Abstract
The autocrine/paracrine peptide signaling molecules such as growth factors have many promising biologic activities for clinical applications. However, one cannot expect specific therapeutic effects of the factors administered by ordinary drug delivery systems as they have limited target specificity and short half-lives in vivo. To overcome the difficulties in using growth factors as therapeutic agents, we have produced fusion proteins consisting of growth factor moieties and a collagen-binding domain (CBD) derived from Clostridium histolyticum collagenase. The fusion proteins carrying the epidermal growth factor (EGF) or basic fibroblast growth factor (bFGF) at the N terminal of CBD (CBEGF/CBFGF) tightly bound to insoluble collagen and stimulated the growth of BALB/c 3T3 fibroblasts as much as the unfused counterparts. CBEGF, when injected subcutaneously into nude mice, remained at the sites of injection for up to 10 days, whereas EGF was not detectable 24 h after injection. Although CBEGF did not exert a growth-promoting effect in vivo, CBFGF, but not bFGF, strongly stimulated the DNA synthesis in stromal cells at 5 days and 7 days after injection. These results indicate that CBD may be used as an anchoring unit to produce fusion proteins nondiffusible and long-lasting in vivo.
Currently, more than 100 soluble peptide signaling molecules, including peptide hormones, growth factors, and lymphokines, are known. These molecules can be classified into two types: one, like classic hormones, is produced in a specific organ/cell and exerts a characteristic effect on relatively limited target cells via blood flow (endocrine), and the other is produced in a wide variety of tissues and acts in an autocrine or a paracrine manner with low target specificity. In the latter case, the specificity of the action depends for the most part on spatio-temporarily controlled production of the signaling molecule and the local tissue architecture. The former type of molecules (endocrine factors) are suitable as therapeutic agents as they can be delivered systemically without loss of target specificity. In contrast, autocrine/paracrine factors may induce diverse responses in various tissues when present in the blood at concentrations higher than a threshold value. Thus, one cannot expect specific therapeutic effects of the factors administered by ordinary methods, though they have many promising biologic activities for clinical applications.
To overcome the difficulties in using autocrine/paracrine factors as therapeutic agents, some efforts have been made to develop a novel drug delivery system that enables the factors to act on restricted target cells. Kawase et al. (1) constructed a fusion protein from epidermal growth factor (EGF) and the cell-binding domain of fibronectin. The fusion protein (C-EGF) exhibited both cell-adhesive activity and growth factor activity, each of which was indistinguishable from that of the corresponding unfused protein. The immobilization of EGF on a modified glass surface via star poly(ethylene oxide) (PEO) was reported by Kuhl and Griffith-Cima (2). The use of flexible PEO arms permitted the EGF molecule to retain its native conformation and to interact with its receptor in a relatively unrestricted manner. As a result, the immobilized EGF (tethered EGF) showed biologic activities comparable with those of soluble EGF in vitro. In such studies, however, the limited capacity of the cell surface receptors to retain the fusion protein (C-EGF) or the inevitable use of artificial matrices (tethered EGF) may restrict the clinical application of the agents. In addition, the lack of in vivo experiments makes it difficult to evaluate the usefulness of these delivery systems.
The extracellular matrix (ECM) is known to provide a site for the storage of various growth factors (3), making it an attractive target for localizing exogenous peptide signaling molecules. Among a variety of ECM components, collagen is the most abundant and ubiquitous protein in mammals. In a previous study, we identified a gene encoding Clostridium histolyticum collagenase (colH gene) and showed that the collagenase (ColH) consisted of four segments, S1, S2a, S2b, and S3 (4). We also demonstrated that the C-terminal region of ColH (S2b + S3 or S3) was responsible for substrate recognition and that the C-terminal fragment produced in Escherichia coli retained collagen-binding activity in vitro (5).
