Abstract
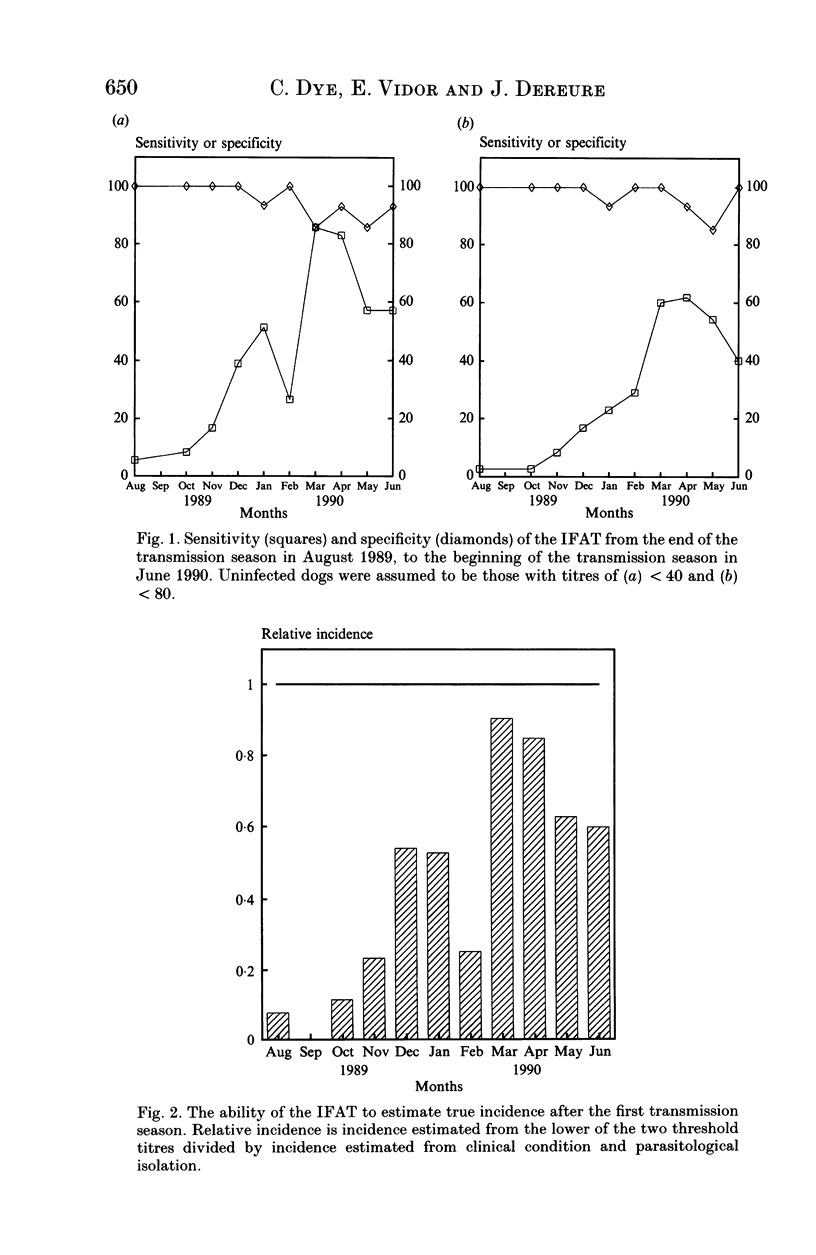
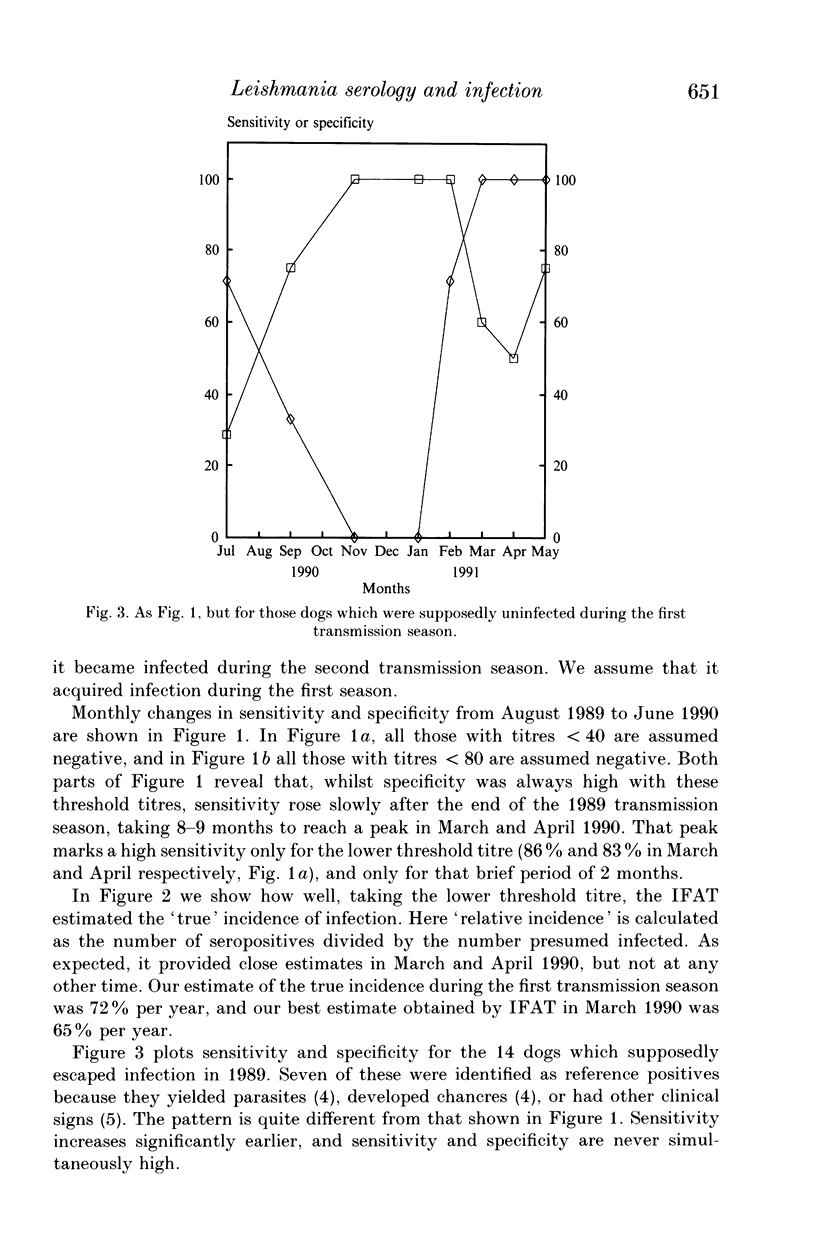
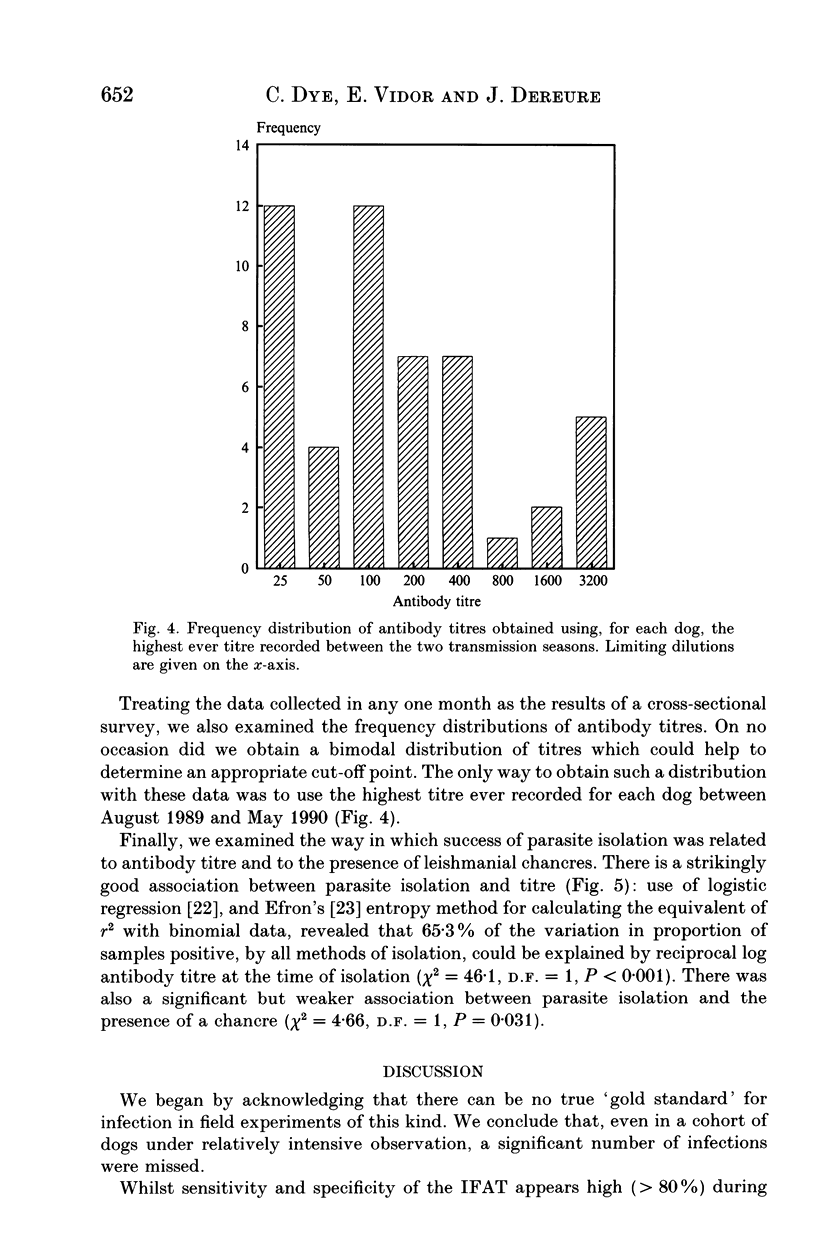
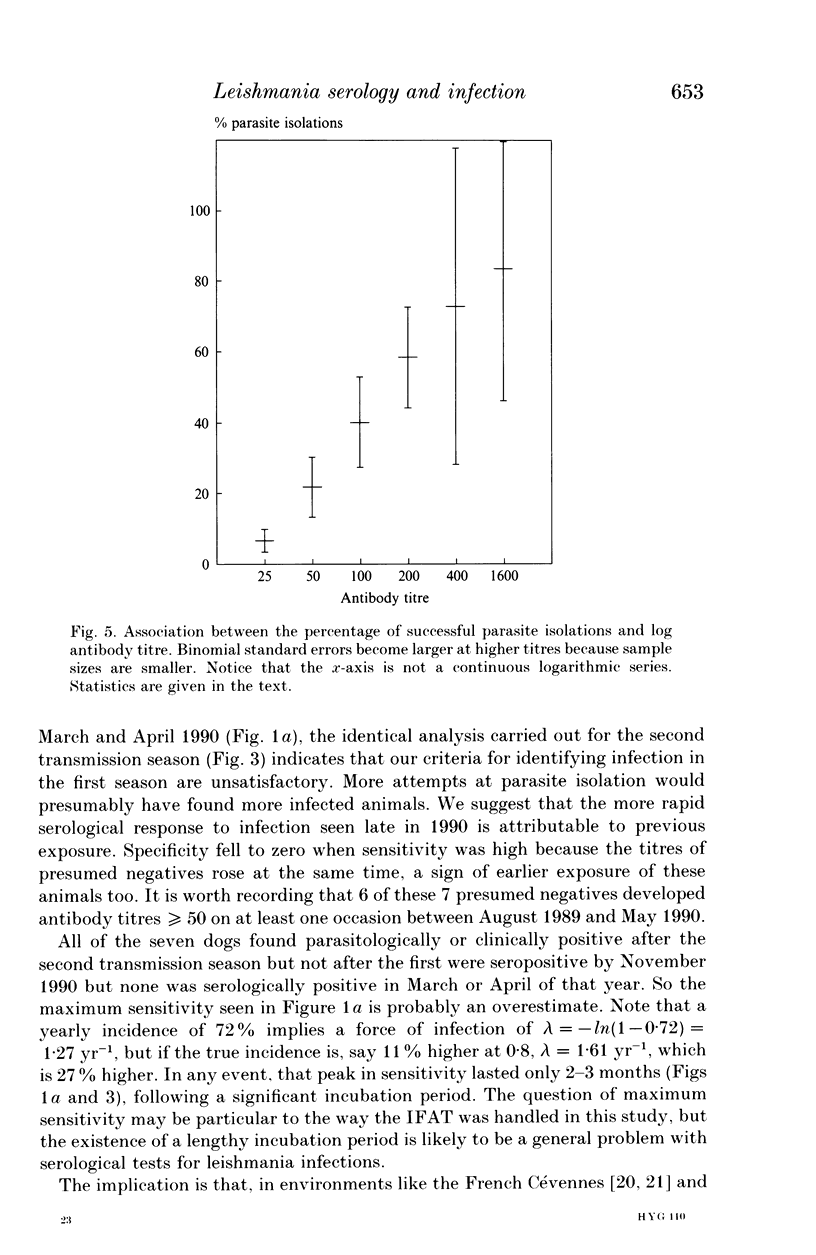
Serological tests are very frequently used in epidemiological surveys of leishmaniasis and other parasitoses. Their sensitivity and specificity are generally defined with respect to parasitism and disease, rather than infection. The reason is that known positives are those individuals most likely to yield parasites, or who have distinctive clinical signs, and concomitantly high antibody titres. This paper investigates the performance of one serological method, the indirect fluorescent antibody test (IFAT), in detecting Leishmania infantum infection during an intensive 2-year cohort study of dogs in southern France. The results show that sensitivity and specificity with respect to infection can be simultaneously high, but maximum sensitivity is probably < 80%, and lasts for a relatively short period of 2-3 months after a lengthy incubation period. The IFAT gave the incidence of infection as 18-65% in the first year, whereas the best estimate of incidence based on parasite isolation and clinical observation was 72%. But data from the second year suggest that the 72% was itself an underestimate. We argue that, during epidemiological surveys, the IFAT in particular, and serological tests for leishmania in general, will underestimate prevalence, incidence and hence the scale of