Abstract
Erythropoietin (EPO) is required for red blood cell development, but whether EPO-specific signals directly instruct erythroid differentiation is unknown. We used a dominant system in which constitutively active variants of the EPO receptor were introduced into erythroid progenitors in mice. Chimeric receptors were constructed by replacing the cytoplasmic tail of constitutively active variants of the EPO receptor with tails of diverse cytokine receptors. Receptors linked to granulocyte or platelet production supported complete erythroid development in vitro and in vivo, as did the growth hormone receptor, a nonhematopoietic receptor. Therefore, EPOR-specific signals are not required for terminal differentiation of erythrocytes. Furthermore, we found that cellular context can influence cytokine receptor signaling.
Hematopoiesis is a process whereby pluripotent stem cells give rise to all blood cells through an exponential expansion of progenitor cells coupled with progressive lineage restriction. These steps ultimately lead to production of terminally differentiated blood cells that exit the bone marrow. Control of these cellular decisions is determined, in part, through the action of specific growth factors or cytokines that can promote cellular proliferation, differentiation, and survival.
Erythropoietin (EPO) is a prototypical hematopoietic cytokine essential for mature red blood cell development (1, 2). Its biologic effects in vivo are remarkably specific for erythroid progenitors due in part to the restricted tissue expression of the EPO receptor (EPOR) (3, 4). In hematopoietic progenitor cell culture assays, the effects of EPO on committed erythroid progenitors occurs at a stage between the earliest burst forming unit-erythroid (BFU-E) and the more mature colony forming unit-erythroid (CFU-E) (5). Early BFU-E, which do not express significant levels of EPOR, lead to mature or late EPO-responsive BFU-E upon continued culture in the presence of stem cell factor (6, 7). Subsequently these cells generate CFU-E that express EPOR and are highly responsive to EPO. Mice with null mutations in the EPOR suffer embryonic death by embryonic day 13.5 due to a profound absence of definitive circulating red blood cells, but they generate normal numbers of BFU-E and slightly reduced levels of CFU-E in ex vivo cultures (1, 2). Thus, these observations suggest that EPO is required neither for erythroid lineage commitment (BFU-E development) nor for the transition from BFU-E to CFU-E but, instead, is required for the survival or proliferation of CFU-E and for their terminal differentiation. Although underscoring the vital role of EPO in complete erythroid development, these observations do not distinguish between either an instructive or a supportive role for EPO in terminal erythroid development. Studies in established cell lines, many nonerythroid in their origin, have suggested that specific differentiation signals may indeed be linked to the EPOR (8–10).
The present studies were undertaken to investigate whether activation of the EPOR confers specific differentiation signals upon primary committed erythroid progenitors (e.g., CFU-E) to complete their developmental programs and form terminally differentiated red blood cells. This work exploited constitutively activated chimeric EPOR variants containing the cytoplasmic domains of structurally related cytokine receptors that are not normally expressed in erythroid progenitors. These chimeric receptors were composed of the extracellular domain of the EPOR containing an R129C point substitution that causes constitutive covalent receptor homodimerization and continuous transmission of signals in the absence of EPO (11, 12). Through retroviral transduction, these ligand-independent activated chimeric receptors were expressed in primary erythroid progenitors ex vivo and in vivo. Our findings suggest that the EPOR cytoplasmic tail does not transmit an exclusive erythroid-specific signal for red blood cell differentiation and imply that cytokine-dependent signaling instead provides a supportive function for erythroid differentiation.
MATERIALS AND METHODS
Constructs and Viruses.
Constructs encoding chimeric receptors were constructed essentially as described for various interleukin receptors (13) and contained a cDNA fragment encoding the extracellular domain of constitutively active variants of the EPO receptor (cEPOR) (12) fused at the unique NheI restriction site to PCR-generated fragments encoding the transmembrane and cytoplasmic domains of murine c-mpl, the rabbit growth hormone receptor, or the human granulocyte colony-stimulating factor receptor (G-CSFR). All constructs were verified by automated DNA sequencing and were subcloned into the previously described proviral plasmid pSFF (11). To construct cEPOR(1–255), a PCR was used to introduce a termination codon at amino acid 256 of full-length cEPOR. This 775-bp DNA piece was then subcloned into the retroviral plasmid pSFF, and the eukaryotic expression vector pMEX.Neo. Retroviruses were generated from these plasmids and characterized as described (11, 14).
Cell Lines.
The interleukin 3-dependent pro-B cell line BaF3 has been described (15). Growth factor independence was assessed by infecting cell cultures with the indicated retroviruses, then culturing the cells in medium containing EPO (2 units/ml) and in medium lacking any additional growth factors. BaF3 cells containing cEPOR(1–255) were generated by electroporation with the plasmid pMEX. cEPOR(1–255), and culturing the cells in interleukin 3 and G418 (Geneticin, GIBCO/BRL Life Technologies) for 10 days. After 10 days it was readily apparent by visual inspection and trypan blue staining which cultures were viable and expanding (designated herein as factor independent) compared with those that had expired.
Expression Analysis and Immunoblotting.