In this study, we constructed fusion proteins consisting of a growth factor moiety and the collagen-binding domain (CBD) of C. histolyticum collagenase, which we expected to act as an anchor to the collagen fibrils in vivo. We chose EGF and basic fibroblast growth factor (bFGF) as parts of the fusion proteins (collagen-binding EGF, CBEGF; collagen-binding bFGF, CBFGF). EGF (6) and some members of the EGF family, i.e., transforming growth factor α (7, 8) and heparin-binding EGF (9), are synthesized as transmembrane precursor forms, and the membrane-bound proteins can activate the EGF receptor through a juxtacrine mechanism, that is, they can act without internalization. EGF is suitable for the present study because of this unique property in addition to its high stability. bFGF is not a juxtacrine factor. However, bFGF is also a favorable growth factor for this study, as it is an effective growth stimulator of stromal cells embedded in ECM. The collagen-binding and growth-stimulating properties of CBEGF and CBFGF were investigated both in vitro and in vivo. We found that the fusion proteins have unique properties that make them potential agents for clinical applications.
MATERIALS AND METHODS
Construction of Recombinant Plasmids.
Plasmid vectors for collagen-binding growth factors were prepared by modifying a plasmid, pCHC302 (5), encoding a fusion protein between glutathione S-transferase (GST) and the collagen-binding domain (S2b + S3) of C. histolyticum collagenase. A rat EGF cDNA encoding a mature form (47 amino acids) (10) was amplified by PCR from first-strand cDNAs prepared from the poly(A)+ RNA fraction of rat submaxillary gland using forward and reverse primers tagged with extra 5′ BamHI (5′-CGTCCTGGATCCAACAGTAACACAGGATGCCCGCCGTCG-3′) and EcoRI (5′-CGACCGGAATTCCTAAGTCTCGGTGCTGACATCGTTCTCC-3′) sequences, respectively, and digested with BamHI and EcoRI. A human bFGF cDNA encoding an 18-kDa form (11) was prepared in the same way by using first-strand cDNAs prepared from a human osteosarcoma cell line (OST-1-PF) (12), and forward and reverse primers tagged with extra 5′ BglII (5′-CGTCCTAGATCTATGGCAGCCGGGAGCATCACCACGCTG-3′) and EcoRI (5′-CGACCGGAATTCCGCTCTTAGCAGACATTGGAAGAAAAAG-3′) sequences, respectively. The amplified cDNA was digested with BglII and EcoRI. The cDNA fragments were inserted individually into the BamHI–EcoRI site of pCHC302, yielding the expression vectors for fusion proteins between GST and collagen-binding EGF (pCHC302-EGF) and collagen-binding bFGF (pCHC302-bFGF). The cDNA fragment of human bFGF also was inserted into the BamHI–EcoRI site of pGEX-4T-2 (Pharmacia), yielding the expression vector for the GST-human bFGF fusion protein (pGEX-bFGF).
Protein Purification.
E. coli BL21 cells carrying pCHC302-EGF, pCHC302-bFGF, or pGEX-bFGF were grown in 2× YT medium supplemented with 10% (wt/vol) glucose and 100 μg/ml of ampicillin to an optical density at 600 nm of 1.0. The expression of fusion proteins was induced by the addition of 0.1 mM isopropyl-β-d-thiogalactopyranoside, and the culture was continued for 2 h at 30°C. The cell pellet obtained from 500-ml culture was suspended in 72 ml of 10 mM Tris⋅HCl (pH 7.5) containing 0.5 M NaCl and 1 mM phenylmethlysulfonyl fluoride, and then sonicated for 40 sec. The sonicate was supplemented with 1% (wt/vol) 3-[(3-cholamidopropyl)dimethylammonio]-1-propane sulfonate (CHAPS), and then stirred for 30 min at 4°C, followed by centrifugation. The resulting supernatant was subjected to affinity chromatography on a glutathione-Sepharose column (Pharmacia). The affinity-purified fusion proteins were digested with thrombin, and the released GST moiety was removed on a glutathione-Sepharose column. The CBEGF fraction was dialyzed against 20 mM Tris⋅HCl (pH 7.5) containing 0.03% CHAPS (TC buffer), and then subjected to ion-exchange chromatography on a Resource Q column (Pharmacia). The dialyzed sample was applied to a column equilibrated with TC buffer. The column was developed with a linear gradient of 0–0.2 M NaCl in TC buffer. CBEGF was eluted with 0.12 M NaCl. CBFGF and human bFGF were further purified by affinity chromatography on a heparin-Sepharose column (Pharmacia). The samples were applied to a column equilibrated with TC buffer containing 0.5 M NaCl. The column was developed with a linear gradient of 0.5–2.1 M NaCl in TC buffer. The fusion proteins were eluted with 1.7 M NaCl. Rat EGF was purified from submaxillary gland as described previously (13).
