Abstract
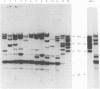
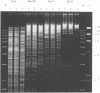
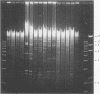
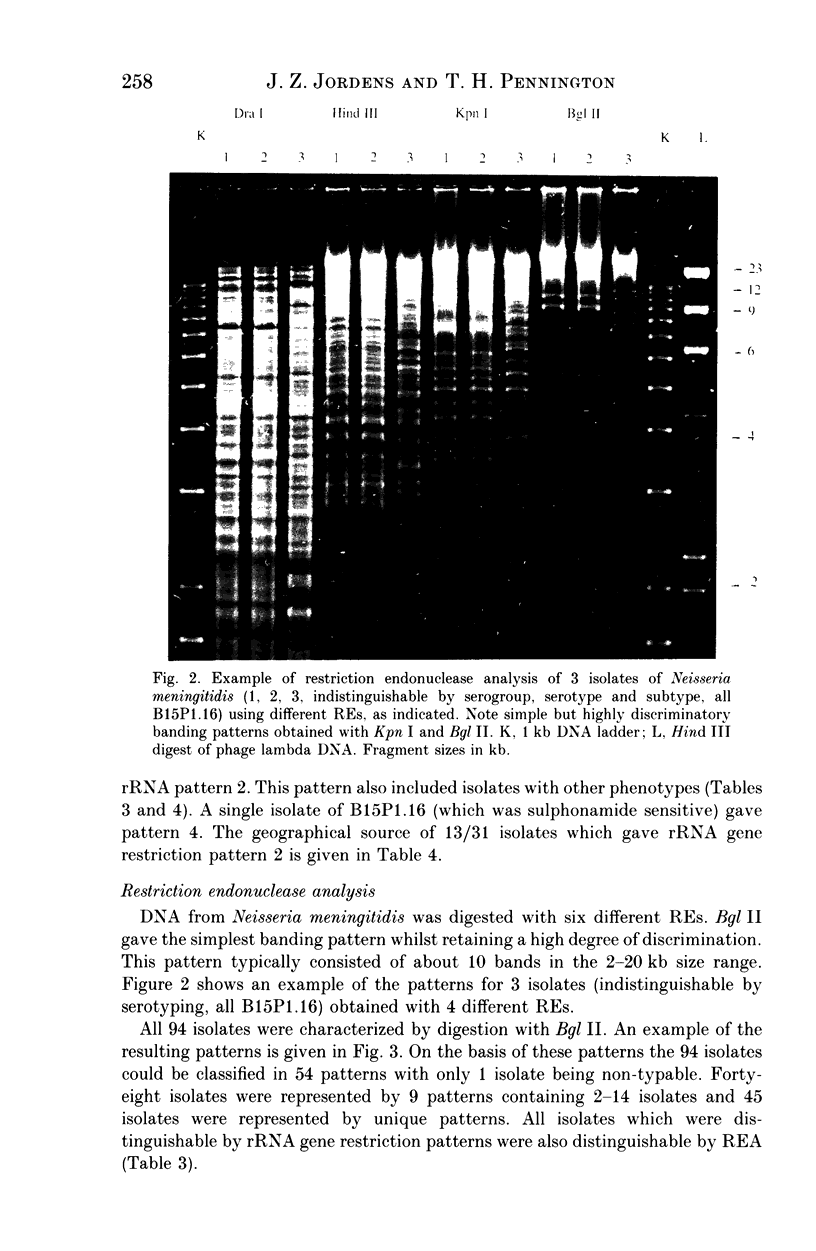
The use of ribosomal RNA (rRNA) gene restriction patterns to study the molecular epidemiology of Neisseria meningitidis was investigated. Ninety-four isolates of Neisseria meningitidis were characterized by their rRNA gene restriction patterns with 16 + 23 S rRNA from Escherichia coli as a probe. Thirteen rRNA gene restriction patterns were recognized; each of these patterns represented between 1 and 30 isolates. Isolated with the outbreak-associated phenotype B15P1.16 (sulphonamide resistant) all gave a single rRNA gene restriction pattern but this pattern also contained isolates with other phenotypes. Further discrimination between isolates was achieved by comparison of banding patterns resulting from restriction endonuclease digestion of chromosomal DNA with Bgl II. This gave a banding pattern consisting of about ten bands which was simple to interpret. Using this technique 94 isolates were classified in 54 patterns containing between 1 and 14 isolates. Restriction endonuclease analysis with Bgl II characterized outbreak-associated isolates with the phenotype B15P1.16 and enabled strains not typable by conventional methods to be identified as probable outbreak-associated isolates. The techniques should prove useful for epidemiological studies.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bjorvatn B., Lund V., Kristiansen B. E., Korsnes L., Spanne O., Lindqvist B. Applications of restriction endonuclease fingerprinting of chromosomal DNA of Neisseria meningitidis. J Clin Microbiol. 1984 Jun;19(6):763–765. doi: 10.1128/jcm.19.6.763-765.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwell C. C., Weir D. M., James V. S., Todd W. T., Banatvala N., Chaudhuri A. K., Gray H. G., Thomson E. J., Fallon R. J. Secretor status, smoking and carriage of Neisseria meningitidis. Epidemiol Infect. 