the control problem. However, there is evidence that tests for canine leishmaniasis employing high threshold titres will identify the most infectious animals, allowing selective treatment or culling of those which contribute disproportionately to transmission.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abranches P., Lopes F. J., Silva F. M., Ribeiro M. M., Pires C. A. Le kala-azar au Portugal. III. Résultats d'une enquête sur la leishmaniose canine réalisée dans les environs de Lisbonne. Comparaison des zones urbaines et rurales. Ann Parasitol Hum Comp. 1983;58(4):307–315. [PubMed] [Google Scholar]
- Abranches P., Silva-Pereira M. C., Conceiço-Silva F. M., Santos-Gomes G. M., Janz J. G. Canine leishmaniasis: pathological and ecological factors influencing transmission of infection. J Parasitol. 1991 Aug;77(4):557–561. [PubMed] [Google Scholar]
- Badaro R., Jones T. C., Carvalho E. M., Sampaio D., Reed S. G., Barral A., Teixeira R., Johnson W. D., Jr New perspectives on a subclinical form of visceral leishmaniasis. J Infect Dis. 1986 Dec;154(6):1003–1011. doi: 10.1093/infdis/154.6.1003. [DOI] [PubMed] [Google Scholar]
- Badaró R., Jones T. C., Lorenço R., Cerf B. J., Sampaio D., Carvalho E. M., Rocha H., Teixeira R., Johnson W. D., Jr A prospective study of visceral leishmaniasis in an endemic area of Brazil. J Infect Dis. 1986 Oct;154(4):639–649. doi: 10.1093/infdis/154.4.639. [DOI] [PubMed] [Google Scholar]