Assay of Collagen-Binding and Growth Factor Activities.
Collagen-binding activity was assayed as described elsewhere (5). Briefly, proteins dissolved in 50 μl of 50 mM Tris⋅HCl (pH 7.5) containing 5 mM CaCl2 were incubated at 25°C for 30 min in the absence or presence of 15 mg of insoluble type I collagen (Sigma) in a filter cup placed in a microfuge tube (Suprec-01, Takara, Kyoto), followed by centrifugation. The resulting filtrate containing unbound proteins was analyzed by SDS/PAGE.
Growth factor activity was assayed on BALB/c 3T3 A31 cells obtained from the Riken Gene Bank. The cells (2 × 104 cells in 2 ml of DMEM-2% calf serum) were inoculated onto 6-well multiwell plates, and 7 h later test samples were added. The cell number was determined with a Coulter counter after 4 days culture.
In Vivo Studies.
Rat EGF (10 μg), CBEGF (50 μg), human bFGF (20 μg), and CBFGF (50 μg) were dissolved in 75 μl of PBS and then injected subcutaneously into adult male BALB/c-nu mice. Six animals were used for each protein, and each animal was injected with the protein at four different sites (75 μl/site). After the period described in the text, the animals were killed by cervical dislocation (each group consisted of three mice). The animals received an i.p. injection of bromodeoxyuridine (BrdU, 10 mg/100 g body weight) 24 h before death.
Immunohistochemical Localization.
Skin tissue was fixed in 4% paraformaldehyde and embedded in paraffin. Immunolocalization was performed on 5-μm sections by means of the streptoavidin-biotin-alkaline phosphatase complex technique using a Histofine SAB-AP kit (Nichirei, Tokyo). Affinity-purified anti-rat EGF antibodies (14), anti-human bFGF antibodies (R & D Systems) and anti-BrdU mAbs (Progen, Heidelberg) were used as the primary antibodies. The paraffin sections used for the detection of BrdU were treated with Proteinase K (30 μg/ml) for 45 min at 37°C before incubation with the primary antibodies.
RESULTS
Preparation of Collagen-Binding Growth Factors.
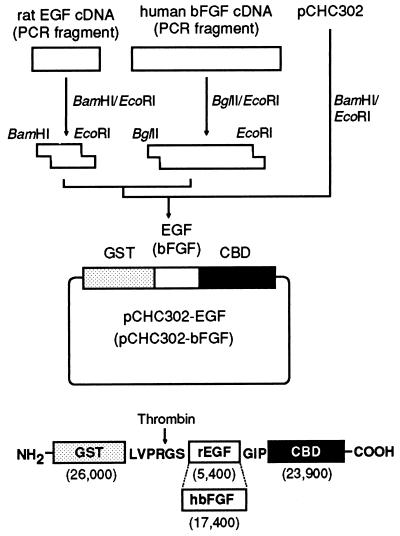
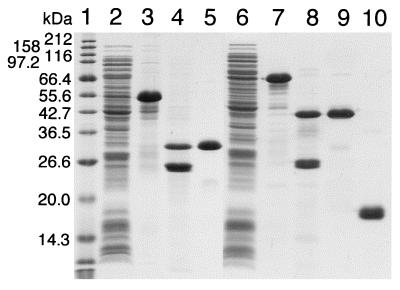
The structures of the expression plasmids of CBEGF and CBFGF as fusions with GST, and the translation products of the plasmids are shown in Fig. 1. E. coli BL21 cells carrying the plasmids, pCHC302-EGF and pCHC302-bFGF, produced a 55-kDa protein (GST-CBEGF) and a 68-kDa protein (GST-CBFGF), respectively (Fig. 2). The fusion proteins each were isolated from a cell extract by glutathione-Sepharose affinity chromatography, and then digested with thrombin. After removal of GST from the digest on a glutathione-Sepharose column, CBEGF and CBFGF were further purified by ion-exchange chromatography and heparin-Sepharose affinity chromatography, respectively (Fig. 2).
Figure 1.
