Abstract
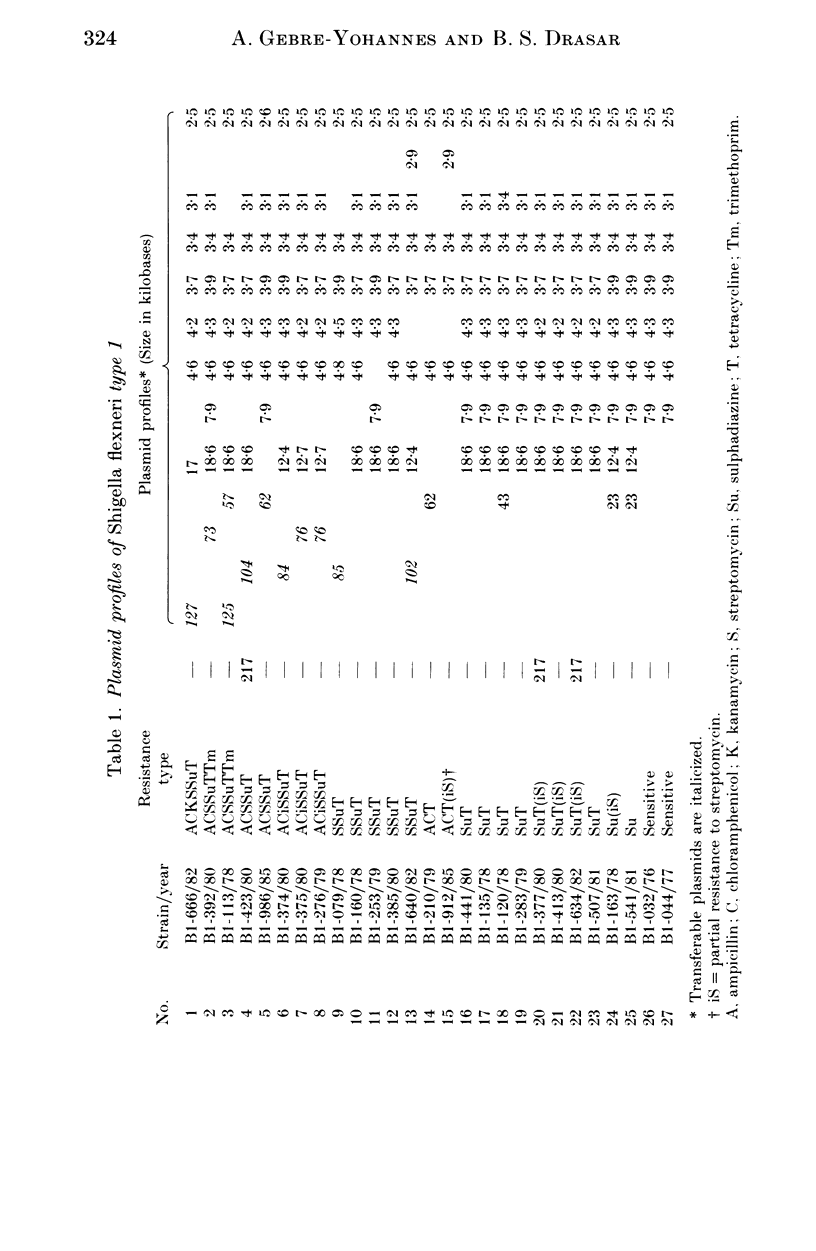
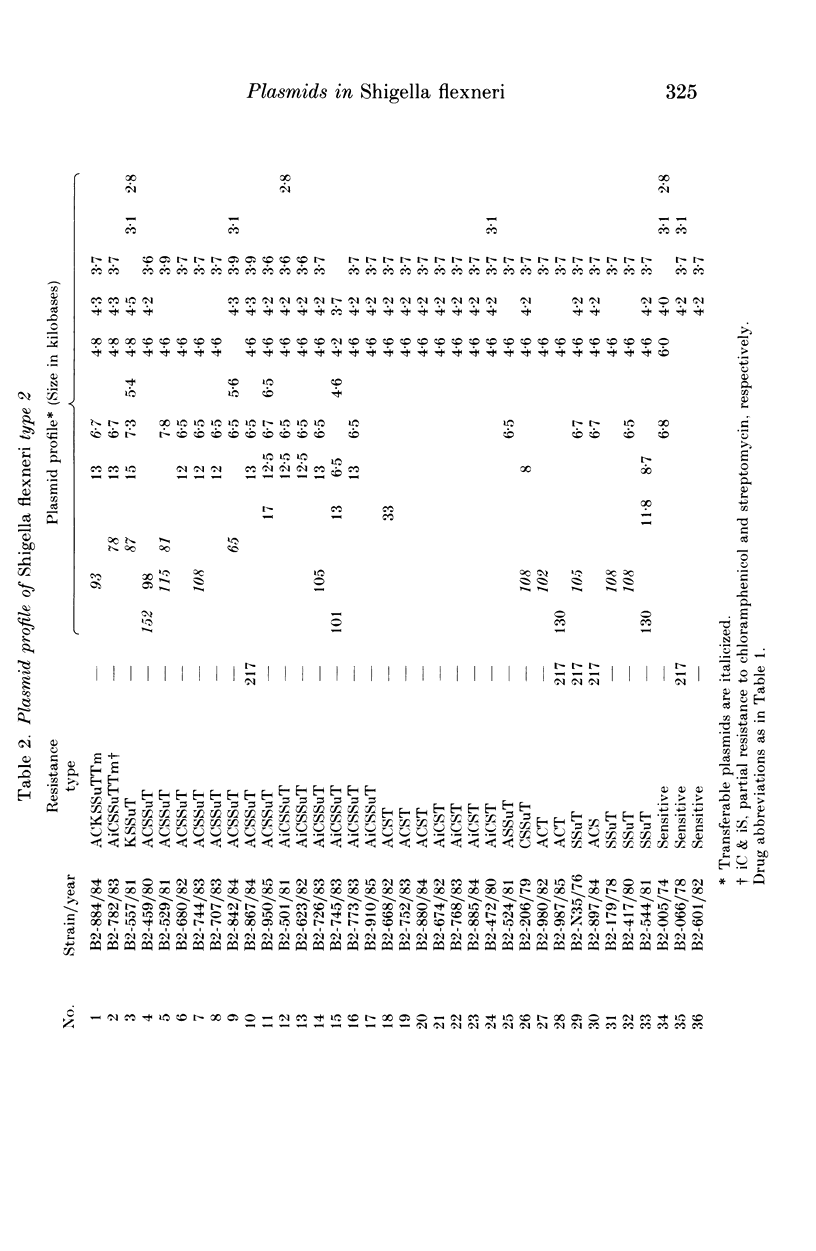
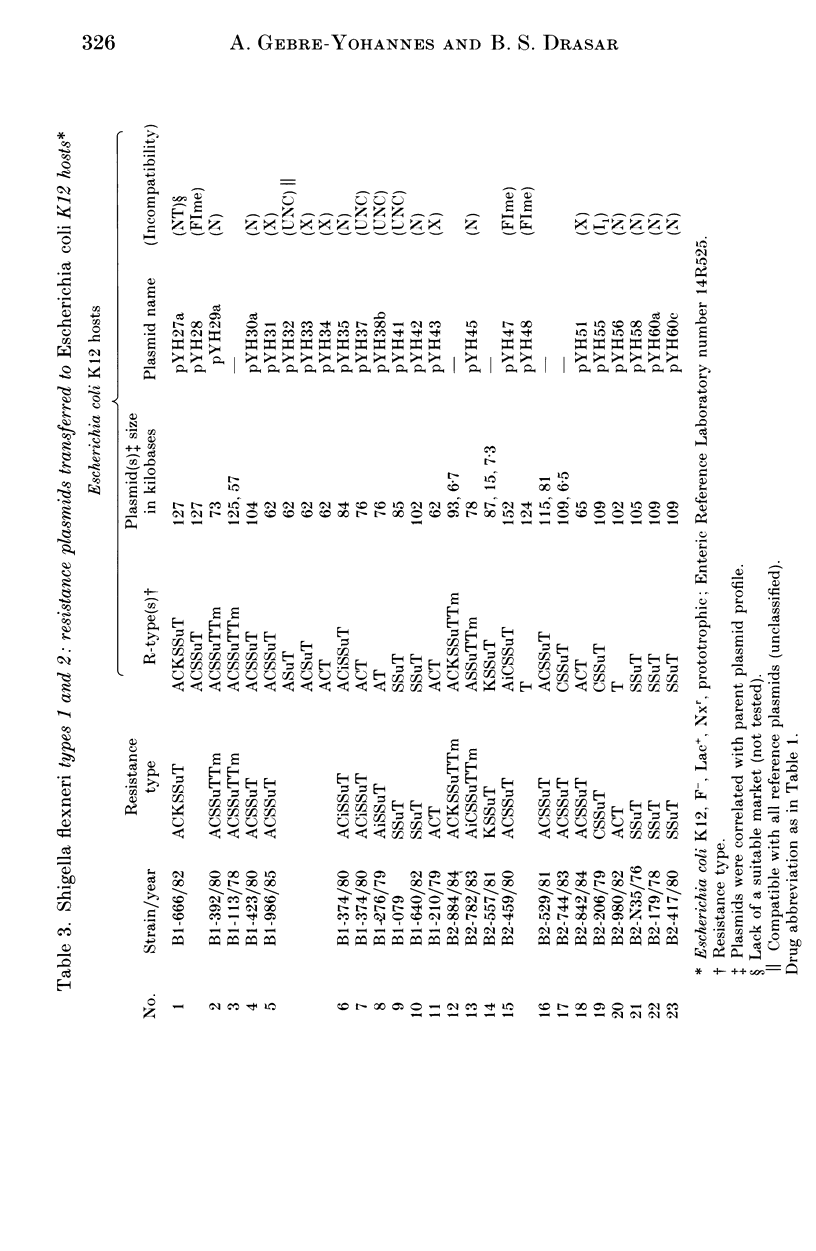
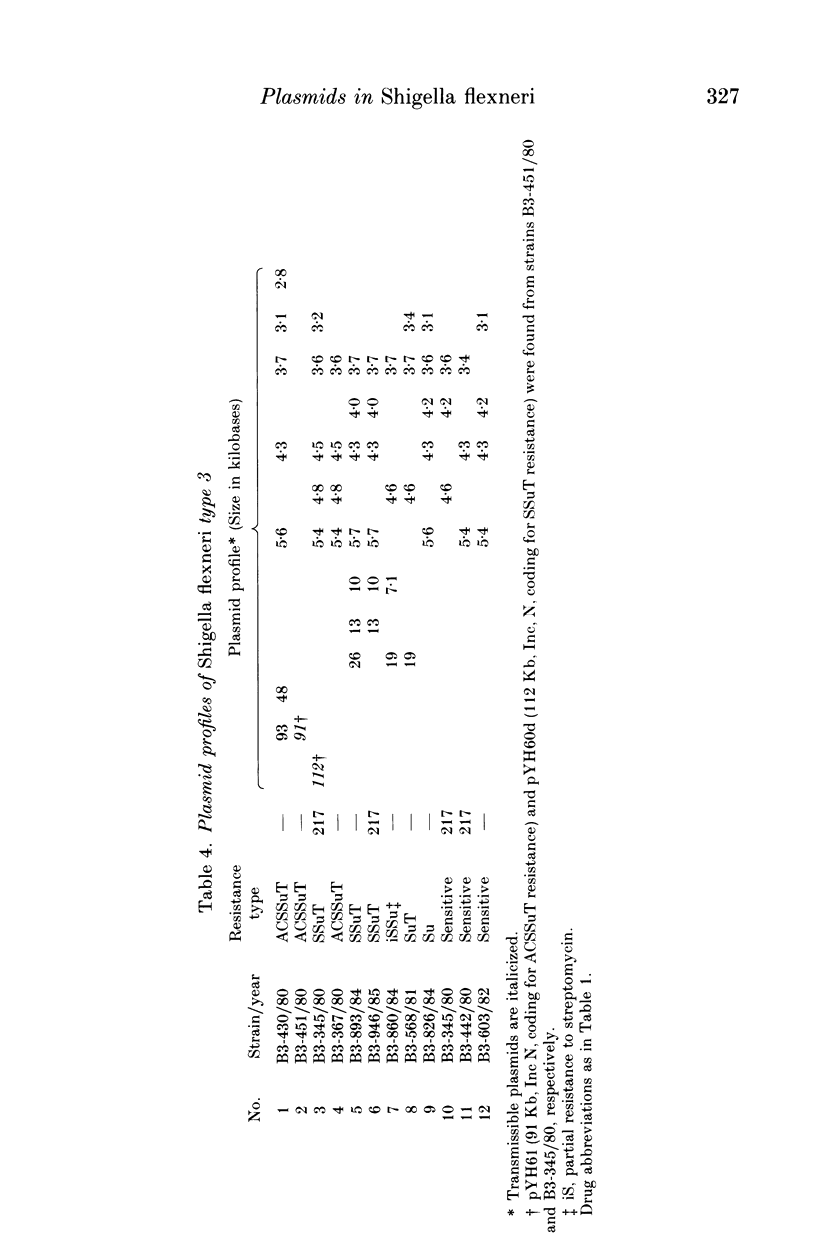
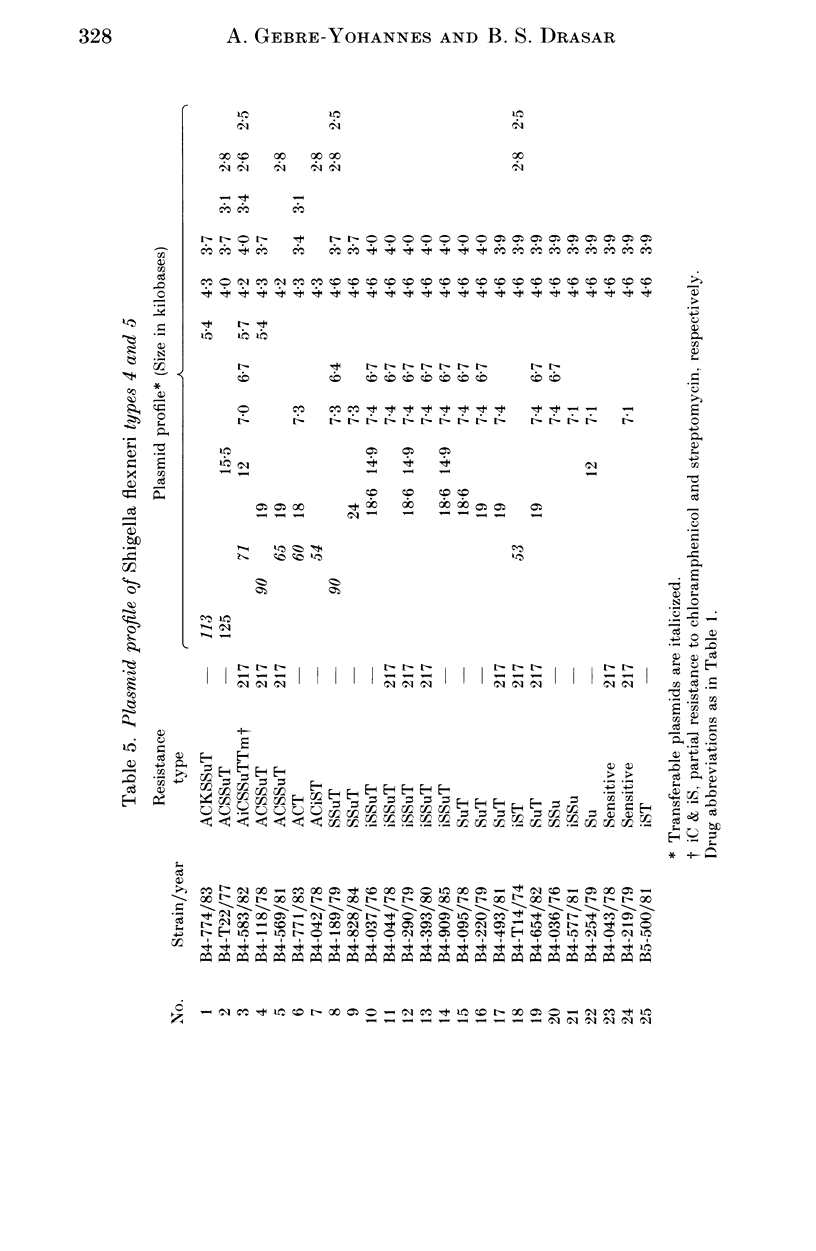
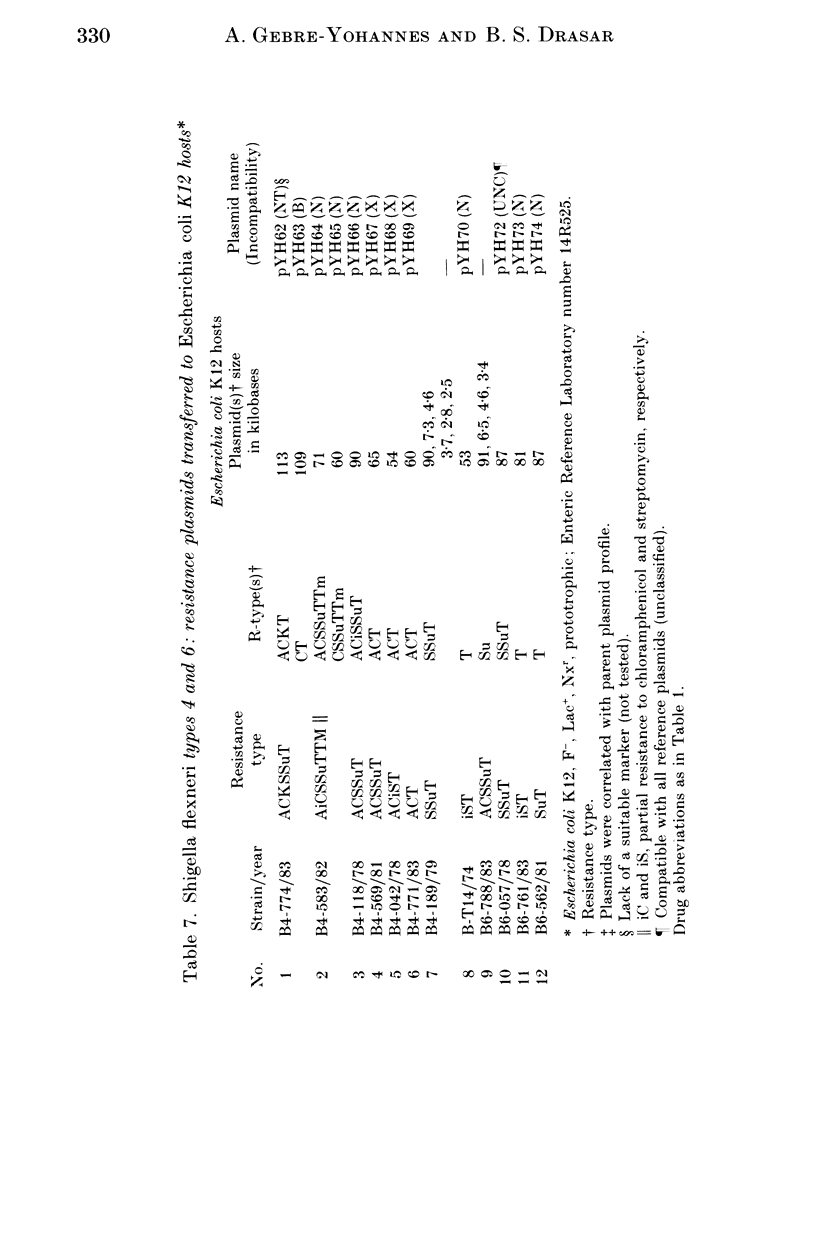
A total of 123 drug-resistant and drug-sensitive Shigella flexneri types 1-6, and their Escherichia coli K12 transconjugants were used for plasmid profile analysis by agarose gel electrophoresis. Resistance factors (R-factors) were further characterized by incompatibility testing. The overall distribution of small plasmids in S. flexneri showed that a cryptic plasmid of about 4.6 Kb was found in all serotypes, and a plasmid of about 4.2 Kb was found in serotypes 1-4. Shigella flexneri types 2, 4 and 6 showed a 6.5 Kb plasmid which correlated with SSu-resistance. All S. flexneri serotypes harboured large plasmids of about 217 Kb. Plasmid profile analysis of S. flexneri in Ethiopia showed a high degree of uniformity within individual serotypes. However, there was a limited variability which, at times, could be useful for epidemiological investigation. Shigella flexneri serotypes 1-6 harboured resistance plasmids with diverse molecular weights but mostly belonging to incompatibility groups N and X.