Whole cell lysates prepared from BaF3 transfectants described above were analyzed by immunoblotting with an antiserum directed against the N-terminal extracellular segment of the EPOR as described (16). For analysis of viral titers, approximately 5 × 105 NIH 3T3 fibroblast cells in a P100 plate were infected with 4 ml of culture supernatant from retroviral producer cells. After infection, cells grown to confluence were harvested and lysed. Protein (150 μg) from the detergent-soluble extract was resolved by SDS/PAGE (8% gels), transferred to nitrocellulose, and immunoblotted with antiserum raised against the N-terminal extracellular segment of the EPOR (see above). The amount of cEPOR chimera proteins expressed were compared with NIH 3T3 cells infected with the well-characterized spleen focus-forming viruses (SFFV) cEPOR virus (11). For splenic extracts, single cell suspensions of virally infected or uninfected spleen cells were prepared. The cells were washed once with PBS and lysed in 1% Triton X-100/150 mM NaCl/20 mM Tris⋅HCl, pH 7.4/1 mM EDTA/0.5% Nonidet P-40, containing aprotinin and phenylmethylsulfonyl fluoride at 4°C for 15 min. Samples were clarified by centrifugation at 10,000 × g for 15 min. Soluble protein concentration was determined by using the Bio-Rad BCA protein determination kit. Equal amounts of protein from each extract was applied to an 8% SDS/PAGE gel and products were resolved under reducing conditions, transferred to nitrocellulose, and immunoblotted with antiserum against the N-terminal segment of the EPOR. In addition immunoblotting with antiserum against the cytoplasmic tail of the G-CSFR (Santa Cruz Biotechnology) was also performed on selected samples. To detect activation of the Janus kinase/signal transducer and activator of transcription (STAT) pathway, electrophoretic mobility shift assays were performed as described (17), using an FcgR1 32P-end labeled double-stranded DNA oligonucleotides as a probe.
Infection and Culture of Hematopoietic Progenitor Cells.
Single cell suspensions of fetal liver were prepared from day 13 pregnant DBA-2 mice (Charles River Breeding Laboratories). Cells were washed three times in α medium. Cells (104) were resuspended in medium containing fresh virus, and Polybrene (4 μg/ml) was added; cells were then incubated at 37°C for 3 h. After infection, samples were washed in α medium and replated in α medium containing 30% fetal bovine serum (Sterile Systems, Logan, UT), 1% crystallized BSA (Sigma), 1.2% 1,500 centipoise methylcellulose (1 poise = 0.1 Pa⋅sec; Shinetsu Chemical, Tokyo), recombinant murine stem cell factor (20 ng/ml, Genzyme), and 50 μM 2-mercaptoethanol (Sigma) at a cell concentration of 2 × 104 cells per ml. Partially purified human urinary EPO (specific activity = 250 units/mg), a generous gift from M. Kawakita (Kumamoto University, Japan), was used where indicated. Polyclonal anti-human EPO antiserum was obtained from R & D Systems. Antiserum at 1–3 μg/ml will neutralize 50% of the bioactivity due to recombinant human EPO at 0.3 unit/ml.
Retroviral Infection of Mice.
Fresh culture supernatant was harvested from replication-defective SFFV retroviral producer cells and mixed (7:3, vol/vol) with culture supernatant from a clone of NIH 3T3 cells producing replication competent Rauscher murine leukemia virus helper virus. The mixture was passed through a 0.45-μm (pore size) sterile filter and 0.6 ml was injected intravenously into 6-week-old female NIH Swiss mice. Weekly hematocrit was determined and at 5–6 weeks mice were sacrificed. At termination, blood smears were made and the spleens were removed for analysis.
RESULTS
Design and Expression of Activated Chimeric Cytokine Receptors.
We have described a dominant gain-of-function approach to studying EPOR signaling in vivo (18). To test whether the cytoplasmic domain of structurally related cytokine receptors not typically expressed in erythroid progenitors could support terminal erythroid development, we expressed constitutively activated chimeric receptors in erythroid progenitors. To develop receptors that are ligand-independent, chimeras were generated containing the complete extracellular domain of cEPOR (an EPOR containing the R129C mutation) fused to the transmembrane and cytoplasmic segments of the G-CSFR (cEPOR/G-CSFR), the growth hormone receptor (cEPOR/GHR), or the thrombopoietin receptor (cEPOR/Mpl). In addition, a severe C-terminal truncation mutant of the cEPOR [cEPOR(1–255)] retaining only the membrane-proximal 6 amino acids of the cytoplasmic domain was also prepared as a negative control. Each chimeric cDNA was introduced into a proviral plasmid (pSFF) and replication-defective SFFVs were generated: SFFV-cEPOR, SFFV-cEPOR/G-CSFR, SFFV-cEPOR/GHR, SFFV-cEPOR/Mpl, and SFFV-cEPOR(1–255). The capacity of the viruses to transduce their respective chimeric receptors into NIH 3T3 cells (i.e., titer) was found to be comparable (data not shown).
