Abstract
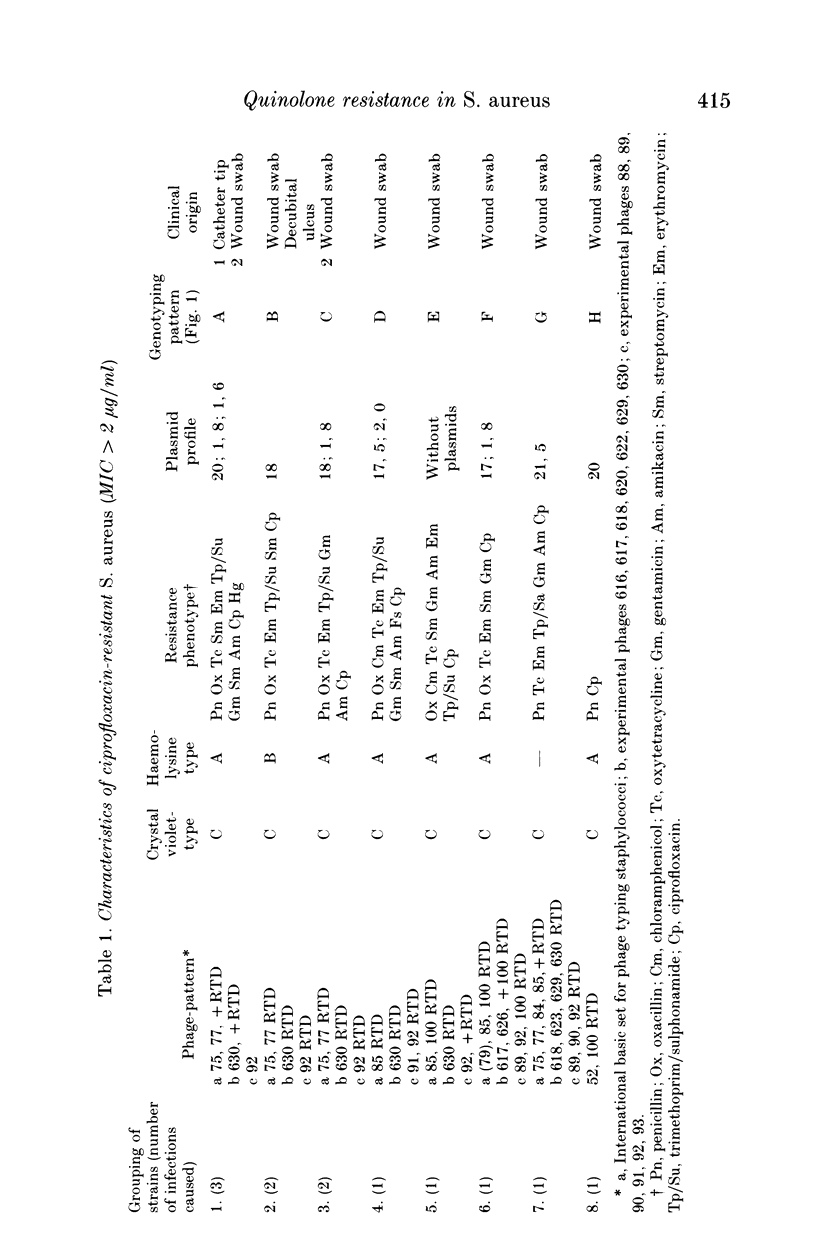
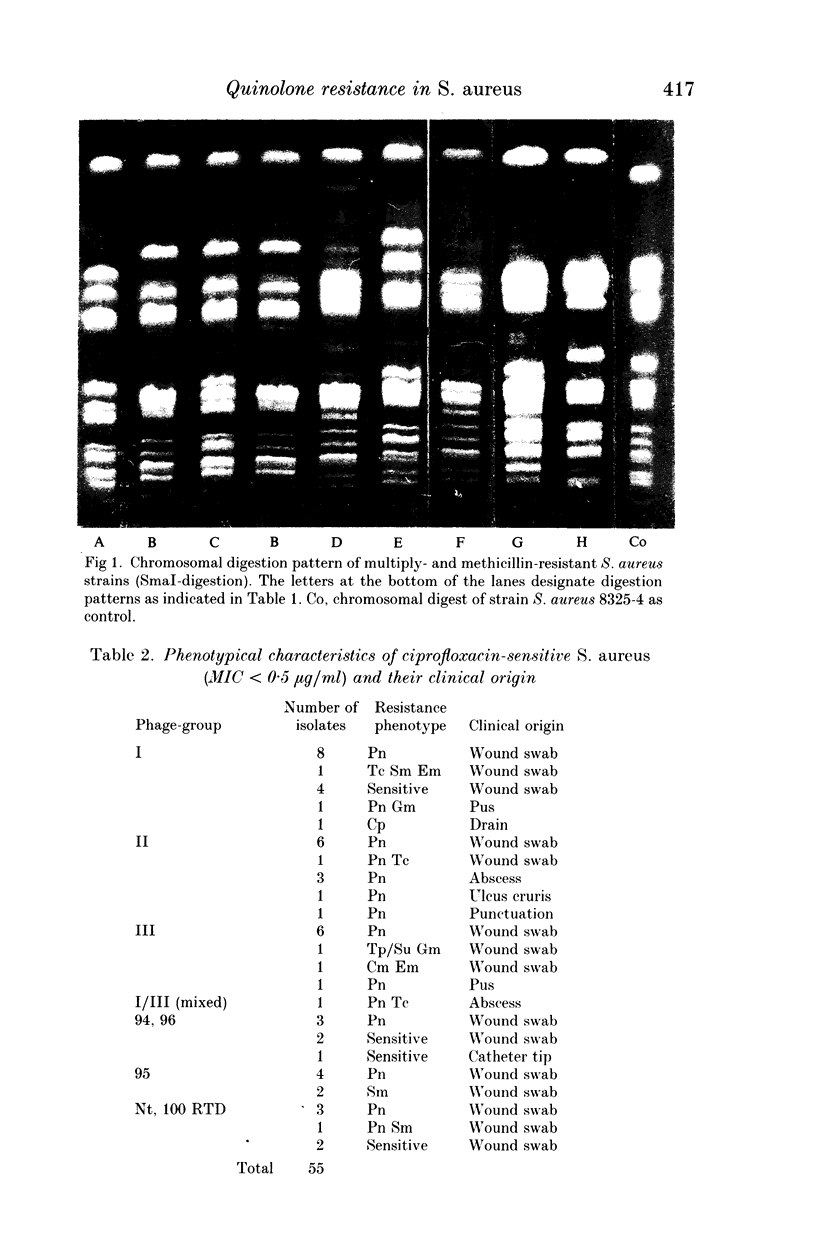
Among 63 Staphylococcus aureus isolates (one isolate per one patient) counted from infections (from August to November 1991) in hospital T., eight exhibited resistance to fluoroquinolones. Seven of these quinolone-resistant isolates were multiply- and methicillin-resistant S. aureus (QR-MRSA). The results of phage-, plasmid- and genotyping (pulsed field electrophoresis) revealed that six different strain-clones of these MRSA were spread in the hospital. In vitro spontaneous mutants resistant to fluoroquinolones are 10-100-fold more frequent in MRSA than in other S. aureus when selected on isosensitest-agar containing 1 microgram/ml of ciprofloxacin. However, the same mutant frequencies were found in strain 8325-4 with and without the mecA-determinant. The resistance phenotype was stable over 30 generations of subculture in nutrient broth as well in natural quinolone resistant MRSA as in mutants of other types of S. aureus selected in vitro. The phenotypic association of quinolone resistance and MRSA is rather likely due to a higher frequency of spontaneous resistant mutants which are present in natural populations of MRSA. Data of chemotherapy prior to the isolation of S. aureus show that three of seven patients from whom QR-MRSA were isolated were treated with a quinolone. In eight cases of infections with non-MRSA and quinolone treatment the isolated S. aureus strains were in vitro sensitive to quinolones.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beck W. D., Berger-Bächi B., Kayser F. H. Additional DNA in methicillin-resistant Staphylococcus aureus and molecular cloning of mec-specific DNA. J Bacteriol. 1986 Feb;165(2):373–378. doi: 10.1128/jb.165.2.373-378.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergan T., Thorsteinsson S. B., Kolstad I. M., Johnsen S. Pharmacokinetics of ciprofloxacin after intravenous and increasing oral doses. Eur J Clin Microbiol. 1986 Apr;5(2):187–192. doi: 10.1007/BF02013984. [DOI] [PubMed] [Google Scholar]