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arai T., Sasahira T., Sakaguchi T., Sakaguchi S., Nakamura I., Kudo Y., Wang F., Zhang Y. X., Liu Y. K., Sima H. L. Plasmid DNA survey of clinically isolated Shigella strains in Shanghai area. Chin Med J (Engl) 1988 May;101(5):346–352. [PubMed] [Google Scholar]
- Bauer A. W., Kirby W. M., Sherris J. C., Turck M. Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol. 1966 Apr;45(4):493–496. [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabbert Y. A., Roussel A. Taxonomy and epidemiology of R plasmids as molecular species. J Antimicrob Chemother. 1977 Nov;3 (Suppl 100):25–33. doi: 10.1093/jac/3.suppl_c.25. [DOI] [PubMed] [Google Scholar]
- Ebright J. R., Moore E. C., Sanborn W. R., Schaberg D., Kyle J., Ishida K. Epidemic Shiga bacillus dysentery in Central Africa. Am J Trop Med Hyg. 1984 Nov;33(6):1192–1197. doi: 10.4269/ajtmh.1984.33.1192. [DOI] [PubMed] [Google Scholar]
- Formal S. B., Gemski P., Baron L. S., Labrec E. H. A Chromosomal Locus Which Controls the Ability of Shigella flexneri to Evoke Keratoconjunctivitis. Infect Immun. 1971 Jan;3(1):73–79. doi: 10.1128/iai.3.1.73-79.