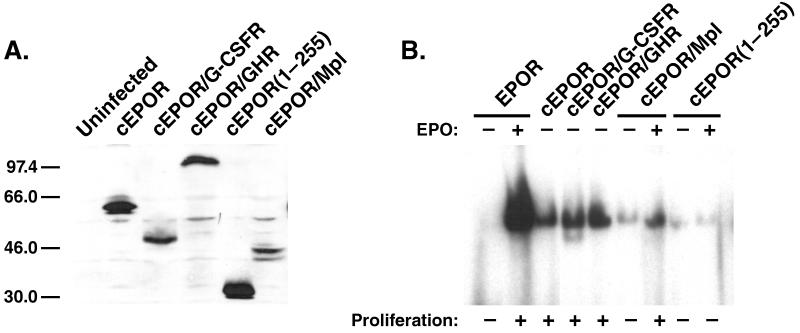
To verify expression and growth signaling function of the experimental receptors, these retroviruses were used to infect Ba/F3 cells, a cell line in which diverse cytokine receptors are known to support cellular proliferation. Immunoblotting of cell lysates from each line with antiserum recognizing the extracellular N terminus of the EPOR demonstrated significant expression of proteins with the expected relative sizes for the cEPOR (66 kDa), cEPOR/G-CSFR (52 kDa), cEPOR/GHR (100 kDa), cEPOR/Mpl (50 kDa), and cEPOR(1–255) (35 kDa) variants (Fig. 1A). Ba/F3 cells containing cEPOR, cEPOR/G-CSFR, or cEPOR/GHR exhibited continuous cytokine-independent growth, but the cEPOR/Mpl line required addition of exogenous EPO (Fig. 1B). Ba/F3 cells containing cEPOR(1–255) did not survive or proliferate in either the presence or absence of EPO. In addition, nuclear extracts were prepared from cytokine-starved cells and analyzed for DNA-binding activity to an oligonucleotide representing a STAT-responsive element present in the FcγR1 promoter. Although cells expressing native EPOR demonstrated detectable STAT activity only upon the addition of EPO, cells expressing the cEPOR, cEPOR/G-CSFR, and cEPOR/GHR exhibited constitutive activity (Fig. 1B), indicating that these receptors are functionally coupled to the JAK/STAT pathway. The cEPOR/Mpl chimera exhibited only low-level STAT activity in the absence of EPO; however, addition of EPO stimulated the STAT activity in cells containing this chimera (Fig. 1B). As expected, no detectable STAT activity was observed in cells containing cEPOR(1–255) (Fig. 1B). Therefore, although extensive prior studies by us and others (for examples, see refs. 4, 19–23) have delineated the specific signal transduction properties of the native receptors represented in this series in cell lines, the present experiments verify the functional integrity of the chimeras built on the cEPOR backbone.
Figure 1.
Expression and signaling function of constitutive chimeras. (A) BaF3 cell lines transduced with the indicated expression constructs were analyzed by immunoblotting with anti-N-terminal EPOR antiserum. (B) To verify the integrity of the signaling function of these receptors, the indicated cell lines were rested or exposed to EPO (50 units/ml, 10 min) followed by preparation of nuclear extracts and electrophoretic mobility shift assay for STAT DNA-binding activity.
Chimeric Non-EPOR Cytokine Receptors Support Erythroid Development of Primary Erythroid Progenitor Cells ex Vivo.
To determine whether these viruses could support development of immature (BFU-E) and mature (CFU-E) committed erythroid progenitor cells, hematopoietic precursor cells from embryonic day 13 fetal liver were infected and cultured in methylcellulose. To negate any contribution from endogenous wild-type EPOR to the responses, cultures were performed in the absence of added EPO. Also, because the methylcellulose culture system contains 30% serum, which contains trace amounts of EPO, we added EPO-neutralizing antiserum to the cultures. In addition, cEPOR, although functional in the absence of EPO, can exhibit an enhanced response in the presence of EPO (24, 25). Thus, the presence of neutralizing EPO antiserum in cultures also ensured that EPO-independent cEPOR function would be measured.
As shown in Table 1, addition of EPO-neutralizing antiserum completely abolished the CFU-E response observed when EPO at 0.3 unit/ml was added to uninfected control cultures. This concentration of EPO exceeds by 10- to 100-fold the concentration EPO detected in undiluted serum (26). As demonstrated (24), SFFV-cEPOR promoted robust EPO-independent generation of erythroid progenitor colonies, which was suppressed only marginally by the addition of anti-EPO antiserum (Table 1); SFFV-cEPOR(1–255) exhibited no significant activity in this assay (Table 1). Interestingly, SFFV-cEPOR/G-CSFR and SFFV-cEPOR/GHR exhibited potent EPO-independent erythropoietic function. SFFV-cEPOR/Mpl also appeared to be active but only in cultures lacking EPO-neutralizing antiserum (Table 1). These findings indicate that hematopoietic receptors normally associated with nonerythroid lineages (e.g., G-CSFR and Mpl) can readily support erythoid development and that this interchangeability also extends to a nonhematopoietic cytokine receptor (e.g., GHR) as well.
Table 1.