- Crook S. M., Selkon J. B., McLardy Smith P. D. Clinical resistance to long-term oral ciprofloxacin. Lancet. 1985 Jun 1;1(8440):1275–1275. doi: 10.1016/s0140-6736(85)92343-8. [DOI] [PubMed] [Google Scholar]
- Goering R. V., Ruff E. A. Comparative analysis of conjugative plasmids mediating gentamicin resistance in Staphylococcus aureus. Antimicrob Agents Chemother. 1983 Sep;24(3):450–452. doi: 10.1128/aac.24.3.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman B. J., Tomasz A. Low-affinity penicillin-binding protein associated with beta-lactam resistance in Staphylococcus aureus. J Bacteriol. 1984 May;158(2):513–516. doi: 10.1128/jb.158.2.513-516.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopf G., Böcker R., Estler C. J., Radtke H. J., Floh W. Concentration of ciprofloxacin in human serum, lung and pleural tissues and fluids during and after lung surgery. Infection. 1988;16(1):29–30. doi: 10.1007/BF01646928. [DOI] [PubMed] [Google Scholar]
- Kaatz G. W., Seo S. M., Ruble C. A. Mechanisms of fluoroquinolone resistance in Staphylococcus aureus. J Infect Dis. 1991 May;163(5):1080–1086. doi: 10.1093/infdis/163.5.1080. [DOI] [PubMed] [Google Scholar]
- Maple P., Hamilton-Miller J., Brumfitt W. Ciprofloxacin resistance in methicillin- and gentamicin-resistant Staphylococcus aureus. Eur J Clin Microbiol Infect Dis. 1989 Jul;8(7):622–624. doi: 10.1007/BF01968141. [DOI] [PubMed] [Google Scholar]
- Piercy E. A., Barbaro D., Luby J. P., Mackowiak P. A. Ciprofloxacin for methicillin-resistant Staphylococcus aureus infections. Antimicrob Agents Chemother. 1989 Jan;33(1):128–130. doi: 10.1128/aac.33.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefler S. Methicillin-resistant strains of Staphylococcus aureus resistant to quinolones. J Clin Microbiol. 1989 Feb;27(2):335–336. doi: 10.1128/jcm.27.2.335-336.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah P. M. The role of quinolones in staphylococcal infection. J Chemother. 1989 Aug;1(4):253–256. doi: 10.1080/1120009x.1989.11738902. [DOI] [PubMed] [Google Scholar]