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost J. A., Rowe B. Plasmid-determined antibiotic resistance in Shigella flexneri isolated in England and Wales between 1974 and 1978. J Hyg (Lond) 1983 Feb;90(1):27–32. doi: 10.1017/s0022172400063798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebre-Yohannes A. Changing patterns of drug resistance in Shigella flexneri serotypes (1978-82). East Afr Med J. 1984 Aug;61(8):600–605. [PubMed] [Google Scholar]
- Gebre-Yohannes A., Drasar B. S. Plasmid mediated drug resistance in Shigella flexneri serotypes 1-6 during 1974-85. Indian J Med Res. 1988 Dec;88:480–487. [PubMed] [Google Scholar]
- Gebre-Yohannes A., Drasar B. S. Plasmid profiles of Shigella dysenteriae type 1 isolates from Ethiopia with special reference to R-plasmids. J Med Microbiol. 1990 Oct;33(2):101–106. doi: 10.1099/00222615-33-2-101. [DOI] [PubMed] [Google Scholar]
- Gebre-Yohannes A., Drasar B. S. Transferable or mobilisable antibiotic resistance in Shigella dysenteriae types 1, 2, 3, 4, 6 and 7 isolated in Ethiopia during 1974-85. J Med Microbiol. 1988 Dec;27(4):285–289. doi: 10.1099/00222615-27-4-285. [DOI] [PubMed] [Google Scholar]