Scheme for the construction of the expression plasmids and the structures of their translation products. cDNAs for rat EGF and human bFGF were amplified by PCR from first-strand cDNAs prepared from the poly(A)+ RNA fraction of rat submaxillary gland and a human osteosarcoma cell line, respectively, using primers tagged with extra 5′ restriction sites. Each amplified cDNA was digested with restriction enzymes and then inserted into the BamHI–EcoRI site of pCHC302, giving pCHC302-EGF and pCHC302-bFGF, which express fusion proteins between GST and collagen-binding EGF (GST-CBEGF) and collagen-binding bFGF (GST-CBFGF), respectively. In the structure of GST-CBEGF (GST-CBFGF), amino acid residues derived from the pGEX-4T-2 plasmid vector are given in the single-letter code, and the thrombin-cleavage site is indicated by an arrow. The numbers in parentheses are the molecular weights of the domains.
Figure 2.
Purification profiles of CBEGF and CBFGF on SDS/PAGE. Samples at each purification step for CBEGF (lanes 2–5) and CBFGF (lanes 6–9), and the final preparation of human recombinant bFGF (lane 10) were electrophoretically separated in a SDS/13% polyacrylamide gel under reducing conditions and then stained with Coomassie brilliant blue R-250. Lane 1, molecular weight markers; lane 2, E. coli BL21/pCHC302-EGF crude extract; lanes 3 and 7, eluate from glutathione-Sepharose; lanes 4 and 8, thrombin digest of the eluate from glutathione-Sepharose; lane 5, eluate from Resource Q (purified CBEGF); lane 6, E. coli BL21/pCHC302-bFGF crude extract; lane 9, eluate from heparin-Sepharose (purified CBFGF); lane 10, purified human recombinant bFGF.
Binding of CBEGF and CBFGF to an Insoluble Collagen Preparation.
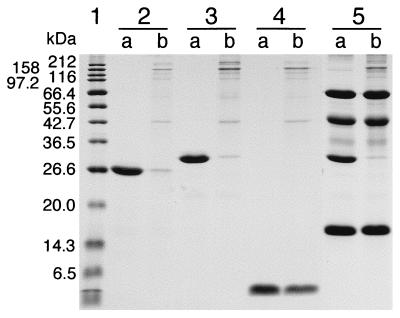
To examine its collagen-binding ability, CBEGF was incubated with insoluble type I collagen at room temperature for 30 min. The unbound fraction was obtained by centrifugation and analyzed by SDS/PAGE. Like CBD, CBEGF, but not rat EGF, bound to collagen almost completely (Fig. 3). The time course of binding and the binding capacity of collagen for CBEGF were indistinguishable from those for CBD described previously (5) (data not shown). Unlike CBEGF, control proteins (BSA, ovalbumin, and myoglobin) did not bind to collagen, nor did they inhibit the binding of CBEGF to collagen (Fig. 3). The binding of CBEGF was partly inhibited by 5 mM EDTA, as in the case of CBD, but not by 1 M NaCl or 1% Triton X-100 (data not shown). The collagen-binding ability of CBFGF was closely similar to that of CBEGF (data not shown).
Figure 3.
Collagen-binding of CBD and CBEGF. The proteins dissolved in 50 μl of 50 mM Tris⋅HCl (pH 7.5) containing 5 mM CaCl2 were incubated at 25°C for 30 min in the absence (a) or presence (b) of 15 mg of insoluble type I collagen in a Suprec-01 microcentrifugal device, followed by centrifugation. The resulting filtrate containing unbound proteins was analyzed by SDS/PAGE. Lane 1, molecular weight markers; lanes 2a and 2b, CBD (20 μg); lanes 3a and 3b, CBEGF (20 μg); lanes 4a and 4b, rat EGF (20 μg); lanes 5a and 5b; CBEGF (20 μg) + BSA (20 μg) + ovalbumin (20 μg) + myoglobin (20 μg).
Growth Factor Activity of CBEGF and CBFGF on Cultured Fibroblasts.
