Abstract
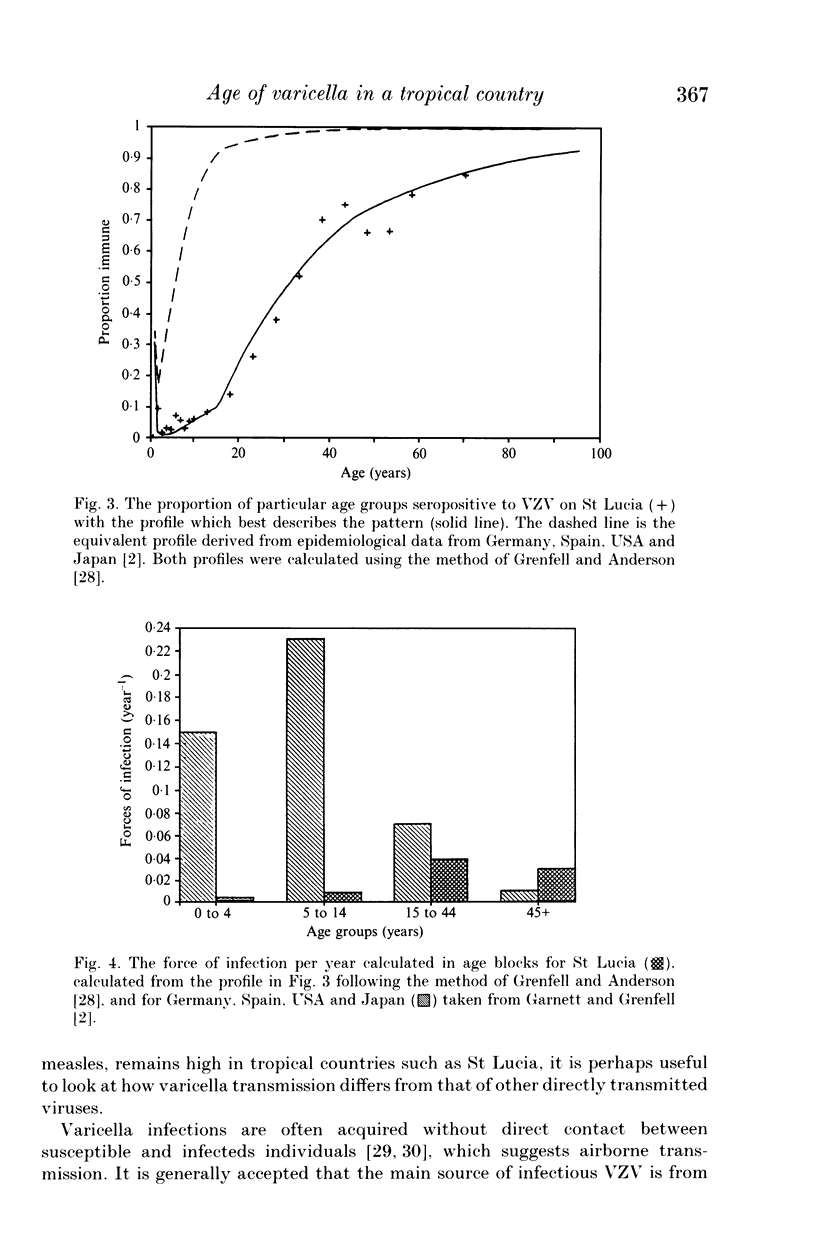
Sera from an age-stratified sample of 1810 people from the Caribbean island of St Lucia were tested for antibodies against varicella-zoster virus. The results indicate that very few infections occur in childhood, which agrees with clinical survey data from other tropical countries, but contrasts with the observed high case rate in children in temperate countries. The alternative hypotheses which may explain these results are discussed, and it is suggested that high ambient temperatures interfere with the transmission of the virus. Irrespective of the cause, the pattern of varicella incidence observed has important implications for any vaccination policy adopted in tropical countries.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asano Y., Iwayama S., Miyata T., Yazaki T., Ozaki T., Tsuzuki K., Ito S., Takahashi M. Spread of varicella in hospitalized children having no direct contact with an indicator zoster case and its prevention by a live vaccine. Biken J. 1980 Sep;23(3):157–161. [PubMed] [Google Scholar]
- Beale A. J. Polio vaccines: time for a change in immunisation policy? Lancet. 1990 Apr 7;335(8693):839–842. doi: 10.1016/0140-6736(90)90945-2. [DOI] [PubMed] [Google Scholar]
- Black F. L., Hierholzer W. J., Pinheiro F., Evans A. S., Woodall J. P., Opton E. M., Emmons J. E., West B. S., Edsall G., Downs W. G. Evidence for persistence of infectious agents in isolated human populations. Am J Epidemiol. 1974 Sep;100(3):230–250. doi: 10.1093/oxfordjournals.aje.a112032. [DOI] [PubMed] [Google Scholar]
- Cox M. J., Anderson R. M., Bundy D. A., Nokes D. J., Didier J. M., Simmons I., St Catherine J. Seroepidemiological study of the transmission of the mumps virus in St. Lucia, West Indies. Epidemiol Infect. 1989 Feb;102(1):147–160. doi: 10.1017/s0950268800029770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edson C. M., Hosler B. A., Respess R. A., Waters D. J., Thorley-Lawson D. A. Cross-reactivity between herpes simplex virus glycoprotein B and a 63,000-dalton varicella-zoster virus envelope glycoprotein. J Virol. 1985 Oct;56(1):333–336. doi: 10.1128/jvi.56.1.333-336.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans A. S., Cook J. A., Kapikian A. Z., Nankervis G., Smith A. L., West B. A serological survey of St Lucia. Int J Epidemiol. 1979 Dec;8(4):327–332. doi: 10.1093/ije/8.4.327. [DOI] [PubMed] [Google Scholar]
- Forghani B., Schmidt N. J., Dennis J. Antibody assays for varicella-zoster virus: comparison of enzyme immunoassay with neutralization, immune adherence hemagglutination, and complement fixation. J Clin Microbiol. 1978 Nov;8(5):545–552. doi: 10.1128/jcm.8.5.545-552.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GORDON J. E. Chickenpox: an epidemiological review. Am J Med Sci. 1962 Sep;244:362–389. [PubMed] [Google Scholar]