- Badaró R., Reed S. G., Carvalho E. M. Immunofluorescent antibody test in American visceral leishmaniasis: sensitivity and specificity of different morphological forms of two Leishmania species. Am J Trop Med Hyg. 1983 May;32(3):480–484. doi: 10.4269/ajtmh.1983.32.480. [DOI] [PubMed] [Google Scholar]
- Dye C., Guy M. W., Elkins D. B., Wilkes T. J., Killick-Kendrick R. The life expectancy of phlebotomine sandflies: first field estimates from southern France. Med Vet Entomol. 1987 Oct;1(4):417–425. doi: 10.1111/j.1365-2915.1987.tb00372.x. [DOI] [PubMed] [Google Scholar]
- Dye C., Killick-Kendrick R., Vitutia M. M., Walton R., Killick-Kendrick M., Harith A. E., Guy M. W., Cañavate M. C., Hasibeder G. Epidemiology of canine leishmaniasis: prevalence, incidence and basic reproduction number calculated from a cross-sectional serological survey on the island of Gozo, Malta. Parasitology. 1992 Aug;105(Pt 1):35–41. doi: 10.1017/s0031182000073662. [DOI] [PubMed] [Google Scholar]
- Evans T. G., Vasconcelos I. A., Lima J. W., Teixeira J. M., McAullife I. T., Lopes U. G., Pearson R. D., Vasconcelos A. W. Canine visceral leishmaniasis in northeast Brazil: assessment of serodiagnostic methods. Am J Trop Med Hyg. 1990 Feb;42(2):118–123. doi: 10.4269/ajtmh.1990.42.118. [DOI] [PubMed] [Google Scholar]