1990 Apr;104(2):203–209. doi: 10.1017/s0950268800059367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce K. D., Jordens J. Z. Characterization of noncapsulate Haemophilus influenzae by whole-cell polypeptide profiles, restriction endonuclease analysis, and rRNA gene restriction patterns. J Clin Microbiol. 1991 Feb;29(2):291–296. doi: 10.1128/jcm.29.2.291-296.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartwright K. A., Stuart J. M., Noah N. D. An outbreak of meningococcal disease in Gloucestershire. Lancet. 1986 Sep 6;2(8506):558–561. doi: 10.1016/s0140-6736(86)90124-8. [DOI] [PubMed] [Google Scholar]
- Caugant D. A., Bøvre K., Gaustad P., Bryn K., Holten E., Høiby E. A., Frøholm L. O. Multilocus genotypes determined by enzyme electrophoresis of Neisseria meningitidis isolated from patients with systemic disease and from healthy carriers. J Gen Microbiol. 1986 Mar;132(3):641–652. doi: 10.1099/00221287-132-3-641. [DOI] [PubMed] [Google Scholar]
- Crowe B. A., Wall R. A., Kusecek B., Neumann B., Olyhoek T., Abdillahi H., Hassan-King M., Greenwood B. M., Poolman J. T., Achtman M. Clonal and variable properties of Neisseria meningitidis isolated from cases and carriers during and after an epidemic in The Gambia, West Africa. J Infect Dis. 1989 Apr;159(4):686–700. doi: 10.1093/infdis/159.4.686. [DOI] [PubMed] [Google Scholar]
- Etienne J., Renaud F., Bes M., Brun Y., Greenland T. B., Freney J., Fleurette J. Instability of characteristics amongst coagulase-negative staphylococci causing endocarditis. J Med Microbiol. 1990 Jun;32(2):115–122. doi: 10.1099/00222615-32-2-115. [DOI] [PubMed] [Google Scholar]
- Grimont F., Grimont P. A. Ribosomal ribonucleic acid gene restriction patterns as potential taxonomic tools. Ann Inst Pasteur Microbiol. 1986 Sep-Oct;137B(2):165–175. doi: 10.1016/s0769-2609(86)80105-3. [DOI] [PubMed] [Google Scholar]
