Abstract
Background
Participation in diabetic retinopathy screening is suboptimal. The Vision is Precious study (2001–2005) tested the hypothesis that a tailored telephone intervention in urban minority diabetes populations, offered in English or Spanish, would result in greater screening for retinopathy than a standard print intervention.
Design
Randomized controlled trial
Setting/participants
Subjects (N=598) were adults with diabetes without a dilated fundus examination (DFE) in >1 year from three healthcare centers in Bronx NY.
Intervention
A tailored telephone intervention to promote retinopathy screening compared to a standard print intervention over a 6-month period.
Main outcome measures
Documentation of a DFE within 6 months was the main outcome. Data on risk perceptions using the Risk Perception Survey for Diabetes were collected pre- and post-intervention. Electronic databases were used to obtain hemoglobin A1c information.
Results
Subjects were 40% men, mean age 57 years; 39% reported household incomes as <$15K; 45% reported their race as black, and 42% reported ethnicity as Hispanic/Latino; 23% chose Spanish as their preferred language. Data were analyzed in 2006. There was a 74% increase in retinopathy screening in the telephone versus print group (p<0.0005), with no differences by intervention language or by gender. Predictors of undergoing a DFE included: telephone intervention, baseline risk-perception scores indicating less worry and more realism about diabetes complications, and the interaction of self-reported worry and being in the telephone intervention. Subjects who had poor diabetes control responded with greater success to telephone interventions.
Conclusions
A limited telephone intervention can improve significantly participation in retinopathy screening in a minority, low-income population. This intervention influenced risk perceptions about diabetes complications. Further research is needed to develop effective risk communications to prevent the complications of diabetes.
Background
Diabetic retinopathy is the primary cause of new-onset blindness in adults aged 20–64 years.1 Primary and secondary preventive measures for visual problems related to diabetes include both diabetes management and early ophthalmic screening. Improvements in metabolic control decrease the risk of retinopathy in both type 12,3 and type 2 diabetes.4 Dilated fundus examinations (DFE) can detect retinal disease in the absence of symptoms and early enough for treatments to effectively prevent serious eye complications or blindness.5,6 However, meeting the standard of care for annual diabetic retinopathy screening continues to be a challenge, with only about two thirds of all individuals with diabetes reporting an annual DFE.7,8
Interventions to increase retinopathy screening in disadvantaged populations have produced some positive results in the face of many obstacles,9–11 but wide adoption of such programs has not yet been realized. This may be due to limited resources for the implementation of these programs, lack of prioritization of this ophthalmic screening standard of care by providers or patients, or cultural appropriateness of interventions for non–English-speaking populations. The purpose of the Vision is Precious (VIP) study was to evaluate the incremental effects of a tailored telephone intervention compared to a standard print intervention on obtaining retinopathy screening in low-income, minority individuals with diabetes. Secondary questions included: What are the differences in the effect of the intervention by gender and by preferred language (English or Spanish)? What are the associations between risk perceptions related to diabetes or the level of metabolic control with the primary outcome of having a DFE?
Methods
The VIP study was a randomized controlled behavioral intervention study from 2001 to 2005, whose participants were primarily residents of Bronx NY recruited by telephone through three healthcare centers. To be eligible for the study, subjects had to be aged >18 years, diagnosed with diabetes, able to speak and read (or be read to in) English or Spanish, capable of providing informed consent, have access to a telephone, and report not having had a DFE in the previous 12 months. The study received approval from the IRBs of the Albert Einstein College of Medicine and each participating health center.
Participants (N=598) were randomized to receive a limited, tailored telephone intervention over 6 months or a mailed print intervention. Using a Solomon 4-group design12 to assess the potential effects of the pre-intervention (PRE) survey on the study outcome, half of the participants were randomized to receive both a PRE and post-intervention (POST) survey and the other half to receive only a POST survey. The PRE and POST surveys were administered by a professional telephone survey group using computer-assisted telephone interview technology. Response data were electronically transferred to study investigators.
