Abstract
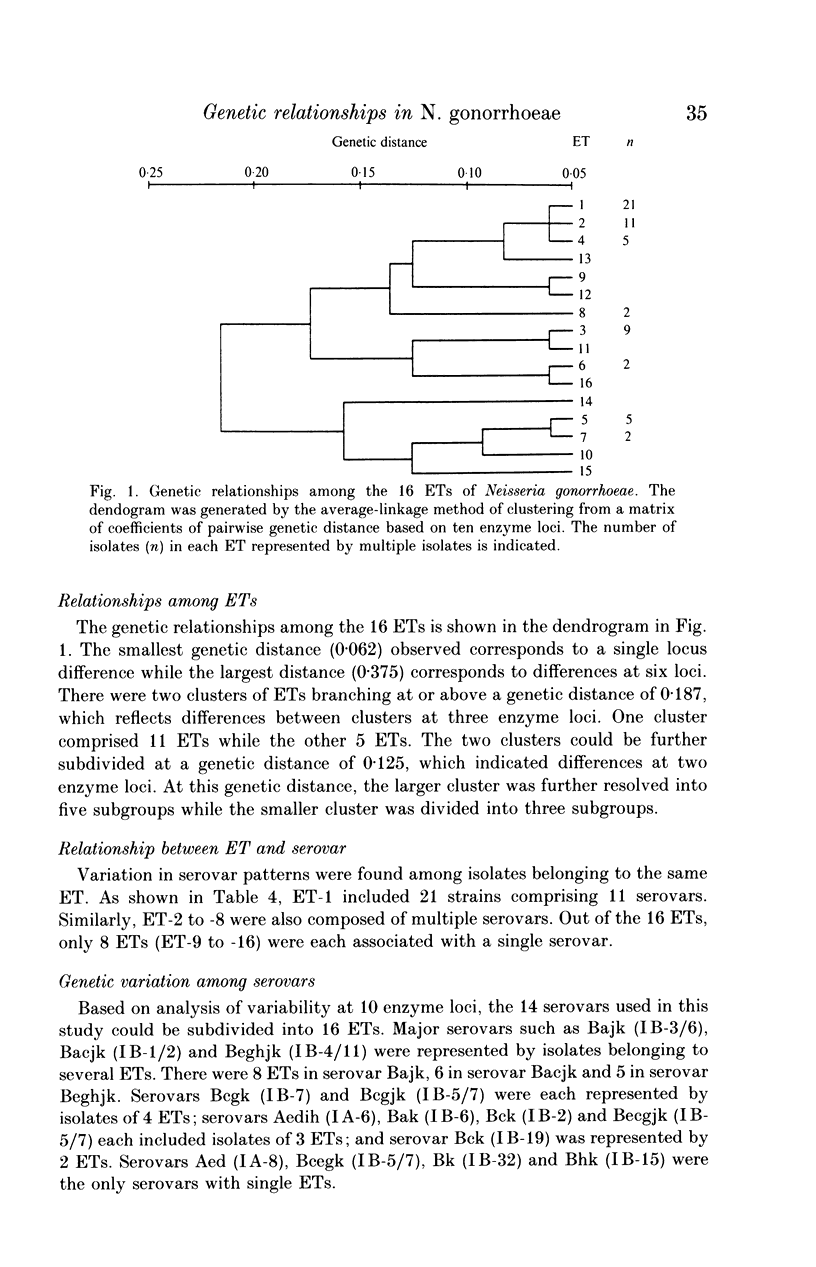
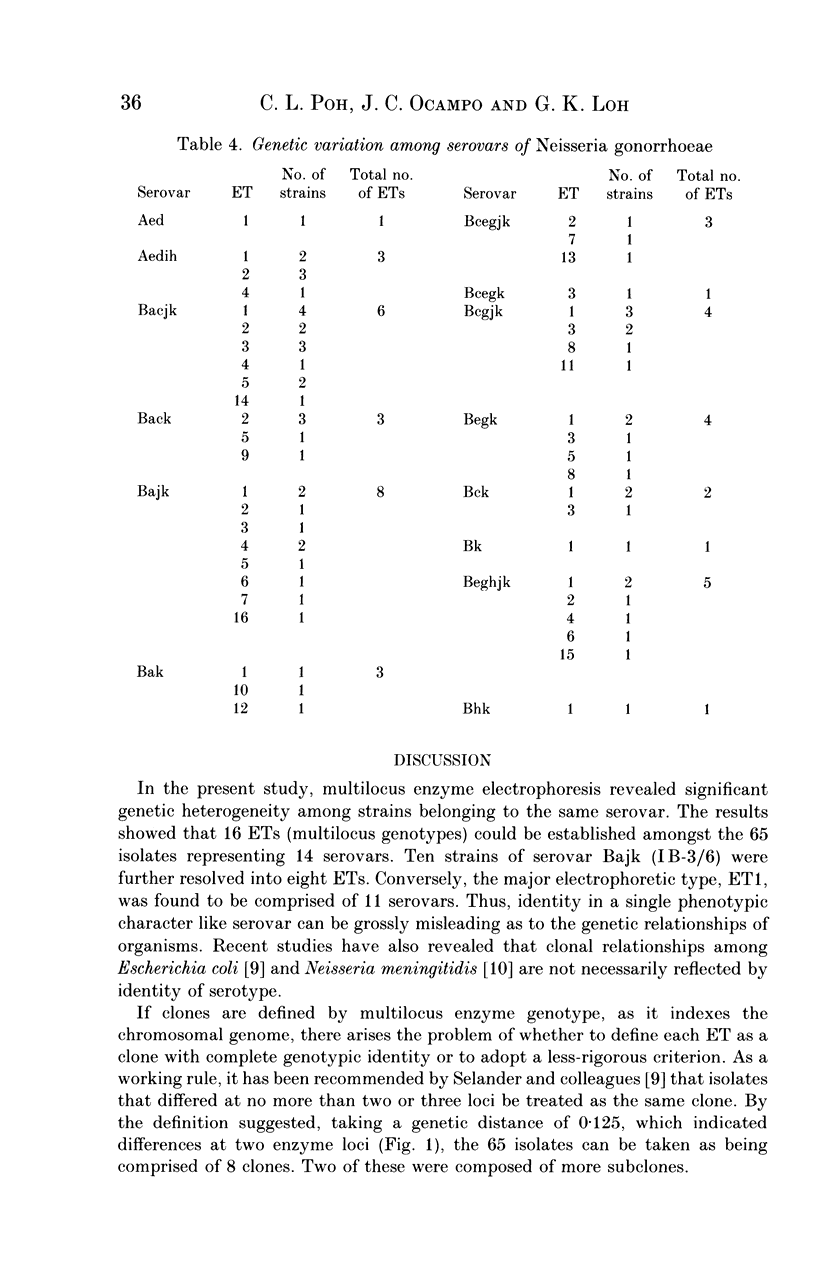
Multilocus enzyme electrophoretic analysis was employed to assess the genetic relatedness of Neisseria gonorrhoeae. Based on the diversity of electromorphs at 9 enzyme loci, 16 electrophoretic types (ETs) were established amongst the 65 isolates. The average number of alleles per enzyme locus was 1.7 and the mean genetic diversity per locus was 0.212. The majority of isolates belonged to either ET1 (32.3%) or ET2 (16.9%). No specific correlation of ETs was seen with serovars as the major types, ETs 1 and 2, were found distributed amongst the various serovars. Major serovars such as Bacjk (IB-1/2) and Bajk (IB-3/6) were each represented by 6 or 8 ETs respectively. Analysis of the genetic relationships of ETs to each other showed some clustering of subgroups that were more closely related than others.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bygdeman S., Danielsson D., Sandström E. Gonococcal W serogroups in Scandinavia. A study with polyclonal and monoclonal antibodies. Acta Pathol Microbiol Immunol Scand B. 1983 Oct;91(5):293–305. [PubMed] [Google Scholar]
- Caugant D. A., Mocca L. F., Frasch C. E., Frøholm L. O., Zollinger W. D., Selander R. K. Genetic structure of Neisseria meningitidis populations in relation to serogroup, serotype, and outer membrane protein pattern. J Bacteriol. 1987 Jun;169(6):2781–2792. doi: 10.1128/jb.169.6.2781-2792.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Knapp J. S., Tam M. R., Nowinski R. C., Holmes K. K., Sandström E. G. Serological classification of Neisseria gonorrhoeae with use of monoclonal antibodies to gonococcal outer membrane protein I. J Infect Dis. 1984 Jul;150(1):44–48. doi: 10.1093/infdis/150.1.44. [DOI] [PubMed] [Google Scholar]