- Haimanot R. T., Caugant D. A., Fekadu D., Bjune G., Belete B., Frøholm L. O., Høiby E. A., Rosenqvist E., Selander R. K., Bjorvatn B. Characteristics of serogroup A Neisseria meningitidis responsible for an epidemic in Ethiopia, 1988-89. Scand J Infect Dis. 1990;22(2):171–174. doi: 10.3109/00365549009037898. [DOI] [PubMed] [Google Scholar]
- Irino K., Grimont F., Casin I., Grimont P. A. rRNA gene restriction patterns of Haemophilus influenzae biogroup aegyptius strains associated with Brazilian purpuric fever. J Clin Microbiol. 1988 Aug;26(8):1535–1538. doi: 10.1128/jcm.26.8.1535-1538.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordens J. Z., Hall L. M. Chromosomally-encoded gentamicin resistance in 'epidemic' methicillin-resistant Staphylococcus aureus: detection with a synthetic oligonucleotide probe. J Antimicrob Chemother. 1989 Mar;23(3):327–334. doi: 10.1093/jac/23.3.327. [DOI] [PubMed] [Google Scholar]
- Kristiansen B. E., Sørensen B., Bjorvatn B., Falk E. S., Fosse E., Bryn K., Frøholm L. O., Gaustad P., Bøvre K. An outbreak of group B meningococcal disease: tracing the causative strain of Neisseria meningitidis by DNA fingerprinting. J Clin Microbiol. 1986 Apr;23(4):764–767. doi: 10.1128/jcm.23.4.764-767.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LiPuma J. J., Stull T. L., Dasen S. E., Pidcock K. A., Kaye D., Korzeniowski O. M. DNA polymorphisms among Escherichia coli isolated from bacteriuric women. J Infect Dis. 1989 Mar;159(3):526–532. doi: 10.1093/infdis/159.3.526. [DOI] [PubMed] [Google Scholar]
- Mendelman P. M., Caugant D. A., Kalaitzoglou G., Wedege E., Chaffin D. O., Campos J., Saez-Nieto J. A., Viñas M., Selander R. K. Genetic diversity of penicillin G-resistant Neisseria meningitidis from Spain. Infect Immun. 1989 Apr;57(4):1025–1029. doi: 10.1128/iai.57.4.1025-1029.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore P. S., Reeves M. W., Schwartz B., Gellin B. G., Broome C. V. Intercontinental spread of an epidemic group A Neisseria meningitidis strain. Lancet. 1989 Jul 29;2(8657):260–263. doi: 10.1016/s0140-6736(89)90439-x. [DOI] [PubMed] [Google Scholar]
