iron limitation. Infect Immun. 1993 Oct;61(10):4473–4479. doi: 10.1128/iai.61.10.4473-4479.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deighton M., Pearson S., Capstick J., Spelman D., Borland R. Phenotypic variation of Staphylococcus epidermidis isolated from a patient with native valve endocarditis. J Clin Microbiol. 1992 Sep;30(9):2385–2390. doi: 10.1128/jcm.30.9.2385-2390.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devriese L. A., Laevens H., Haesebrouck F., Hommez J. A simple identification scheme for coagulase negative staphylococci from bovine mastitis. Res Vet Sci. 1994 Sep;57(2):240–244. doi: 10.1016/0034-5288(94)90064-7. [DOI] [PubMed] [Google Scholar]
- Dickinson G. M., Bisno A. L. Infections associated with indwelling devices: concepts of pathogenesis; infections associated with intravascular devices. Antimicrob Agents Chemother. 1989 May;33(5):597–601. doi: 10.1128/aac.33.5.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson D. A., Jr, Veringa E. M., Mayberry W. R., Overbeek B. P., Lambe D. W., Jr, Verhoef J. Bacteroides and Staphylococcus glycocalyx: chemical analysis, and the effects on chemiluminescence and chemotaxis of human polymorphonuclear leucocytes. Microbios. 1992;69(278):53–65. [PubMed] [Google Scholar]
- Finlay B. B., Falkow S. Common themes in microbial pathogenicity. Microbiol Rev. 1989 Jun;53(2):210–230. doi: 10.1128/mr.53.2.210-230.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleer A., Senders R. C., Visser M. R., Bijlmer R. P., Gerards L. J., Kraaijeveld C. A., Verhoef J. Septicemia due to coagulase-negative staphylococci in a neonatal intensive care unit: clinical and bacteriological features and contaminated parenteral fluids as a source of sepsis. Pediatr Infect Dis. 1983 Nov-Dec;2(6):426–431. doi: 10.1097/00006454-198311000-00003. [DOI] [PubMed] [Google Scholar]
- Goldmann D. A., Pier G. B. Pathogenesis of infections related to intravascular catheterization. Clin Microbiol Rev. 1993 Apr;6(2):176–192. doi: 10.1128/cmr.6.2.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann M., Vaudaux P. E., Pittet D., Auckenthaler R., Lew P. D., Schumacher-Perdreau F., Peters G., Waldvogel F. A. Fibronectin, fibrinogen, and laminin act as mediators of adherence of clinical staphylococcal isolates to foreign material. J Infect Dis. 1988 Oct;158(4):693–701. doi: 10.1093/infdis/158.4.693. [DOI] [PubMed] [Google Scholar]
- Hébert G. A., Hancock G. A. Synergistic hemolysis exhibited by species of staphylococci. J Clin Microbiol. 1985 Sep;22(3):409–415. doi: 10.1128/jcm.22.3.409-415.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis W. R., Martone W. J. Predominant pathogens in hospital infections. J Antimicrob Chemother. 1992 Apr;29 (Suppl A):19–24. doi: 10.1093/jac/29.suppl_a.19. [DOI] [PubMed] [Google Scholar]
- Johnson G. M., Lee D. A., Regelmann W. E., Gray E. D., Peters G., Quie P. G. Interference with granulocyte function by Staphylococcus epidermidis slime. Infect Immun. 1986 Oct;54(1):13–20. doi: 10.1128/iai.54.1.13-20.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristinsson K. G., Hastings J. G., Spencer R. C. The role of extracellular slime in opsonophagocytosis of Staphylococcus epidermidis. J Med Microbiol. 1988 Nov;27(3):207–213. doi: 10.1099/00222615-27-3-207. [DOI] [PubMed] [Google Scholar]
- Lambe D. W., Jr, Ferguson K. P., Keplinger J. L., Gemmell C. G., Kalbfleisch J. H. Pathogenicity of Staphylococcus lugdunensis, Staphylococcus schleiferi, and three other coagulase-negative staphylococci in a mouse model and possible virulence factors. Can J Microbiol. 1990 Jul;36(7):455–463. doi: 10.1139/m90-080. [DOI] [PubMed] [Google Scholar]