- Garnett G. P., Grenfell B. T. The epidemiology of varicella-zoster virus infections: a mathematical model. Epidemiol Infect. 1992 Jun;108(3):495–511. doi: 10.1017/s0950268800050007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershon A. A., LaRussa P., Hardy I., Steinberg S., Silverstein S. Varicella vaccine: the American experience. J Infect Dis. 1992 Aug;166 (Suppl 1):S63–S68. doi: 10.1093/infdis/166.supplement_1.s63. [DOI] [PubMed] [Google Scholar]
- Gershon A. A., Raker R., Steinberg S., Topf-Olstein B., Drusin L. M. Antibody to Varicella-Zoster virus in parturient women and their offspring during the first year of life. Pediatrics. 1976 Nov;58(5):692–696. [PubMed] [Google Scholar]
- Gold E. Serologic and virus-isolation studies of patients with varicella or herpes-zoster infection. N Engl J Med. 1966 Jan 27;274(4):181–185. doi: 10.1056/NEJM196601272740403. [DOI] [PubMed] [Google Scholar]
- Grenfell B. T., Anderson R. M. The estimation of age-related rates of infection from case notifications and serological data. J Hyg (Lond) 1985 Oct;95(2):419–436. doi: 10.1017/s0022172400062859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyun F. Chickenpox occurrence in Ibadan City: a geographic perspective. Geogr Med. 1984;14:73–96. [PubMed] [Google Scholar]
- KAPSENBERG J. G. POSSIBLE ANTIGENIC RELATIONSHIP BETWEEN VARICELLA ZOSTER VIRUS AND HERPES SIMPLEX VIRUS. Arch Gesamte Virusforsch. 1964 Sep 9;15:67–73. doi: 10.1007/BF01241422. [DOI] [PubMed] [Google Scholar]
- Leventon-Kriss S., Yoffe R., Rannon L., Modan M. Seroepidemiologic aspects of varicella zoster virus infections in an Israeli Jewish population. Isr J Med Sci. 1978 Jul;14(7):766–770. [PubMed] [Google Scholar]
- MARETIC Z., COORAY M. P. COMPARISONS BETWEEN CHICKENPOX IN A TROPICAL AND A EUROPEAN COUNTRY. J Trop Med Hyg. 1963 Dec;66:311–315. [PubMed] [Google Scholar]
- Marco Macián A., Casani Martínez C., Gutiérrez San-Román C., Segarra Llidó V., Velázquez Terróns J., Segade Andrade R., Ruiz Company S. Tricobezoar en la infancia. A propósito de dos casos. An Esp Pediatr. 1989 May;30(5):410–413. [PubMed] [Google Scholar]
- Margalith M., Fattal B., Shuval H. I., Morag A. Prevalence of antibodies to enteroviruses and varicella-zoster virus among residents and overseas volunteers at agricultural settlements in Israel. J Med Virol. 1986 Oct;20(2):189–197. doi: 10.1002/jmv.1890200211. [DOI] [PubMed] [Google Scholar]
- McLean A. R., Anderson R. M. Measles in developing countries. Part I. Epidemiological parameters and patterns. Epidemiol Infect. 1988 Feb;100(1):111–133. doi: 10.1017/s0950268800065614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson A. M., Geme J. W., Jr On the respiratory spread of varicella-zoster virus. Pediatrics. 1966 Jun;37(6):1007–1009. [PubMed] [Google Scholar]
- Preblud S. R. Age-specific risks of varicella complications. Pediatrics. 1981 Jul;68(1):14–17. [PubMed] [Google Scholar]
- Ross C. A., Subak Sharpe J. H., Ferry P. Antigenic relationship of varicella-zoster and herpes simplex. Lancet. 1965 Oct 9;2(7415):708–711. doi: 10.1016/s0140-6736(65)90452-6. [DOI] [PubMed] [Google Scholar]