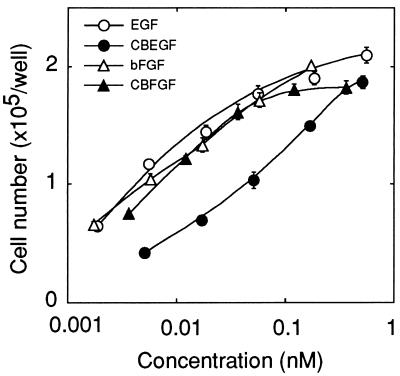
The growth-stimulating activity of the fusion proteins was examined on BALB/c 3T3 fibroblasts and compared with that of the parental growth factors. CBEGF, at a concentration of 0.51 nM (15 ng/ml), stimulated the growth of the fibroblasts about 6-fold over the control level, which was comparable with the maximum stimulation induced by rat EGF (Fig. 4). However, the half-maximum stimulation was attained with CBEGF and rat EGF at concentrations of about 78 pM (2.3 ng/ml) and about 7.0 pM (0.038 ng/ml), respectively, that is, the specific activity of CBEGF was about 1/10 that of rat EGF. The dose–response curves for CBFGF and human bFGF were similar to each other, indicating that the growth-stimulating activity of bFGF was almost fully retained by CBFGF (Fig. 4).
Figure 4.
Dose–response curves for the growth factor activity of rat EGF, CBEGF, human bFGF, and CBFGF. BALB/c 3T3 A31 cells (2 × 104 cells in 2 ml of DMEM-2% calf serum) were inoculated onto 6-well multiwell plates, and 7 h later test samples were added. The cell number was determined with a Coulter counter after 4 days culture. The cell numbers in the absence of test samples and in the presence of 10% calf serum were 29,100 ± 1,600 and 239,600 ± 9,900, respectively. Each point represents the mean value ± SEM for triplicate experiments.
Many kinds of collagen preparations and collagen-coated plasticware are now available for tissue culture experiments. We tried to determine whether or not the collagen-binding growth factors can exert their growth-promoting activity from the solid phase in vitro by using collagen goods. The fusion proteins, however, did not bind in a specific manner to any of the substrata tested, including collagen gel, collagen film, and collagen-coated tissue culture dishes.
Collagen-Binding and Growth-Promoting Activity of CBEGF and CBFGF in Vivo.
As it was not possible to examine the growth-promoting activity of the fusion proteins bound to collagen in vitro, we examined in vivo effects of the proteins by injecting them subcutaneously into nude mice. The DNA synthesis activity of cells in the vicinity of the injection sites was estimated as 24-h BrdU incorporation.
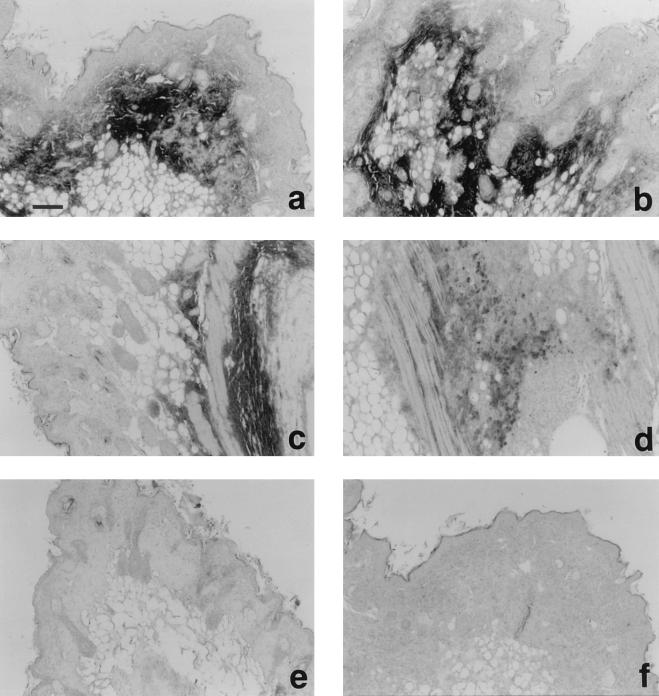
In most of the injection sites, CBEGF was detected in the subcutaneous tissue and the deeper part of the dermis with anti-rat EGF antibodies 5 days after injection (Fig. 5). EGF immunostaining was observed only in the extracellular space, and the stained area was rich in collagen, as revealed on Azan staining (data not shown). CBEGF was detectable even 10 days after injection at some injection sites (Fig. 5). Contrary to CBEGF, rat EGF decreased to an undetectable level as early as 24 h after injection in all of the specimens examined (Fig. 5). The incorporation of BrdU into stromal cell nuclei was hardly observed in either CBEGF-positive and rat EGF-treated, or control specimens. Some basal keratinocytes and hair follicle cells incorporated BrdU. But there was no indication of up-regulation of DNA synthesis in these cells caused by CBEGF (data not shown).