Differentiation of primary hematopoietic precursor cells
| Exp. | Virus | EPO antiserum | No. CFU-E | No. CFU-E (−uninfected control) | No. BFU-E | No. BFU-E (−uninfected control) |
|---|---|---|---|---|---|---|
| A | Uninfected | − | 5 ± 1 | 0 | 5 ± 2 | 0 |
| + | 0 | 0 | 0 | 0 | ||
| Uninfected + EPO | − | 71 ± 10 | 66 | 8 ± 3 | 3 | |
| + | 7 ± 4 | 7 | 2 ± 1 | 2 | ||
| cEPOR | − | 141 ± 23 | 136 | 42 ± 2 | 37 | |
| + | 79 ± 11 | 79 | 30 ± 7 | 30 | ||
| cEPOR (1–255) | − | 20 ± 8 | 15 | 15 ± 3 | 10 | |
| + | 9 ± 2 | 9 | 2 ± 2 | 2 | ||
| cEPOR/G-CSFR | − | 97 ± 9 | 92 | 17 ± 3 | 12 | |
| + | 76 ± 22 | 76 | 9 ± 1 | 9 | ||
| cEPOR/GHR | − | 79 ± 6 | 74 | 13 ± 2 | 8 | |
| + | 63 ± 12 | 63 | 8 ± 2 | 8 | ||
| B | Uninfected | − | 4 ± 2 | 0 | 9 ± 6 | 0 |
| + | 0 | 0 | 2 ± 1 | 0 | ||
| cEPOR | − | 67 ± 12 | 63 | 28 ± 5 | 19 | |
| + | 39 ± 9 | 39 | 12 ± 3 | 10 | ||
| cEPOR/Mpl | − | 33 ± 10 | 29 | 8 ± 2 | 0 | |
| + | 5 ± 2 | 5 | 1 ± 0.4 | 0 |
Embryonic day 13 fetal liver cells were prepared and infected. To each dish, either normal rabbit IgG (0.5 μg/ml) or rabbit polyclonal antierythropoietin antibody (+, 5 μg/ml) were added. In one condition (+EPO) human EPO was added to the cultures at 0.3 unit/ml. CFU-E numbers were determined at day 2 of culture; BFU-E numbers were determined at day 7. Data are expressed as mean ± SD (n = 4). Experiments A and B were performed on separate days.
Chimeric Non-EPOR Cytokine Receptors Support Erythroid Development in Vivo.
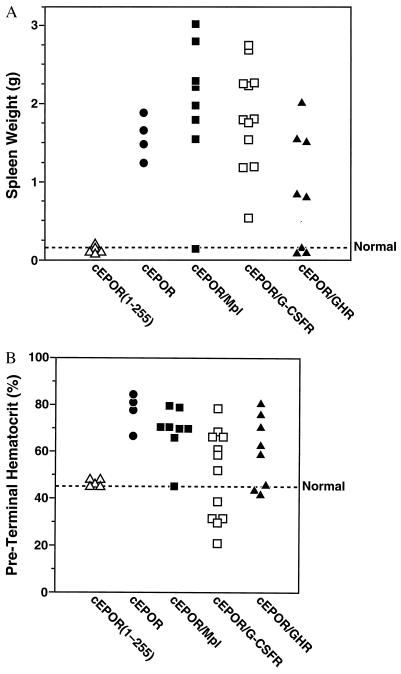
To investigate the function of the constitutive chimeras in a physiologic context, NIH Swiss mice were inoculated intravenously with these recombinant viruses in the presence of replication competent Rauscher helper virus and monitored by serial hematocrit testing and measurement of spleen weight upon termination at 5–6 weeks. As expected from previous studies (14, 18), SFFV-cEPOR uniformly caused marked expansion in early erythroid progenitors (e.g., splenomegaly) and mature terminally differentiated red cells (elevation in hematocrit; Fig. 2A). With virtually indistinguishable kinetics, SFFV-cEPOR/Mpl caused significant splenomegaly and elevation in hematocrits in nearly all recipients, indicating that the Mpl cytoplasmic tail can deliver the necessary signals in erythroid progenitor cells to support red cell development (Fig. 2A). SFFV-cEPOR/GHR also caused marked splenomegaly and erythrocytosis in most animals, although disease was not observed in a minority of recipients within this time frame (Fig. 2A). No effect upon spleen size or red cell numbers was detected in mice infected with SFFV-cEPOR(1–255) (Fig. 2A), indicating that the presence of extracellular cEPOR sequences did not recruit other cell surface receptors. Therefore, signals generated from Mpl and GHR readily supported erythropoiesis in vivo.
Figure 2.
Expansion of hematopoietic compartment in vivo. (A) The spleen weight of each animal infected with an SFFV recombinant expressing a constitutive chimera (as indicated) was determined at the time of termination, and each individual animal from the experiment is represented. Dashed line represents spleen weight of normal uninfected adult mice. (B) The hematocrit of each animal infected with an SFFV recombinant expressing a constitutive chimera (as indicated) was determined immediately before termination (always within 5–6 weeks of infection), and each individual animal from the experiment is represented. Dashed line represents hematocrit of normal uninfected adult mice.