- Mack D., Siemssen N., Laufs R. Parallel induction by glucose of adherence and a polysaccharide antigen specific for plastic-adherent Staphylococcus epidermidis: evidence for functional relation to intercellular adhesion. Infect Immun. 1992 May;60(5):2048–2057. doi: 10.1128/iai.60.5.2048-2057.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller E., Hübner J., Gutierrez N., Takeda S., Goldmann D. A., Pier G. B. Isolation and characterization of transposon mutants of Staphylococcus epidermidis deficient in capsular polysaccharide/adhesin and slime. Infect Immun. 1993 Feb;61(2):551–558. doi: 10.1128/iai.61.2.551-558.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myrvik Q. N., Wagner W., Barth E., Wood P., Gristina A. G. Effects of extracellular slime produced by Staphylococcus epidermidis on oxidative responses of rabbit alveolar macrophages. J Invest Surg. 1989;2(4):381–389. doi: 10.3109/08941938909018263. [DOI] [PubMed] [Google Scholar]
- Noble M. A., Reid P. E., Park C. M., Chan V. Y. Inhibition of human neutrophil bacteriocidal activity by extracellular substance from slime-producing Staphylococcus epidermidis. Diagn Microbiol Infect Dis. 1986 Apr;4(4):335–339. doi: 10.1016/0732-8893(86)90074-x. [DOI] [PubMed] [Google Scholar]
- Patrick C. C., Plaunt M. R., Hetherington S. V., May S. M. Role of the Staphylococcus epidermidis slime layer in experimental tunnel tract infections. Infect Immun. 1992 Apr;60(4):1363–1367. doi: 10.1128/iai.60.4.1363-1367.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters G., Locci R., Pulverer G. Adherence and growth of coagulase-negative staphylococci on surfaces of intravenous catheters. J Infect Dis. 1982 Oct;146(4):479–482. doi: 10.1093/infdis/146.4.479. [DOI] [PubMed] [Google Scholar]
- Peters G. New considerations in the pathogenesis of coagulase-negative staphylococcal foreign body infections. J Antimicrob Chemother. 1988 Apr;21 (Suppl 100):139–148. doi: 10.1093/jac/21.suppl_c.139. [DOI] [PubMed] [Google Scholar]
- Stout R. D., Ferguson K. P., Li Y. N., Lambe D. W., Jr Staphylococcal exopolysaccharides inhibit lymphocyte proliferative responses by activation of monocyte prostaglandin production. Infect Immun. 1992 Mar;60(3):922–927. doi: 10.1128/iai.60.3.922-927.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda S., Pier G. B., Kojima Y., Tojo M., Muller E., Tosteson T., Goldmann D. A. Protection against endocarditis due to Staphylococcus epidermidis by immunization with capsular polysaccharide/adhesin. Circulation. 1991 Dec;84(6):2539–2546. doi: 10.1161/01.cir.84.6.2539. [DOI] [PubMed] [Google Scholar]
- Timmerman C. P., Fleer A., Besnier J. M., De Graaf L., Cremers F., Verhoef J. Characterization of a proteinaceous adhesin of Staphylococcus epidermidis which mediates attachment to polystyrene. Infect Immun. 1991 Nov;59(11):4187–4192. doi: 10.1128/iai.59.11.4187-4192.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tojo M., Yamashita N., Goldmann D. A., Pier G. B. Isolation and characterization of a capsular polysaccharide adhesin from Staphylococcus epidermidis. J Infect Dis. 1988 Apr;157(4):713–722. doi: 10.1093/infdis/157.4.713. [DOI] [PubMed] [Google Scholar]
- van Bronswijk H., Verbrugh H. A., Heezius H. C., Renders N. H., Fleer A., van der Meulen J., Oe P. L., Verhoef J. Heterogeneity in opsonic requirements of Staphylococcus epidermidis: relative importance of surface hydrophobicity, capsules and slime. Immunology. 1989 May;67(1):81–86. [PMC free article] [PubMed] [Google Scholar]