- Gebre-Yohannes A., Habte-Gabr E. Shigellosis in Ethiopia. I. Prevalent Shigella serogroups and serotypes. J Diarrhoeal Dis Res. 1984 Jun;2(2):79–82. [PubMed] [Google Scholar]
- Gebre-Yohannes A., Habte-Gabr E. Shigellosis in Ethiopia: II. Patterns of drug resistance in Shigella serotypes. J Diarrhoeal Dis Res. 1984 Dec;2(4):212–216. [PubMed] [Google Scholar]
- Gedebou M., Tassew A. Shigella species from Addis Ababa: frequency of isolation and in vitro drug sensitivity. J Hyg (Lond) 1982 Feb;88(1):47–55. doi: 10.1017/s0022172400069886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grindley N. D., Grindley J. N., Anderson E. S. R factor compatibility groups. Mol Gen Genet. 1972;119(4):287–297. doi: 10.1007/BF00272087. [DOI] [PubMed] [Google Scholar]
- Haider K., Huq M. I., Samadi A. R., Ahmad K. Plasmid characterization of Shigella spp. isolated from children with shigellosis and asymptomatic excretors. J Antimicrob Chemother. 1985 Dec;16(6):691–698. doi: 10.1093/jac/16.6.691. [DOI] [PubMed] [Google Scholar]
- Haider K., Kay B. A., Talukder K. A., Huq M. I. Plasmid analysis of Shigella dysenteriae type 1 isolates obtained from widely scattered geographical locations. J Clin Microbiol. 1988 Oct;26(10):2083–2086. doi: 10.1128/jcm.26.10.2083-2086.