- Nei M. Estimation of average heterozygosity and genetic distance from a small number of individuals. Genetics. 1978 Jul;89(3):583–590. doi: 10.1093/genetics/89.3.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandstrom E. G., Chen K. C., Buchanan T. M. Serology of Neisseria gonorrhoeae: coagglutination serogroups WI and WII/III correspond to different outer membrane protein I molecules. Infect Immun. 1982 Nov;38(2):462–470. doi: 10.1128/iai.38.2.462-470.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selander R. K., Caugant D. A., Ochman H., Musser J. M., Gilmour M. N., Whittam T. S. Methods of multilocus enzyme electrophoresis for bacterial population genetics and systematics. Appl Environ Microbiol. 1986 May;51(5):873–884. doi: 10.1128/aem.51.5.873-884.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selander R. K., Korhonen T. K., Väisänen-Rhen V., Williams P. H., Pattison P. E., Caugant D. A. Genetic relationships and clonal structure of strains of Escherichia coli causing neonatal septicemia and meningitis. Infect Immun. 1986 Apr;52(1):213–222. doi: 10.1128/iai.52.1.213-222.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam M. R., Buchanan T. M., Sandström E. G., Holmes K. K., Knapp J. S., Siadak A. W., Nowinski R. C. Serological classification of Neisseria gonorrhoeae with monoclonal antibodies. Infect Immun. 1982 Jun;36(3):1042–1053. doi: 10.1128/iai.36.3.1042-1053.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]



