Abstract
Oral administration to newly hatched chickens or to chicks up to 5 days of age with an avirulent, rough, spectinomycin-resistant mutant of Salmonella typhimurium strain F98 inhibited the colonization of a nalidixic acid-resistant mutant of the same strain administered by the same route 1 day later. The second strain passed rapidly through the alimentary tract and persisted in the caeca of only a few chickens. Resistance to colonization did not develop until 24 h after inoculation of the first strain but was still evident if the second strain was inoculated up to 7 days later. Resistance occurred in 5 different breeds of chicken and in chickens reared on 5 different diets. Protection was evident against a very high challenge dose and could be produced by the introduction of small numbers of the first strain. Pre-colonization of chicks with the first strain of F98 reduced faecal excretion of the second strain over many weeks, whether chickens were challenged directly or by contact with other infected chickens. The rough strain F98 produced protection against only a few S. typhimurium strains and not against other serotypes. However, strains of S. infantis and S. heidelberg, chosen because they colonized the chicken alimentary tract better than did F98, produced inhibition of a wider range of serotypes.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnes E. M., Mead G. C., Barnum D. A., Harry E. G. The intestinal flora of the chicken in the period 2 to 6 weeks of age, with particular reference to the anaerobic bacteria. Br Poult Sci. 1972 May;13(3):311–326. doi: 10.1080/00071667208415953. [DOI] [PubMed] [Google Scholar]
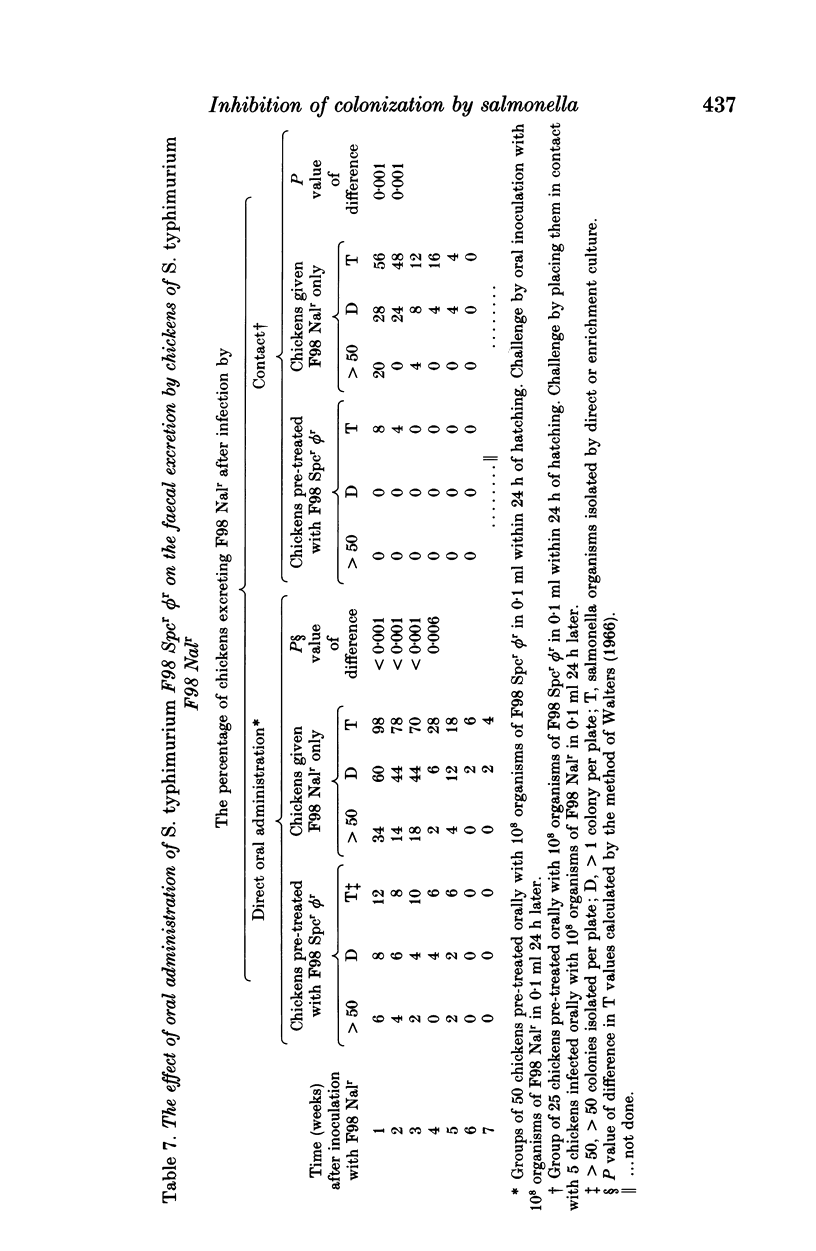
- Barrow P. A., Hassan J. O., Berchieri A., Jr Reduction in faecal excretion of Salmonella typhimurium strain F98 in chickens vaccinated with live and killed S. typhimurium organisms. Epidemiol Infect. 1990 Jun;104(3):413–426. doi: 10.1017/s0950268800047439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrow P. A., Tucker J. F. Inhibition of colonization of the chicken caecum with Salmonella typhimurium by pre-treatment with strains of Escherichia coli. J Hyg (Lond) 1986 Apr;96(2):161–169. doi: 10.1017/s0022172400065931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrow P. A., Tucker J. F., Simpson J. M. Inhibition of colonization of the chicken alimentary tract with Salmonella typhimurium gram-negative facultatively anaerobic bacteria. Epidemiol Infect. 1987 Jun;98(3):311–322. doi: 10.1017/s0950268800062063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borriello S. P., Barclay F. E. Protection of hamsters against Clostridium difficile ileocaecitis by prior colonisation with non-pathogenic strains. J Med Microbiol. 1985 Jun;19(3):339–350. doi: 10.1099/00222615-19-3-339. [DOI] [PubMed] [Google Scholar]
- Coloe P. J., Bagust T. J., Ireland L. Development of the normal gastrointestinal microflora of specific pathogen-free chickens. J Hyg (Lond) 1984 Feb;92(1):79–87. doi: 10.1017/s0022172400064056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtiss R., 3rd, Kelly S. M. Salmonella typhimurium deletion mutants lacking adenylate cyclase and cyclic AMP receptor protein are avirulent and immunogenic. Infect Immun. 1987 Dec;55(12):3035–3043. doi: 10.1128/iai.55.12.3035-3043.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson J. N., Hirsh D. C. Bacterial competition as a means of preventing neonatal diarrhea in pigs. Infect Immun. 1976 Jun;13(6):1773–1774. doi: 10.1128/iai.13.6.1773-1774.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duval-Iflah Y., Chappuis J. P., Ducluzeau R., Raibaud P. Intraspecific interactions between Escherichia coli strains in human newborns and in gnotobiotic mice and piglets. Prog Food Nutr Sci. 1983;7(3-4):107–116. [PubMed] [Google Scholar]