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan M., Curlin G. T., Huq I. Epidemiology of Shigella dysenteriae, type 1 infections, in Dacca urban area. Trop Geogr Med. 1979 Jun;31(2):213–223. [PubMed] [Google Scholar]
- Kopecko D. J., Washington O., Formal S. B. Genetic and physical evidence for plasmid control of Shigella sonnei form I cell surface antigen. Infect Immun. 1980 Jul;29(1):207–214. doi: 10.1128/iai.29.1.207-214.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy S. B., Hedges R. W., Sullivan F., Medeiros A. A., Sosroseputro H. Multiple antibiotic resistance plasmids in Enterobacteriaceae isolated from diarrhoeal specimens of hospitalized children in Indonesia. J Antimicrob Chemother. 1985 Jul;16(1):7–16. doi: 10.1093/jac/16.1.7. [DOI] [PubMed] [Google Scholar]
- Ling J., Chau P. Y. Plasmids mediating resistance to chloramphenicol, trimethoprim, and ampicillin in Salmonella typhi strains isolated in the Southeast Asian region. J Infect Dis. 1984 Apr;149(4):652–652. doi: 10.1093/infdis/149.4.652. [DOI] [PubMed] [Google Scholar]
- Macrina F. L., Kopecko D. J., Jones K. R., Ayers D. J., McCowen S. M. A multiple plasmid-containing Escherichia coli strain: convenient source of size reference plasmid molecules. Plasmid. 1978 Jun;1(3):417–420. doi: 10.1016/0147-619x(78)90056-2. [DOI] [PubMed] [Google Scholar]