- Harith A. E., Kolk A. H., Kager P. A., Leeuwenburg J., Faber F. J., Muigai R., Kiugu S., Laarman J. J. Evaluation of a newly developed direct agglutination test (DAT) for serodiagnosis and sero-epidemiological studies of visceral leishmaniasis: comparison with IFAT and ELISA. Trans R Soc Trop Med Hyg. 1987;81(4):603–606. doi: 10.1016/0035-9203(87)90423-8. [DOI] [PubMed] [Google Scholar]
- Harith A. E., Kolk A. H., Kager P. A., Leeuwenburg J., Muigai R., Kiugu S., Kiugu S., Laarman J. J. A simple and economical direct agglutination test for serodiagnosis and sero-epidemiological studies of visceral leishmaniasis. Trans R Soc Trop Med Hyg. 1986;80(4):583–536. doi: 10.1016/0035-9203(86)90149-5. [DOI] [PubMed] [Google Scholar]
- Hasibeder G., Dye C., Carpenter J. Mathematical modelling and theory for estimating the basic reproduction number of canine leishmaniasis. Parasitology. 1992 Aug;105(Pt 1):43–53. doi: 10.1017/s0031182000073674. [DOI] [PubMed] [Google Scholar]
- Ho M., Siongok T. K., Lyerly W. H., Smith D. H. Prevalence and disease spectrum in a new focus of visceral leishmaniasis in Kenya. Trans R Soc Trop Med Hyg. 1982;76(6):741–746. doi: 10.1016/0035-9203(82)90095-5. [DOI] [PubMed] [Google Scholar]
- Lainson R., Dye C., Shaw J. J., Macdonald D. W., Courtenay O., Souza A. A., Silveira F. T. Amazonian visceral leishmaniasis--distribution of the vector Lutzomyia longipalpis (Lutz & Neiva) in relation to the fox Cerdocyon thous (linn.) and the efficiency of this reservoir host as a source of infection. Mem Inst Oswaldo Cruz. 1990 Jan-Mar;85(1):135–137. doi: 10.1590/s0074-02761990000100027. [DOI] [PubMed] [Google Scholar]