Figure 5.
Immunohistochemical staining of CBEGF and rat EGF injected subcutaneously into nude mice. Immunolocalization was performed on 5-μm paraffin sections by the streptoavidin-biotin-alkaline phosphatase complex technique. Affinity-purified anti-rat EGF antibodies (a-e) or preimmune rabbit serum (f) was used as the primary antibodies. (a, b and f) 5 days after injection of CBEGF (50 μg). (c and d) 10 days after injection of CBEGF. (e) 1 day after injection of rat EGF (10 μg). (Bar, 100 μm.)
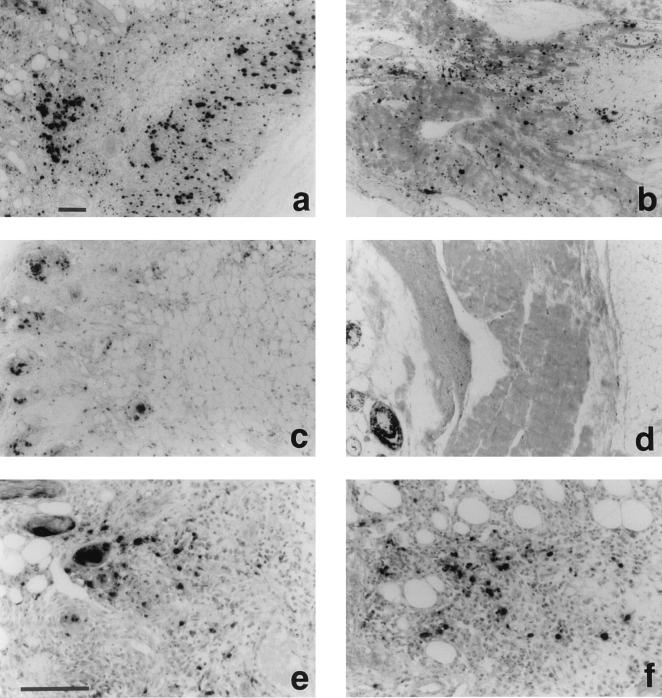
On the other hand, CBFGF administration resulted in a massive increase in BrdU incorporation into stromal cell nuclei at 5 days and 7 days after injection (Fig. 6). In bFGF-treated specimens, only hair follicle cells were labeled with BrdU considerably. bFGF showed negligible effect even at the highest dose examined (40 μg/injection site, data not shown). We next examined whether or not the localization of CBFGF coincided with that in BrdU-positive stromal cells by using serial sections prepared from two injection sites. Unexpectedly, only stromal cell nuclei were clearly stained with anti-human bFGF antibodies at both sites (Fig. 6). There were far fewer CBFGF (human bFGF)-positive cells than BrdU-positive ones, and they were located near the center of the BrdU-positive cell cluster.
Figure 6.
Detection of S-phase cells as BrdU incorporation and immunohistochemical staining of CBFGF in subcutaneous tissue of nude mice injected with CBFGF and human bFGF. The animals received an i.p. injection of BrdU (10 mg/100 g body weight) 24 h before death. Immunolocalization was performed on 5-μm paraffin sections by the streptoavidin-biotin-alkaline phosphatase complex technique using anti-BrdU mAbs (a-d) or anti-human bFGF antibodies (e and f) as the primary antibodies. (a, e, and f) 5 days after injection of CBFGF (50 μg). (b) 7 days after injection of CBFGF. (c and d) 5 days and 7 days, respectively, after injection of human bFGF (20 μg). (Bars, 100 μm.)
DISCUSSION
We have shown that fusion proteins consisting of growth factor moieties and CBD retained both growth factor activity and collagen-binding activity in vitro. Each of the activities was closely similar to that of the corresponding parental protein except for the low specific growth factor activity of CBEGF. The impaired growth factor activity of CBEGF may be caused by interference with the interaction between the EGF moiety and the EGF receptor by CBD and/or misfolding (formation of incorrect disulfide bonds) of the EGF moiety itself. In either case, it is not an inevitable consequence associated with the formation of the fusion protein but may be avoidable or correctable. The successful formation of a fusion protein consisting of CBD and bFGF, a relatively unstable growth factor, indicates that it is possible to produce collagen-binding fusion proteins with a variety of functional domains.
