Abstract
The present study was undertaken to investigate the occurrence of antibiotic resistance in enteric flora in 64 children in rural Bangladesh over a 12-month period. The antibiotic resistance pattern of the isolates varied throughout the year and multiple resistance was highest during the post monsoon period. Seventy-three percent of children had isolates resistant to more than three antibiotics throughout the year. Resistance to streptomycin was highest (78%), followed closely by ampicillin (72%). Of 82 multiply resistant isolates, plasmid DNA was demonstrated in 75%. Plasmid sizes ranged between 3.7 and 110 MDa, the commonest plasmids were of 70, 98 and 110 MDa. Complete or partial resistance was transferred by conjugation from 52% of the isolates, most frequently by single plasmids. The commonest plasmid incompatibility group was F11-A (46%) followed by incompatibility group P (22%). Plasmids of molecular weight 98 MDa most often hybridized with F11-A probes and those of 110 MDa with H11 probes. Plasmids from 10 transconjugants were digested with restriction enzymes and digest patterns demonstrated the presence of common plasmids. The findings show that there is a diverse, and mobile, genetic pool of resistance genes in this rural community. This genetic reservoir is potentially transferable to enteric pathogens, with major implications for public health and diarrhoeal disease control.
Full text
PDF




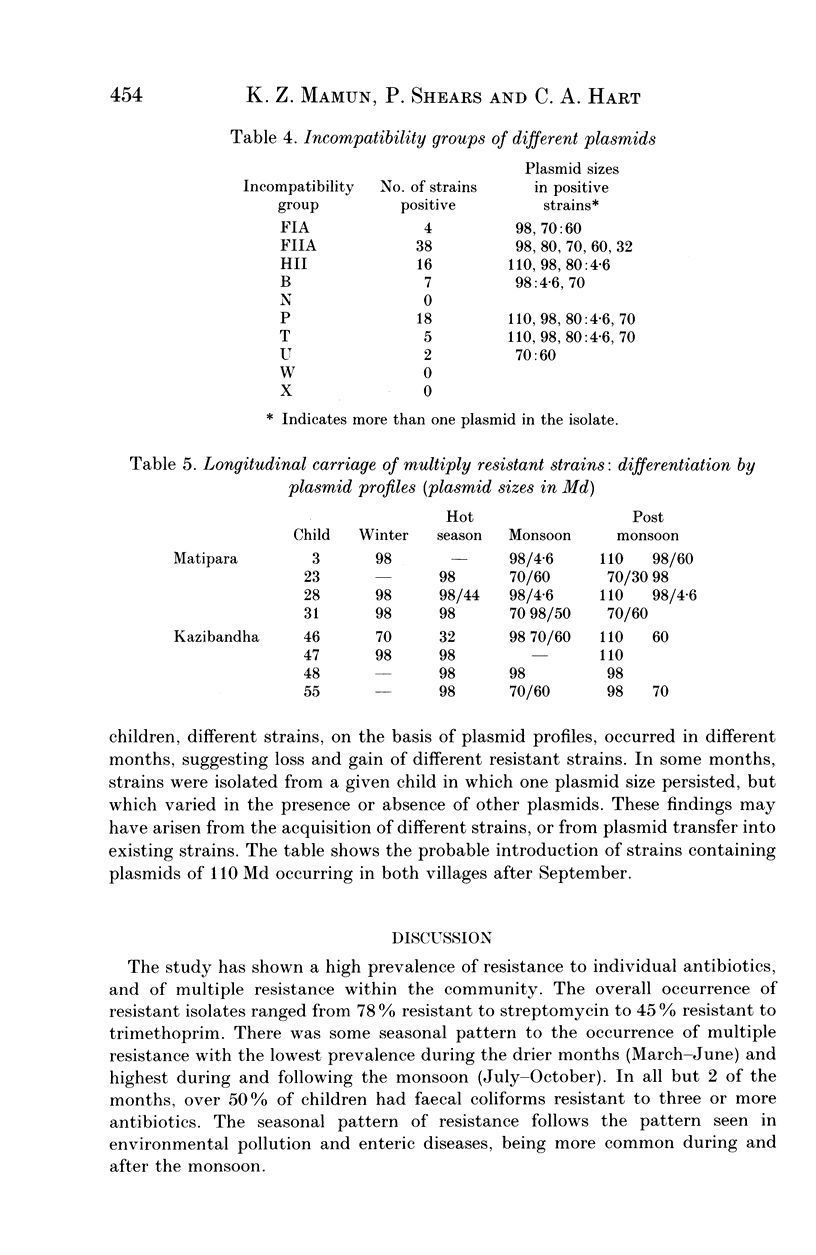




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amyes S. G., Tait S., Thomson C. J., Payne D. J., Nandivada L. S., Jesudason M. V., Mukundan U. D., Young H. K. The incidence of antibiotic resistance in aerobic faecal flora in south India. J Antimicrob Chemother. 1992 Apr;29(4):415–425. doi: 10.1093/jac/29.4.415. [DOI] [PubMed] [Google Scholar]
- Ashenafi M., Gedebou M. Salmonella and Shigella in adult diarrhoea in Addis Ababa--prevalence and antibiograms. Trans R Soc Trop Med Hyg. 1985;79(5):719–721. doi: 10.1016/0035-9203(85)90201-9. [DOI] [PubMed] [Google Scholar]
- Bennish M., Eusof A., Kay B., Wierzba T. Multiresistant Shigella infections in Bangladesh. Lancet. 1985 Aug 24;2(8452):441–441. doi: 10.1016/s0140-6736(85)92756-4. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couturier M., Bex F., Bergquist P. L., Maas W. K. Identification and classification of bacterial plasmids. Microbiol Rev. 1988 Sep;52(3):375–395. doi: 10.1128/mr.52.3.375-395.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrar W. E. Antibiotic resistance in developing countries. J Infect Dis. 1985 Dec;152(6):1103–1106. doi: 10.1093/infdis/152.6.1103. [DOI] [PubMed] [Google Scholar]
- Frost J. A., Rowe B., Vandepitte J. Acquisition of trimethoprim resistance in epidemic strain of Shigella dysenteriae type 1 from Zaire. Lancet. 1982 Apr 24;1(8278):963–963. doi: 10.1016/s0140-6736(82)91960-2. [DOI] [PubMed] [Google Scholar]