- Lanotte G., Rioux J. A., Perieres J., Vollhardt Y. Ecologie des leishmanioses dans le sud de la France. 10. Les formes évolutives de la leishmaniose viscérale canine. Elaboration d'une typologie bio-clinique à finalité épidémiologique. Ann Parasitol Hum Comp. 1979 May-Jun;54(3):277–295. [PubMed] [Google Scholar]
- Pozio E., Gradoni L., Bettini S., Gramiccia M. Leishmaniasis in Tuscany (Italy): VI. Canine leishmaniasis in the focus of Monte Argentario (Grosseto). Acta Trop. 1981 Dec;38(4):383–393. [PubMed] [Google Scholar]
- Reed S. G., Shreffler W. G., Burns J. M., Jr, Scott J. M., Orge M. da G., Ghalib H. W., Siddig M., Badaro R. An improved serodiagnostic procedure for visceral leishmaniasis. Am J Trop Med Hyg. 1990 Dec;43(6):632–639. doi: 10.4269/ajtmh.1990.43.632. [DOI] [PubMed] [Google Scholar]
- de Korte P. M., Harith A. E., Dereure J., Huigen E., Faucherre V., van der Kaay H. J. Introduction of an improved direct agglutination test for the detection of Leishmania infantum infection in southern France. Parasitol Res. 1990;76(6):526–530. doi: 10.1007/BF00931059. [DOI] [PubMed] [Google Scholar]
- el Harith A., Slappendel R. J., Reiter I., van Knapen F., de Korte P., Huigen E., Kolk A. H. Application of a direct agglutination test for detection of specific anti-Leishmania antibodies in the canine reservoir. J Clin Microbiol. 1989 Oct;27(10):2252–2257. doi: 10.1128/jcm.27.10.2252-2257.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]