It is anticipated that the fusion proteins have the ability to exert a growth-promoting effect from a solid phase, that is, they can activate the corresponding receptor in a collagen-bound form. To examine this point, we carried out in vivo experiments, because the fusion proteins did not bind to collagen preparations ordinarily used in cell culture. When injected into subcutaneous tissue, CBEGF, but not rat EGF, remained at the injection sites for an extraordinarily long period. This result shows that CBEGF can bind to not only the insoluble collagen preparation used in the collagen-binding assay but also “living collagen,” and that the binding is fairly stable in vivo. CBD probably recognizes the native structure of the collagen triple helix or the higher-order structure of the molecule. In spite of this long-lasting nature of CBEGF in vivo, we did not obtain firm evidence of increased DNA synthesis activity at the injection sites in cells. The inability of CBEGF to induce DNA synthesis may be caused by its low specific growth factor activity. However, this is not probable because a considerable amount of CBEGF was detected at 5 days after administration, and in vitro experiments showed that the maximum growth factor activity of CBEGF was comparable with that of rat EGF. Another possibility is that the EGF moiety of collagen-bound CBEGF cannot interact properly with the EGF receptor because of the distance between most of the collagen fibers and the surface of cells embedded in ECM. If this is the case, the insertion of an appropriate linker peptide between EGF and CBD moieties may permit the interaction. Chemical linking of the two domains with a long flexible polymer like a poly(ethylene oxide) chain may be another possibility. The susceptibility to growth factors of cells at the injection sites also affects the results: Although EGF can stimulate the growth of a variety of cells, including fibroblastic cells like BALB/c 3T3, in vitro, it is possible that stromal cells of normal subcutaneous tissue lack sensitivity to EGF in vivo. The cellular responses to growth factors may partly explain the difference between CBEGF and CBFGF in the DNA synthesis stimulating effect in vivo. Unlike CBEGF, CBFGF induced DNA synthesis in numerous stromal cells at 5 days and 7 days after injection. As bFGF showed a negligible effect in this period, the long-standing action of CBFGF probably depends on the collagen-binding activity of the molecule. bFGF and other members of the FGF family are known to show high affinity for heparan sulfate and related molecules associated with the cell surface and ECM (15). The interaction of heparan sulfate with FGFs modulates their extracellular distribution, stability, and receptor binding. Because sulfated polysaccharides are thought to function as a reservoir for FGFs, the inability of exogenous bFGF to induce sustained DNA synthesis may result from the limited capacity of the molecules to retain bFGF and/or the lower stability of the binding between sulfated polysaccharides and bFGF when compared with that between collagen and CBFGF in vivo. It is possible that bFGF is capable of inducing DNA synthesis in stromal cells shortly after injection and the effect diminishes rapidly as the molecule disappears by diffusion. Immunohistochemical localization of CBFGF using anti-human bFGF, however, showed that immunostaining did not occur in the extracellular space but in the stromal cell nuclei (Fig. 6). The mitogenic signals of growth factors are transmitted to the cell nucleus via cell surface receptors and intracellular signal transduction systems. In addition to this authentic pathway, some growth factors, especially the members of the FGF family, have been shown to be localized and to act in the cell nucleus (16). Therefore, it is most probable that the bFGF moiety released from CBFGF is internalized and transported to the stromal cell nuclei. The low abundance of bFGF-positive cells compared with BrdU-positive ones may result from the low sensitivity of the antibodies used. Although the mechanism of the cleavage is obscure, we can consider that the CBFGF molecule functions like a time-release capsule for bFGF, and it may be possible to control the release by modulating the structure around the junction between bFGF and CBD moieties.
The unique characters of CBD and collagen-binding growth factors described in this study suggest that a novel drug delivery system, which makes proteineous agents nondiffusible and long-standing in vivo, can be constructed by using CBD as an anchoring unit as to collagen fibers. There are some limitations in the application of such a delivery system: Only those proteins that act in or from the extracellular space can be used as the functional domain. The method of administration may be restricted to direct injection into the affected tissue or to use as an ointment. However, such a delivery system may provide the additional option of the use of proteineous drugs.
