Abstract
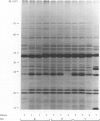
A quantitative carbon source growth assay, comprising ten carbon sources, was used to compare acinetobacter strains from three hospitals. The strains had been obtained during episodes of increased prevalence of isolations and were, for each hospital, assumed to be epidemiologically related. This assumption was supported by the electrophoretic protein profiles of the strains. Univariate analysis of growth data showed significant differences between strains from the three hospitals. Moreover, cluster analysis revealed that the major pattern in the data was related to the epidemiological origin of the strains. Exceptions to the epidemic-related pattern were observed. Thus, apart from epidemiological factors, other factors might contribute to carbon source growth profiles of the strains. It is concluded that the carbon growth assay may be useful to distinguish roughly between acinetobacter strains from different sites of origin. Further studies are required to analyse additional factors which influence carbon source growth of strains.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander M., Ismail F., Jackman P. J., Noble W. C. Fingerprinting Acinetobacter strains from clinical sources by numerical analysis of electrophoretic protein patterns. J Med Microbiol. 1984 Aug;18(1):55–64. doi: 10.1099/00222615-18-1-55. [DOI] [PubMed] [Google Scholar]
- Allen K. D., Green H. T. Hospital outbreak of multi-resistant Acinetobacter anitratus: an airborne mode of spread? J Hosp Infect. 1987 Mar;9(2):110–119. doi: 10.1016/0195-6701(87)90048-x. [DOI] [PubMed] [Google Scholar]
- Andrews H. J. Acinetobacter bacteriocin typing. J Hosp Infect. 1986 Mar;7(2):169–175. doi: 10.1016/0195-6701(86)90060-5. [DOI] [PubMed] [Google Scholar]
- Baumann P., Doudoroff M., Stanier R. Y. A study of the Moraxella group. II. Oxidative-negative species (genus Acinetobacter). J Bacteriol. 1968 May;95(5):1520–1541. doi: 10.1128/jb.95.5.1520-1541.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouvet P. J., Grimont P. A. Identification and biotyping of clinical isolates of Acinetobacter. Ann Inst Pasteur Microbiol. 1987 Sep-Oct;138(5):569–578. doi: 10.1016/0769-2609(87)90042-1. [DOI] [PubMed] [Google Scholar]
- Crombach W. H., Dijkshoorn L., van Noort-Klaassen M., Niessen J., van Knippenberg-Gordebeke G. Control of an epidemic spread of a multi-resistant strain of Acinetobacter calcoaceticus in a hospital. Intensive Care Med. 1989;15(3):166–170. doi: 10.1007/BF01058568. [DOI] [PubMed] [Google Scholar]
- Dijkshoorn L., Michel M. F., Degener J. E. Cell envelope protein profiles of Acinetobacter calcoaceticus strains isolated in hospitals. J Med Microbiol. 1987 Jun;23(4):313–319. doi: 10.1099/00222615-23-4-313. [DOI] [PubMed] [Google Scholar]
- Gerner-Smidt P., Hansen L., Knudsen A., Siboni K., Søgaard I. Epidemic spread of Acinetobacter calcoaceticus in a neurosurgical department analyzed by electronic data processing. J Hosp Infect. 1985 Jun;6(2):166–174. doi: 10.1016/s0195-6701(85)80094-3. [DOI] [PubMed] [Google Scholar]
- Gilardi G. L. Carbon assimilation by the Achromobacter-Moraxella group (Debord's tribe Mimease). Am J Med Technol. 1968 Jul;34(7):388–392. [PubMed] [Google Scholar]
- Glew R. H., Moellering R. C., Jr, Kunz L. J. Infections with Acinetobacter calcoaceticus (Herellea vaginicola): clinical and laboratory studies. Medicine (Baltimore) 1977 Mar;56(2):79–97. doi: 10.1097/00005792-197703000-00001. [DOI] [PubMed] [Google Scholar]
- Sneath P. H., Johnson R. The influence on numerical taxonomic similarities of errors in microbiological tests. J Gen Microbiol. 1972 Sep;72(2):377–392. doi: 10.1099/00221287-72-2-377. [DOI] [PubMed] [Google Scholar]
- Snell J. J., Lapage S. P. Carbon source utilization tests as an aid to the classification of non-fermenting gram-negative bacteria. J Gen Microbiol. 1973 Jan;74(1):9–20. doi: 10.1099/00221287-74-1-9. [DOI] [PubMed] [Google Scholar]
- Stanier R. Y., Palleroni N. J., Doudoroff M. The aerobic pseudomonads: a taxonomic study. J Gen Microbiol. 1966 May;43(2):159–271. doi: 10.1099/00221287-43-2-159. [DOI] [PubMed] [Google Scholar]