- Impey C. S., Mead G. C. Fate of salmonellas in the alimentary tract of chicks pre-treated with a mature caecal microflora to increase colonization resistance. J Appl Bacteriol. 1989 Jun;66(6):469–475. doi: 10.1111/j.1365-2672.1989.tb04567.x. [DOI] [PubMed] [Google Scholar]
- Impey C. S., Mead G. C., George S. M. Competitive exclusion of salmonellas from the chick caecum using a defined mixture of bacterial isolates from the caecal microflora of an adult bird. J Hyg (Lond) 1982 Dec;89(3):479–490. doi: 10.1017/s0022172400071047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILNER K. C., SHAFFER M. F. Bacteriologic studies of experimental Salmonella infections in chicks. J Infect Dis. 1952 Jan-Feb;90(1):81–96. doi: 10.1093/infdis/90.1.81. [DOI] [PubMed] [Google Scholar]
- Noble W. C., Willie J. A. Interactions between antibiotic-producing and non-producing staphylococci in skin surface and sub-surface models. Br J Exp Pathol. 1980 Jun;61(3):339–343. [PMC free article] [PubMed] [Google Scholar]
- Ochi Y., Mitsuoka T., Sega T. Untersuchungen über die Darmflora des Huhnes. III. Die Entwicklung der Darmflora von Küken bis zum Huhn. Zentralbl Bakteriol Orig. 1964 Jun;193(1):80–95. [PubMed] [Google Scholar]
- Onderdonk A., Marshall B., Cisneros R., Levy S. B. Competition between congenic Escherichia coli K-12 strains in vivo. Infect Immun. 1981 Apr;32(1):74–79. doi: 10.1128/iai.32.1.74-79.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertsson J. A., Lindberg A. A., Hoiseth S., Stocker B. A. Salmonella typhimurium infection in calves: protection and survival of virulent challenge bacteria after immunization with live or inactivated vaccines. Infect Immun. 1983 Aug;41(2):742–750. doi: 10.1128/iai.41.2.742-750.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHINEFIELD H. R., RIBBLE J. C., BORIS M., EICHENWALD H. F. Bacterial interference: its effect on nursery-acquired infection with Staphylococcus aureus. I. Preliminary observations on artificial colonzation of newborns. Am J Dis Child. 1963 Jun;105:646–654. [PubMed] [Google Scholar]
- Smith H. W. The development of the flora of the alimentary tract in young animals. J Pathol Bacteriol. 1965 Oct;90(2):495–513. [PubMed] [Google Scholar]
- Smith H. W., Tucker J. F. The effect of antibiotic therapy on the faecal excretion of Salmonella typhimurium by experimentally infected chickens. J Hyg (Lond) 1975 Oct;75(2):275–292. doi: 10.1017/s0022172400047306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soerjadi A. S., Lloyd A. B., Cumming R. B. Streptococcus faecalis, a bacterial isolate which protects young chickens from enteric invasion by Salmonellae. Aust Vet J. 1978 Nov;54(11):549–550. doi: 10.1111/j.1751-0813.1978.tb00337.x. [DOI] [PubMed] [Google Scholar]
- Sprunt K., Leidy G. The use of bacterial interference to prevent infection. Can J Microbiol. 1988 Mar;34(3):332–338. doi: 10.1139/m88-061. [DOI] [PubMed] [Google Scholar]
- Williams Smith H., Tucker J. F. The virulence of salmonella strains for chickens: their excretion by infected chickens. J Hyg (Lond) 1980 Jun;84(3):479–488. doi: 10.1017/s0022172400027017. [DOI] [PMC free article] [PubMed] [Google Scholar]