Acknowledgments
This work was supported in part by a Grant-in-Aid from the Ministry of Education, Science, Sport, and Culture of Japan.
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: CBD, collagen-binding domain; EGF, epidermal growth factor; bFGF, basic fibroblast growth factor; CBEGF, collagen-binding EGF; CBFGF, collagen-binding bFGF; GST, glutathione S-transferase; BrdU, bromodeoxyuridine; ECM, extracellular matrix.
References
- 1.Kawase Y, Ohdate Y, Shimojo T, Taguchi Y, Kimizuka F, Kato I. FEBS Lett. 1992;298:126–128. doi: 10.1016/0014-5793(92)80037-h. [DOI] [PubMed] [Google Scholar]
- 2.Kuhl P R, Griffith-Cima L G. Nat Med. 1996;2:1022–1027. doi: 10.1038/nm0996-1022. [DOI] [PubMed] [Google Scholar]
- 3.Field S L, Khachigian L M, Sleigh M J, Yang G, Vandermark S E, Hogg P J, Chesterman C N. J Cell Physiol. 1996;168:322–332. doi: 10.1002/(SICI)1097-4652(199608)168:2<322::AID-JCP11>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 4.Yoshihara K, Matsushita O, Minami J, Okabe A. J Bacteriol. 1994;176:6489–6496. doi: 10.1128/jb.176.21.6489-6496.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matsushita O, Jung C-M, Minami J, Katayama S, Nishi N, Okabe A. J Biol Chem. 1998;273:3643–3648. doi: 10.1074/jbc.273.6.3643. [DOI] [PubMed] [Google Scholar]
- 6.Kroczkowski B, Reich M, Chen K, Bell G I, Cohen S. Mol Cell Biol. 1989;9:2771–2778. doi: 10.1128/mcb.9.7.2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brachmann R, Lindquist P B, Nagashima M, Kohr W, Lipari T, Napier M, Derynck R. Cell. 1989;56:691–700. doi: 10.1016/0092-8674(89)90591-6. [DOI] [PubMed] [Google Scholar]
- 8.Wong S T, Winchell L F, McCune B K, Earp H S, Teixidó J, Massagué J, Herman B, Lee D C. Cell. 1989;56:495–506. doi: 10.1016/0092-8674(89)90252-3. [DOI] [PubMed] [Google Scholar]
- 9.Higashiyama S, Iwamoto R, Goishi K, Taniguchi N, Klagsbrun M, Mekada E. J Cell Biol. 1995;128:929–938. doi: 10.1083/jcb.128.5.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nishi N, Shimizu C, Okutani T, Kagawa Y, Takasuga H, Suno M, Wada F. Biochim Biophys Acta. 1991;1095:268–275. doi: 10.1016/0167-4889(91)90110-j. [DOI] [PubMed] [Google Scholar]
- 11.Prats H, Kaghad M, Prats A C, Klagsbrun M, Lélias J M, Liauzun P, Chalon P, Tauber J P, Amalric F, Smith J A, Caput D. Proc Natl Acad Sci USA. 1989;86:1836–1840. doi: 10.1073/pnas.86.6.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamada K, Yoshitake Y, Norimatsu H, Nishikawa K. Cell Struct Funct. 1992;17:9–17. doi: 10.1247/csf.17.9. [DOI] [PubMed] [Google Scholar]
- 13.Nishi N, Matuo Y, Wada F. Prostate. 1988;13:209–220. doi: 10.1002/pros.2990130303. [DOI] [PubMed] [Google Scholar]
- 14.Nishi N, Inui M, Miyanaka H, Oya H, Wada F. Anal Biochem. 1995;227:401–402. doi: 10.1006/abio.1995.1302. [DOI] [PubMed] [Google Scholar]
- 15.Vlodavsky I, Miao H Q, Medalion B, Danagher P, Ron D. Cancer Metastasis Rev. 1996;15:177–186. doi: 10.1007/BF00437470. [DOI] [PubMed] [Google Scholar]
- 16.Levine J E, Prystowsky M B. Neuroimmunomodulation. 1995;2:290–298. doi: 10.1159/000097208. [DOI] [PubMed] [Google Scholar]