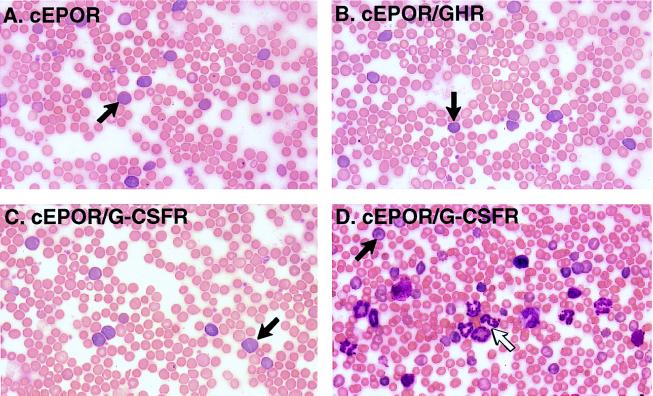
All SFFV-cEPOR/G-CSFR-infected mice also developed splenomegaly. Although the splenomegaly implied relatively common function among these receptors, evaluation of preterminal hematocrits revealed a more complex pattern. As expected, SFFV-cEPOR triggered striking elevation in hematocrit, whereas SFFV-cEPOR(1–255) did not (Fig. 2B). Similarly, all of the SFFV-cEPOR/Mpl and SFFV-cEPOR/GHR animals exhibiting splenomegaly also demonstrated peripheral erythrocytosis (Fig. 2B). Surprisingly, the SFFV-cEPOR/G-CSFR animals exhibited a biphasic response that was not evident from the spleen weights alone. Seven of 11 mice demonstrated erythrocytosis, represented by striking elevations in hematocrit (Fig. 2B); the remaining four animals developed significant suppression in red cell numbers (low hematocrit or anemia) and a concurrent elevation in blood neutrophil numbers (leukocytosis) (Fig. 2B). An anemic or leukocytic response to SFFV-cEPOR has not been observed in a cumulative examination of more than 100 recipient mice. To investigate the mechanism underlying this phenomenon, peripheral blood smears were examined for animals from each group. Consistent with the initial description of the cEPOR response in mice, smears from SFFV-cEPOR mice were notable for the presence of numerous immature erythroid reticulocytes in addition to terminally differentiated erythrocytes (Fig. 3) (11). Similarly, elevated reticulocyte numbers were evident in smears from SFFV-cEPOR/Mpl and SFFV-cEPOR/GHR animals (Fig. 3 and data not shown). However, the smears from anemic and polycythemic SFFV-cEPOR/G-CSFR animals differed substantially from one another: both sets contained increased numbers of reticulocytes, but only the anemic SFFV-cEPOR/G-CSFR animals were found also to contain numerous myeloid cells representing both immature progenitors and mature neutrophils (Fig. 3). The spleens from these anemic animals likewise were densely packed with progenitor cells of the myeloid lineage (data not shown). Thus, although both chimeric cEPOR/GHR and cEPOR/G-CSFR evidently can support erythroid lineage development, cEPOR/G-CSFR can also support myeloid lineage development in this system.
Figure 3.
Constitutive chimera function and expansion of blood cell lineages in the periphery. Peripheral blood smears from various animals were visualized by Wright–Giemsa staining, and representative photomicrographs are presented. (A) Animal transduced with cEPOR. Arrow, reticulocyte. (B) Animal transduced with cEPOR/GHR. Arrow, reticulocyte. (C) Representative animal transduced with cEPOR/G-CSFR and exhibiting marked elevation in hematocrit. Arrow, reticulocyte. (D) Representative animal transduced with cEPOR/G-CSFR and exhibiting marked anemia. Solid arrow, reticulocyte; open arrow, neutrophil.
DISCUSSION
A central question in blood cell development is the specific contributions of hematopoietic cytokines to the growth and differentiation of progenitor cells leading to mature cells of various lineages. A large body of evidence, including recent gene deletion studies, have demonstrated that factors such as EPO, G-CSF, and thrombopoietin and their cellular receptors play major and relatively selective roles in vivo in the production of erythrocytes, leukocytes, and platelets, respectively (1, 2, 27, 28). What has been more difficult to discern experimentally is whether the regulatory effects exerted by these factors are instructive regarding differentiation and cell fate or instead are limited to general supportive (proliferative or antiapoptotic) functions that do not dictate lineage-specific differentiation. Studies in established cell lines have suggested that specific differentiation signals are indeed linked to each receptor in some settings, but these analyses are limited by constraints on the differentiation parameters that can be monitored in the existing model systems using transformed cells (8–10, 20, 29). The present study was undertaken to examine this question in the context of primary progenitor cells both ex vivo and in vivo.
Clearly a dominant source of specificity in the biologic regulation of hematopoiesis by cytokines factors is the lineage-restricted expression of high-affinity receptors that bind and respond selectively to each factor (30–32). These receptors have quite limited primary sequence similarity to one another despite their relationship as products of a cytokine receptor gene superfamily characterized by a cysteine tetrad near the N terminus, fibronectin-type domains, and the WSXWS motif in the extracellular segments, as well as distantly related peptide sequences (Box B1 and Box B2) and sites of tyrosine phosphorylation within the cytoplasmic segments. Moreover, based on extensive analyses in established cell line systems, such receptors appear to be coupled to related signal transduction systems including JAK/STAT pathways and cascades linked to phosphatidylinositol 3-kinase and mitogen-activated phosphorylation kinases (21, 32, 33). Nonetheless, despite their similarity in general structure and functional connections, specific molecular differences have been described such as the coupling of the G-CSFR to STAT-3 or STAT-3/STAT-5 (34–38) and the restricted coupling of the EPOR to STAT-5 (33). Extensive investigation of the specific signal transduction properties of these receptor, in cell lines, has been undertaken by others and was not reproduced in this work. In the present studies, we sought expressly to investigate the question of specificity in erythroid development by testing the capacity of nonerythroid receptors to support red blood cell development in vivo.