- SIMPSON R. E. H. Studies on shingles: is the virus ordinary chickenpox virus? Lancet. 1954 Dec 25;267(6852):1299–1302. doi: 10.1016/s0140-6736(54)90708-4. [DOI] [PubMed] [Google Scholar]
- Salomon J. B., Gordon J. E., Scrimshaw N. S. Studies of diarrheal disease in Central America. X. Associated chickenpox, diarrhea and kwashiorkor in a highland Guatemalan village. Am J Trop Med Hyg. 1966 Nov;15(6):997–1002. doi: 10.4269/ajtmh.1966.15.997. [DOI] [PubMed] [Google Scholar]
- Schenzle D. An age-structured model of pre- and post-vaccination measles transmission. IMA J Math Appl Med Biol. 1984;1(2):169–191. doi: 10.1093/imammb/1.2.169. [DOI] [PubMed] [Google Scholar]
- Schneweis K. E., Krentler C., Wolff M. H. Durchseuchung mit dem Varicella-Zoster-Virus und serologische Feststellung der Erstinfektionsimmunität. Dtsch Med Wochenschr. 1985 Mar 22;110(12):453–457. doi: 10.1055/s-2008-1068845. [DOI] [PubMed] [Google Scholar]
- Shehab Z., Brunell P. A. Enzyme-linked immunosorbent assay for susceptibility to varicella. J Infect Dis. 1983 Sep;148(3):472–476. doi: 10.1093/infdis/148.3.472. [DOI] [PubMed] [Google Scholar]
- Sinha D. P. Chickenpox--a disease predominantly affecting adults in rural West Bengal, India. Int J Epidemiol. 1976 Dec;5(4):367–374. doi: 10.1093/ije/5.4.367. [DOI] [PubMed] [Google Scholar]
- Venkitaraman A. R., John T. J. The epidemiology of varicella in staff and students of a hospital in the tropics. Int J Epidemiol. 1984 Dec;13(4):502–505. doi: 10.1093/ije/13.4.502. [DOI] [PubMed] [Google Scholar]
- Venkitaraman A. R., Seigneurin J. M., Baccard M., Lenoir G. M., John T. J. Measurement of antibodies to varicella-zoster virus in a tropical population by enzyme-linked immunosorbent assay. J Clin Microbiol. 1984 Sep;20(3):582–583. doi: 10.1128/jcm.20.3.582-583.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkitaraman A. R., Seigneurin J. M., Lenoir G. M., John T. J. Infections due to the human herpesviruses in southern India: a seroepidemiological survey. Int J Epidemiol. 1986 Dec;15(4):561–566. doi: 10.1093/ije/15.4.561. [DOI] [PubMed] [Google Scholar]
- WELLER T. H. Serial propagation in vitro of agents producing inclusion bodies derived from varicella and herpes zoster. Proc Soc Exp Biol Med. 1953 Jun;83(2):340–346. doi: 10.3181/00379727-83-20354. [DOI] [PubMed] [Google Scholar]
- Wagenvoort J. H., Harmsen M., Boutahar-Trouw B. J., Kraaijeveld C. A., Winkler K. C. Epidemiology of mumps in the Netherlands. J Hyg (Lond) 1980 Dec;85(3):313–326. doi: 10.1017/s0022172400063385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff M. H., Büchel F., Zoll A. Serological studies on the antigenic relationship between herpes simplex virus and varicella-zoster virus. Immunobiology. 1979 Aug;156(1-2):76–82. [PubMed] [Google Scholar]
- Yorke J. A., London W. P. Recurrent outbreaks of measles, chickenpox and mumps. II. Systematic differences in contact rates and stochastic effects. Am J Epidemiol. 1973 Dec;98(6):469–482. doi: 10.1093/oxfordjournals.aje.a121576. [DOI] [PubMed] [Google Scholar]