- Shalit I., Berger S. A., Gorea A., Frimerman H. Widespread quinolone resistance among methicillin-resistant Staphylococcus aureus isolates in a general hospital. Antimicrob Agents Chemother. 1989 Apr;33(4):593–594. doi: 10.1128/aac.33.4.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S. M., Eng R. H., Bais P., Fan-Havard P., Tecson-Tumang F. Epidemiology of ciprofloxacin resistance among patients with methicillin-resistant Staphylococcus aureus. J Antimicrob Chemother. 1990 Oct;26(4):567–572. doi: 10.1093/jac/26.4.567. [DOI] [PubMed] [Google Scholar]
- Smith S. M., Eng R. H., Berman E. The effect of ciprofloxacin on methicillin-resistant Staphylococcus aureus. J Antimicrob Chemother. 1986 Mar;17(3):287–295. doi: 10.1093/jac/17.3.287. [DOI] [PubMed] [Google Scholar]
- Sreedharan S., Oram M., Jensen B., Peterson L. R., Fisher L. M. DNA gyrase gyrA mutations in ciprofloxacin-resistant strains of Staphylococcus aureus: close similarity with quinolone resistance mutations in Escherichia coli. J Bacteriol. 1990 Dec;172(12):7260–7262. doi: 10.1128/jb.172.12.7260-7262.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte W., Green L., Misra T. K., Silver S. Resistance to mercury and to cadmium in chromosomally resistant Staphylococcus aureus. Antimicrob Agents Chemother. 1986 Apr;29(4):663–669. doi: 10.1128/aac.29.4.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfson J. S., Hooper D. C. The fluoroquinolones: structures, mechanisms of action and resistance, and spectra of activity in vitro. Antimicrob Agents Chemother. 1985 Oct;28(4):581–586. doi: 10.1128/aac.28.4.581. [DOI] [PMC free article] [PubMed] [Google Scholar]