- Ogle J. W., Janda J. M., Woods D. E., Vasil M. L. Characterization and use of a DNA probe as an epidemiological marker for Pseudomonas aeruginosa. J Infect Dis. 1987 Jan;155(1):119–126. doi: 10.1093/infdis/155.1.119. [DOI] [PubMed] [Google Scholar]
- Olyhoek T., Crowe B. A., Achtman M. Clonal population structure of Neisseria meningitidis serogroup A isolated from epidemics and pandemics between 1915 and 1983. Rev Infect Dis. 1987 Jul-Aug;9(4):665–692. doi: 10.1093/clinids/9.4.665. [DOI] [PubMed] [Google Scholar]
- Owen R. J. Chromosomal DNA fingerprinting--a new method of species and strain identification applicable to microbial pathogens. J Med Microbiol. 1989 Oct;30(2):89–99. doi: 10.1099/00222615-30-2-89. [DOI] [PubMed] [Google Scholar]
- Owen R. J., Hernandez J., Bolton F. DNA restriction digest and ribosomal RNA gene patterns of Campylobacter jejuni: a comparison with bio-, sero-, and bacteriophage-types of United Kingdom outbreak strains. Epidemiol Infect. 1990 Oct;105(2):265–275. doi: 10.1017/s0950268800047877. [DOI] [PMC free article] [PubMed] [Google Scholar]
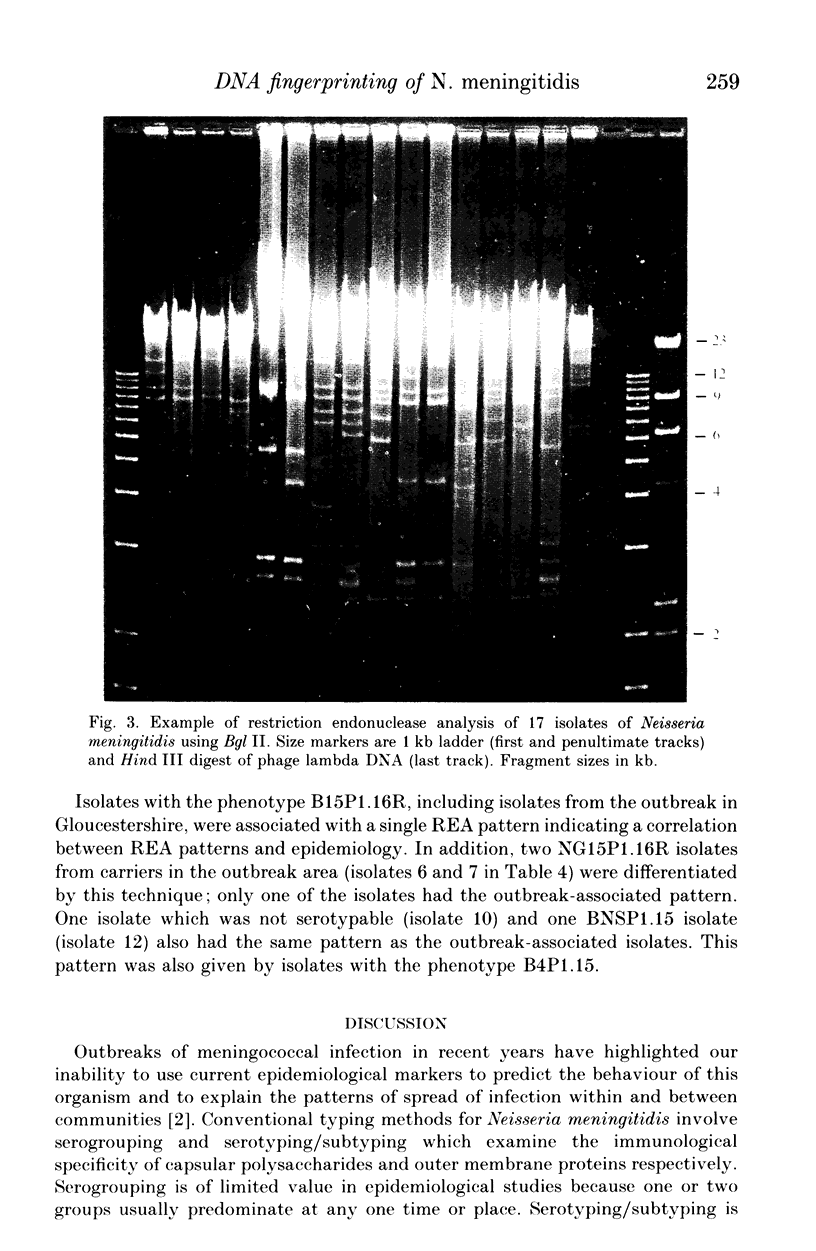
- Selander R. K., Caugant D. A., Ochman H., Musser J. M., Gilmour M. N., Whittam T. S. Methods of multilocus enzyme electrophoresis for bacterial population genetics and systematics. Appl Environ Microbiol. 1986 May;51(5):873–884. doi: 10.1128/aem.51.5.873-884.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stull T. L., LiPuma J. J., Edlind T. D. A broad-spectrum probe for molecular epidemiology of bacteria: ribosomal RNA. J Infect Dis. 1988 Feb;157(2):280–286. doi: 10.1093/infdis/157.2.280. [DOI] [PubMed] [Google Scholar]
- Tinsley C. R., Heckels J. E. Variation in the expression of pili and outer membrane protein by Neisseria meningitidis during the course of meningococcal infection. J Gen Microbiol. 1986 Sep;132(9):2483–2490. doi: 10.1099/00221287-132-9-2483. [DOI] [PubMed] [Google Scholar]