We used an approach built upon viral transduction and expression of constitutively active chimeric receptors that were designed to exert dominant effects within recipient mice. These receptors all contained the extracellular domain of an EPOR variant (cEPOR) characterized by an additional cysteine residue that results in ligand-independent receptor homodimerization and activation. Mice infected with retroviruses expressing the parental cEPOR rapidly and reproducibly develop profound erythrocytosis and erythroleukemia (11). We have used this system recently to investigate the roles of cytoplasmic tyrosine residues of the EPOR in vivo and found that such tyrosines play important yet redundant biologic roles in supporting erythroid development in animals (18). In the present adaptation of the system, the cytoplasmic domain of the cEPOR was replaced by analogous segments from various cytokine receptors normally involved in hematopoietic (nonerythroid) or nonhematopoietic processes. These constitutive chimeras were introduced into the hematopoietic compartment ex vivo or in vivo.
Three major findings emerged from these studies. First, in the ex vivo analyses using hematopoietic progenitor cells from fetal liver, cytoplasmic tails from various cytokine receptors exhibited remarkable interchangeability with the EPOR regarding red blood cell development. For example, both the G-CSFR and Mpl in the chimeric receptor configuration robustly supported red blood cell development, although they are linked normally to leukocyte and platelet production, respectively. Likewise, the GHR potently substituted for the EPOR in the differentiation of red blood cells, although its usual direct biologic effects are limited to somatic nonhematopoietic tissues. Thus, in this culture system designed to measure erythroid development from primary committed erythroid precursors, erythroid-specific signals do not appear to be restricted to the EPOR itself. This finding is consistent with a recent report that the prolactin receptor can similarly substitute for the EPOR (39). These observations are most consistent with the hypothesis that in lineage committed hematopoietic progenitors these cytokine/cytokine receptor interactions predominantly serve to support cellular expansion along lines of differentiation determined largely by other events and/or factors. Whether cytokines also serve similar supportive roles in the development of earlier multipotent progenitors or confer specific instructive signals was not addressed in this study. However, expression of the EPOR or CSF-1R in pluripotent blast cell colonies were shown to confer proliferative signals but did not result in preferential erythroid or monocytic development, respectively, arguing against an instructive role for EPO or CSF-1 in these multipotent cells (40).
Second, infection studies in animals also revealed a high degree of interchangeability of these receptors for blood cell development in vivo. As observed in the ex vivo system, two hematopoietic receptors strongly associated with nonerythroid lineages under normal circumstances rapidly stimulated profound erythrocytosis upon expression in the bone marrow via the retroviral transduction system. Furthermore, the nonhematopoietic cytokine receptor GHR also potently induced expansion of the mature red blood cell compartment. Therefore, both the ex vivo and in vivo experiments demonstrated that red blood cell development can be supported by a range of cytokine receptors not expected to have erythrogenic potential based on prior physiologic, molecular, or genetic analyses.
These findings indicate that the biologic or developmental outcomes regulated by such cytokine receptors is determined principally by the selective cellular environments in which these receptors function rather than by receptor-specific signals delivered upon binding of ligand. Furthermore, the selective expression of individual cytokine receptor types most likely results from lineage commitment and does not function as a prerequisite for specific lineage differentiation. It is not possible yet to distinguish between two alternate models embodying this principle. (i) Production of red blood cells by the erythroid lineage-committed precursors may represent an established program of events that is triggered stochastically (or by other signals) and that simply requires the cellular survival functions provided by the EPOR (or a substitute receptor) to permit completion of the differentiation process. (ii) Red blood cell differentiation may be triggered in part by common generic signals emanating from diverse members of the cytokine receptor superfamily. Although it will be important to distinguish between these specific models through further experimentation, both are nonetheless predicated upon the essential role of the cellular environment rather than specificity in the molecular signals emanating from the EPOR itself.
A third and surprising finding results from the in vivo studies. Although the G-CSFR exhibited a potent capacity to support erythroid development in both experimental systems as described above, it also exhibited another property that appeared to be restricted to the G-CSFR itself: striking induction of white blood cell production. In the animals infected with retroviruses expressing the constitutively active cEPOR/G-CSFR, a biphasic response was observed in the blood compartment. Sixty-four percent of the animals exhibited selective erythroid effects, and 36% exhibited strong myeloid effects. The precise target cell population of the SFFV system is not known, but it is reasonable to assume that viral tropism was not influenced by the cDNA receptor construct embedded within the recombinant viral genomes because the envelope glycoprotein determining target cell specificity was provided in trans by the helper virus in all of these transduction studies. Thus, these findings represent one case in which biologic outcome appeared to be linked to the specific receptor construct, in possible violation of the principle proposed above. However, to reconcile these observations, we would suggest that this apparent conflict may derive from cell-specific restrictions in the functioning of certain cytokine receptors. That is, although the EPOR, G-CSFR, Mpl, and GHR all are able to couple functionally to appropriate signal transduction components in erythroid progenitor cells, myeloid progenitor cells may be less permissive for such diverse receptors (25, 41). Indeed, cell-specific restrictions on cytokine receptor function have been observed previously, such as the relative incompetence of the EPOR in T lymphocytes and granulocytes (25, 42). Although the molecular mechanism of this phenomenon remains unknown, such restrictions could readily explain the selective ability of the G-CSFR to support white blood cell development and the relative permissivity of the red blood cell lineage for diverse receptor types.