- Frost J. A., Rowe B., Vandepitte J., Threlfall E. J. Plasmid characterisation in the investigation of an epidemic caused by multiply resistant Shigella dysenteriae type 1 in Central Africa. Lancet. 1981 Nov 14;2(8255):1074–1076. doi: 10.1016/s0140-6736(81)91277-0. [DOI] [PubMed] [Google Scholar]
- Gebre-Yohannes A., Drasar B. S. Plasmid profiles of Shigella dysenteriae type 1 isolates from Ethiopia with special reference to R-plasmids. J Med Microbiol. 1990 Oct;33(2):101–106. doi: 10.1099/00222615-33-2-101. [DOI] [PubMed] [Google Scholar]
- Goldstein F. W., Chumpitaz J. C., Guevara J. M., Papadopoulou B., Acar J. F., Vieu J. F. Plasmid-mediated resistance to multiple antibiotics in Salmonella typhi. J Infect Dis. 1986 Feb;153(2):261–266. doi: 10.1093/infdis/153.2.261. [DOI] [PubMed] [Google Scholar]
- Hassan H. S. Sensitivity of Salmonella and Shigella to antibiotics and chemotherapeutic agents in Sudan. J Trop Med Hyg. 1985 Aug;88(4):243–247. [PubMed] [Google Scholar]
- Ichinose Y., Ehara M., Watanabe S., Shimodori S., Waiyaki P. G., Kibue A. M., Sang F. C., Ngugi J., Kaviti J. N. The characterization of Vibrio cholerae isolated in Kenya in 1983. J Trop Med Hyg. 1986 Oct;89(5):269–276. [PubMed] [Google Scholar]
- Khan M. U., Shahidullah M., Ahmed W. U., Barua D. K., Begum T., Purification D., Rahman N. Changes in the trend of shigellosis in Dhaka: family study on secondary infection, clinical manifestation and sensitivity pattern: 1980. Trans R Soc Trop Med Hyg. 1984;78(2):151–156. doi: 10.1016/0035-9203(84)90262-1. [DOI] [PubMed] [Google Scholar]
- Khan M., Curlin G. T., Huq I. Epidemiology of Shigella dysenteriae, type 1 infections, in Dacca urban area. Trop Geogr Med. 1979 Jun;31(2):213–223. [PubMed] [Google Scholar]
- Kobari K., Takakura I., Nakatomi M., Sogame S., Uylangco C. Antibiotic-resistant strains of E1 Tor vibrio in the Philippines and the use of furalazine for chemotherapy. Bull World Health Organ. 1970;43(3):365–371. [PMC free article] [PubMed] [Google Scholar]
- Kunin C. M., Johansen K. S., Worning A. M., Daschner F. D. Report of a symposium on use and abuse of antibiotics worldwide. Rev Infect Dis. 1990 Jan-Feb;12(1):12–19. doi: 10.1093/clinids/12.1.12. [DOI] [PubMed] [Google Scholar]
- Levy S. B., Hedges R. W., Sullivan F., Medeiros A. A., Sosroseputro H. Multiple antibiotic resistance plasmids in Enterobacteriaceae isolated from diarrhoeal specimens of hospitalized children in Indonesia. J Antimicrob Chemother. 1985 Jul;16(1):7–16. doi: 10.1093/jac/16.1.7. [DOI] [PubMed] [Google Scholar]
- Linton A. H. Flow of resistance genes in the environment and from animals to man. J Antimicrob Chemother. 1986 Oct;18 (Suppl 100):189–197. doi: 10.1093/jac/18.supplement_c.189. [DOI] [PubMed] [Google Scholar]
- Linton A. H. Flow of resistance genes in the environment and from animals to man. J Antimicrob Chemother. 1986 Oct;18 (Suppl 100):189–197. doi: 10.1093/jac/18.supplement_c.189. [DOI] [PubMed] [Google Scholar]