- Murray B. E., Alvarado T., Kim K. H., Vorachit M., Jayanetra P., Levine M. M., Prenzel I., Fling M., Elwell L., McCracken G. H. Increasing resistance to trimethoprim-sulfamethoxazole among isolates of Escherichia coli in developing countries. J Infect Dis. 1985 Dec;152(6):1107–1113. doi: 10.1093/infdis/152.6.1107. [DOI] [PubMed] [Google Scholar]
- Mutanda L. N., Kaviti J. N., Wamola I. A. Patterns of shigella species and serotypes in East Africa. East Afr Med J. 1979 Aug;56(8):381–387. [PubMed] [Google Scholar]
- Novick R. P., Clowes R. C., Cohen S. N., Curtiss R., 3rd, Datta N., Falkow S. Uniform nomenclature for bacterial plasmids: a proposal. Bacteriol Rev. 1976 Mar;40(1):168–189. doi: 10.1128/br.40.1.168-189.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulou B., Gerbaud G., Courvalin P., Acar J. F., Goldstein F. W. Molecular epidemiology of resistance to trimethoprim in enterobacteria isolated in a Parisian hospital. Ann Inst Pasteur Microbiol. 1986 May-Jun;137A(3):239–251. doi: 10.1016/s0769-2609(86)80031-x. [DOI] [PubMed] [Google Scholar]
- Sansonetti P. J., Kopecko D. J., Formal S. B. Involvement of a plasmid in the invasive ability of Shigella flexneri. Infect Immun. 1982 Mar;35(3):852–860. doi: 10.1128/iai.35.3.852-860.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tacket C. O., Shahid N., Huq M. I., Alim A. R., Cohen M. L. Usefulness of plasmid profiles for differentiation of Shigella isolates in Bangladesh. J Clin Microbiol. 1984 Aug;20(2):300–301. doi: 10.1128/jcm.20.2.300-301.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi S., Nagano Y. Rapid procedure for isolation of plasmid DNA and application to epidemiological analysis. J Clin Microbiol. 1984 Oct;20(4):608–613. doi: 10.1128/jcm.20.4.608-613.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe H., Nakamura A. Large plasmids associated with virulence in Shigella species have a common function necessary for epithelial cell penetration. Infect Immun. 1985 Apr;48(1):260–262. doi: 10.1128/iai.48.1.260-262.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young H. K., Jesudason M. V., Koshi G., Amyes S. G. Trimethoprim resistance amongst urinary pathogens in south India. J Antimicrob Chemother. 1986 May;17(5):615–621. doi: 10.1093/jac/17.5.615. [DOI] [PubMed] [Google Scholar]