Collectively these studies emphasize the importance of cellular context in the response to cytokine/cytokine receptor interactions during hematopoiesis in vivo. The findings suggest that such interaction may not trigger specific receptor-dependent differentiation signals required for red blood cell production. Instead, these receptors may deliver relatively universal intracellular signals that support diverse biologic responses that are driven by the cellular context in which they are received. Such a model is consistent with the recognition that pleiotropic biologic processes are regulated by cytokine receptor systems linked to a relatively small pool of highly related signal transduction options. These results do not exclude the possibility that, within blood cell development, certain differentiation signals do indeed derive from these receptors. However, they strongly imply that such signals must be shared across a wide range of receptors. More importantly, they emphasize the need to identify the major characteristics or factors, both cellular and environmental, that specify lineage commitment and the ability to respond specifically to common signaling events.
Acknowledgments
We acknowledge the excellent assistance of Jessica Diamond, John Carroll, and Stephen Gonzalez in the preparation of this manuscript. We also thank Ann Hofbauer for technical assistance, Drs. W. Wood and F. de Sauvage for plasmids, and Ortho Biotech for the gift of human EPO. K.D.L. is supported by the Program in Biological Sciences and the National Institutes of Health Medical Scientist Training Program. This work was supported in part by National Institutes of Health R01 grants GM54351 (M.A.G.) and CA75315 (G.D.L.).
ABBREVIATIONS
- EPO
erythropoietin
- EPOR
EPO receptor
- cEPOR
constitutively active variants of the EPOR
- BFU-D
blast-forming unit-erythroid
- CFU-E
colony-forming unit-erythroid
- G-CSFR
granulocyte colony-stimulating factor receptor
- STAT
signal transducer and activator of transcription
- SFFV
spleen focus-forming virus
- GHR
growth hormone receptor
Footnotes
A commentary on this article begins on page 6573.
References
- 1.Wu H, Liu X, Jaenisch R, Lodish H F. Cell. 1995;83:59–67. doi: 10.1016/0092-8674(95)90234-1. [DOI] [PubMed] [Google Scholar]
- 2.Lin C-S, Lim S-K, D’Agati V, Costantini F. Genes Dev. 1996;10:154–164. doi: 10.1101/gad.10.2.154. [DOI] [PubMed] [Google Scholar]
- 3.D’Andrea A D, Lodish H F, Wong G G. Cell. 1989;57:277–285. doi: 10.1016/0092-8674(89)90965-3. [DOI] [PubMed] [Google Scholar]
- 4.Youssoufian H, Longmore G D, Neumann D, Yoshimura A, Lodish H F. Blood. 1993;81:2223–2236. [PubMed] [Google Scholar]
- 5.Gregory C J, Eaves A C. Blood. 1978;51:527–537. [PubMed] [Google Scholar]
- 6.Sawada K, Krantz S B, Sawyer S T, Civin C I. J Cell Physiol. 1988;137:337–445. doi: 10.1002/jcp.1041370218. [DOI] [PubMed] [Google Scholar]
- 7.Sawada K, Krantz S B, Dai C H, Koury S T, Horn S T, Glick A D, Civin C I. J Cell Physiol. 1990;142:219–230. doi: 10.1002/jcp.1041420202. [DOI] [PubMed] [Google Scholar]
- 8.Libio E, Carroll M, D’Andrea A, Mathey-Prevot B. Proc Natl Acad Sci USA. 1993;90:11351–11355. doi: 10.1073/pnas.90.23.11351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maruyama K, Miyata K, Yoshimura A. J Biol Chem. 1994;269:5976–5980. [PubMed] [Google Scholar]
- 10.Chiba T, Nagata Y, Kishi A, Sakamaki K, Miyajima A, Yamamoto M, Engel J D, Todokoro K. Proc Natl Acad Sci USA. 1993;90:11593–11597. doi: 10.1073/pnas.90.24.11593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Longmore G D, Lodish H F. Cell. 1991;67:1089–1102. doi: 10.1016/0092-8674(91)90286-8. [DOI] [PubMed] [Google Scholar]
- 12.Watowich S S, Yoshimura A, Longmore G D, Hilton D J, Yoshimura Y, Lodish H F. Proc Natl Acad Sci USA. 1992;89:2140–2144. doi: 10.1073/pnas.89.6.2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goldsmith M A, Lai S Y, Xu W, Amaral M C, Kuczek E S, Parent L J, Mills G B, Tarr K L, Longmore G D, Greene W C. J Biol Chem. 1995;270:21729–21737. doi: 10.1074/jbc.270.37.21729. [DOI] [PubMed] [Google Scholar]