- Martin A. R., Mosley W. H., Sau B. B., Ahmed S., Huq I. Epidemiologic analysis of endemic cholera in urban East Pakistan, 1964-1966. Am J Epidemiol. 1969 May;89(5):572–582. doi: 10.1093/oxfordjournals.aje.a120970. [DOI] [PubMed] [Google Scholar]
- Mata L. J., Gangarosa E. J., Cáceres A., Perera D. R., Mejicanos M. L. Epidemic Shiga bacillus dysentery in Central America. I. Etiologic investigations in Guatemala, 1969. J Infect Dis. 1970 Sep;122(3):170–180. doi: 10.1093/infdis/122.3.170. [DOI] [PubMed] [Google Scholar]
- Montefiore D., Rotimi V. O., Adeyemi-Doro F. A. The problem of bacterial resistance to antibiotics among strains isolated from hospital patients in Lagos and Ibadan, Nigeria. J Antimicrob Chemother. 1989 Apr;23(4):641–651. doi: 10.1093/jac/23.4.641. [DOI] [PubMed] [Google Scholar]
- Montgomery J., West B., Michael A., Kadivaion B. Bacterial resistance in the Eastern Highlands Province. P N G Med J. 1987 Mar;30(1):11–19. [PubMed] [Google Scholar]
- Shahid N. S., Rahaman M. M., Haider K., Banu H., Rahman N. Changing pattern of resistant Shiga bacillus (Shigella dysenteriae type 1) and Shigella flexneri in Bangladesh. J Infect Dis. 1985 Dec;152(6):1114–1119. doi: 10.1093/infdis/152.6.1114. [DOI] [PubMed] [Google Scholar]
- Shears P., Suliman G., Hart C. A. Occurrence of multiple antibiotic resistance and R plasmids in Enterobacteriaceae isolated from children in the Sudan. Epidemiol Infect. 1988 Feb;100(1):73–81. doi: 10.1017/s0950268800065572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabtieng R., Wattanasri S., Echeverria P., Seriwatana J., Bodhidatta L., Chatkaeomorakot A., Rowe B. An epidemic of Vibrio cholerae el tor Inaba resistant to several antibiotics with a conjugative group C plasmid coding for type II dihydrofolate reductase in Thailand. Am J Trop Med Hyg. 1989 Dec;41(6):680–686. doi: 10.4269/ajtmh.1989.41.680. [DOI] [PubMed] [Google Scholar]
- Taylor D. N., Bodhidatta L., Brown J. E., Echeverria P., Kunanusont C., Naigowit P., Hanchalay S., Chatkaeomorakot A., Lindberg A. A. Introduction and spread of multi-resistant Shigella dysenteriae I in Thailand. Am J Trop Med Hyg. 1989 Jan;40(1):77–85. doi: 10.4269/ajtmh.1989.40.77. [DOI] [PubMed] [Google Scholar]
- Towner K. J., Pearson N. J., Mhalu F. S., O'Grady F. Resistance to antimicrobial agents of Vibrio cholerae E1 Tor strains isolated during the fourth cholera epidemic in the United Republic of Tanzania. Bull World Health Organ. 1980;58(5):747–751. [PMC free article] [PubMed] [Google Scholar]
- Vázquez V., Calderón E., Rodríquez R. S. Chloramphenicol-resistant strains of Salmonella typhosa. N Engl J Med. 1972 Jun 1;286(22):1220–1220. [PubMed] [Google Scholar]
- Young H. K., Amyes S. G. Plasmid trimethoprim resistance in Vibrio cholerae: migration of the type I dihydrofolate reductase gene out of the Enterobacteriaceae. J Antimicrob Chemother. 1986 Jun;17(6):697–703. doi: 10.1093/jac/17.6.697. [DOI] [PubMed] [Google Scholar]