- 14.Longmore G D, Pharr P N, Lodish H F. Mol Cell Biol. 1994;14:2266–2277. doi: 10.1128/mcb.14.4.2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Palacios R, Steinmetz M. Cell. 1985;41:727–734. doi: 10.1016/s0092-8674(85)80053-2. [DOI] [PubMed] [Google Scholar]
- 16.Yoshimura A, Longmore G, Lodish H F. Nature (London) 1990;348:647–649. doi: 10.1038/348647a0. [DOI] [PubMed] [Google Scholar]
- 17.Gaffen S L, Lai S Y, Xu W, Gouillex F, Groner B, Goldsmith M A, Greene W C. Proc Natl Acad Sci USA. 1995;92:7192–7196. doi: 10.1073/pnas.92.16.7192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Longmore G, You Y, Molden J, Lai S Y, Goldsmith M A. Blood. 1998;91:870–878. [PubMed] [Google Scholar]
- 19.Gurney A, Wong S, Henzel W, DeSauvage F. Proc Natl Acad Sci USA. 1995;92:5292–5296. doi: 10.1073/pnas.92.12.5292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Avalos B R. Blood. 1996;88:761–777. [PubMed] [Google Scholar]
- 21.Ihle J N, Witthuhn B A, Quelle F W, Yamamoto K, Thierfelder W E, Kreider B, Silvennoinen O. Trends Biochem Sci. 1994;19:222–227. doi: 10.1016/0968-0004(94)90026-4. [DOI] [PubMed] [Google Scholar]
- 22.Colosi P, Wong K, Leong S, Wood W. J Biol Chem. 1993;268:12617–12623. [PubMed] [Google Scholar]
- 23.Pallard C, Gouilleux F, Benit L, Cocault L, Souyri M, Levy D, Groner B, Gisselbrecht S, Dusanter-Fourt I. EMBO J. 1995;14:2847–2856. doi: 10.1002/j.1460-2075.1995.tb07284.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pharr P N, Hankins D, Hofbauer A, Lodish H F, Longmore G D. Proc Natl Acad Sci USA. 1993;90:938–942. doi: 10.1073/pnas.90.3.938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McArthur G A, Longmore G D, Klinger K, Johnson G R. Exp Hematol. 1995;23:645–652. [PubMed] [Google Scholar]
- 26.Wognum A W, Lam V, Goudsmit R, Krystal G. Blood. 1990;76:1323–1329. [PubMed] [Google Scholar]
- 27.Liu F, Yang H, Wesselschmidt R, Kornaga T, Link D. Immunity. 1996;5:491–501. doi: 10.1016/s1074-7613(00)80504-x. [DOI] [PubMed] [Google Scholar]
- 28.Gurney A L, Carver-Moore K, de Sauvage F, Moore M. Science. 1994;265:1445–1447. doi: 10.1126/science.8073287. [DOI] [PubMed] [Google Scholar]
- 29.Pless M, Norga K, Carroll M, Heim M H, D’Andrea A D, Mathey-Prevot B. Blood. 1997;89:3175–3185. [PubMed] [Google Scholar]
- 30.Metcalf D. Nature (London) 1989;339:27–30. doi: 10.1038/339027a0. [DOI] [PubMed] [Google Scholar]
- 31.Nicola N A, Metcalf D. Cell. 1991;67:1–4. doi: 10.1016/0092-8674(91)90564-f. [DOI] [PubMed] [Google Scholar]
- 32.Kishimoto T, Taga T, Akira S. Cell. 1994;76:253–262. doi: 10.1016/0092-8674(94)90333-6. [DOI] [PubMed] [Google Scholar]
- 33.Ihle J N. Nature (London) 1995;377:591–594. doi: 10.1038/377591a0. [DOI] [PubMed] [Google Scholar]
- 34.Tian S, Lamb P, Seidel H M, Stein R B, Rosen J. Blood. 1994;84:1760–1764. [PubMed] [Google Scholar]
- 35.Nicholson S E, Novak U, Ziegler S F, Layton J E. Blood. 1995;86:3698–3704. [PubMed] [Google Scholar]
- 36.Novak U, Ward A C, Hertzog P J, Hamilton J A, Paradiso L. Growth Factors. 1996;13:251–260. doi: 10.3109/08977199609003226. [DOI] [PubMed] [Google Scholar]
- 37.Nicholson S E, Starr R, Novak U, Hilton D J, Layton J E. J Biol Chem. 1996;271:26947–53. doi: 10.1074/jbc.271.43.26947. [DOI] [PubMed] [Google Scholar]
- 38.Tian S S, Tapley P, Sincich C, Stein R B, Rosen J, Lamb P. Blood. 1996;88:4435–4444. [PubMed] [Google Scholar]
- 39.Socolovsky M, Dusanter-Fourt I, Lodish H F. J Biol Chem. 1997;272:14009–14012. doi: 10.1074/jbc.272.22.14009. [DOI] [PubMed] [Google Scholar]
- 40.Pharr P N, Ogawa M, Hofbauer A, Longmore G D. Proc Natl Acad Sci USA. 1994;91:7482–7486. doi: 10.1073/pnas.91.16.7482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McArthur G A, Rohrschneider L R, Johnson G R. Blood. 1994;83:972–980. [PubMed] [Google Scholar]
- 42.Yamamura Y, Kageyama Y, Matuzaki T, Noda M, Ikawa Y. EMBO J. 1992;11:4909–4915. doi: 10.1002/j.1460-2075.1992.tb05597.x. [DOI] [PMC free article] [PubMed] [Google Scholar]