The Risk Perception Survey for Diabetes Mellitus (RPS-DM) includes a 31-item measure; it has been described in detail previously.13 The subscales composing the RPS-DM were developed based on factors that emerged in previous risk-perception research,14,15 and now were applied to diabetes. Table 1 provides a description of the RPS-DM with some examples of items. The complete survey and its scoring are available online at: http://www.aecom.yu.edu/diabetes/surveyinstruments.ae.
Table 1.
Description of the Risk Perception Survey–Diabetes Mellitus (RPS-DM)
| Scales | Number of items | Interpretation | Sample items with response patterns |
|---|---|---|---|
| Composite risk perception | 26 | Higher score indicates greater overall perceived risk related to diabetes and its complications. | Items from 5 subscales: personal control, worry, optimistic bias, comparative disease risk, environmental risk |
| Personal control | 4 | Higher score indicates more perceived control, less perceived risk. | “My own efforts can help control my risks of getting diabetes complications.”
Strongly agree, agree, disagree, strongly disagree |
| Worry | 2 | Higher score indicates more worry Regarding diabetes complications. | “I worry about getting diabetes complications.”
Strongly agree, agree, disagree, strongly disagree |
| Optimistic bias | 2 | Higher score indicates more optimistic bias; lower score is more realism/pessimism. | “Compared to other people with diabetes of my same age and sex, I am less likely than they are to get diabetes complications.”
Strongly agree, agree, disagree, strongly disagree |
| Comparative disease risk | 9 | Higher score indicates greater comparative disease risk of 9 conditions. | How would you rate your risk of: heart attack; blindness; cancer...? |
| Almost no risk, slight risk, moderate risk, high risk | |||
| Environmental risk | 9 | Higher score indicates greater perceived risk from 9 environmental conditions. | How would you rate your risk from: violent crime; extreme weather (hot or cold); air pollution…?
Almost no risk, slight risk, moderate risk, high risk |
| Risk knowledge | 5 | Higher score indicates greater knowledge of diabetes complications. | Having a yearly eye exam, does this…
Increase the risk, have no effect on the risk, or decrease the risk of diabetes complications? |
The primary outcome was documentation of a DFE within 6 months of randomization. To ascertain this outcome, subjects’ self-report of a DFE was verified through chart audit at the patient-identified eye-care practices in the community. The trained chart auditor was masked to the subjects’ group assignment. If a subject could not be reached or their DFE self-report could not be confirmed in medical records after intensive efforts to locate this record, the subject was classified as not having had a DFE. In addition, hemoglobin A1c (HbA1c) results, from a 1-year period encompassing the subjects’ 6-month intervention period, were collected from electronic databases.
Telephone Intervention
This tailored telephone intervention was delivered by bilingual interventionists trained in assessing the participant’s stage of change,16 basic problem-solving skills,17 and diabetes self-management education content.18 According to protocol, the focus of the phone call was to educate and motivate individuals about the importance of having an annual dilated eye exam and to elicit barriers to having this exam. Risk communications, such as the frequent lack of symptoms of retinopathy and that early treatment for retinopathy decreases the risk of blindness, were included. Interventionists used problem-solving approaches to empower patients to navigate through personal, motivational, and institutional barriers that prevented them from getting a DFE. Each telephone call was tailored to the individual’s readiness, choices, and barriers assessed and documented in previous phone calls. Participants were eligible to receive up to seven telephone calls within a 6-month period; when a subject reported that he/she had received a DFE, the telephone calls ceased. Participants in the print-intervention group received a mailing of a colorful, 14-page booklet on preventing diabetes eye problems called Keep Your Eyes Healthy, in English or Spanish, developed by the National Institutes of Health (available at www.niddk.nih.gov).
Analysis Plan
Statistical analyses were carried out in 2006, using Stata version 9.1. Comparability of the randomized groups was explored by examining cross-tabulations of demographic characteristics by treatment assignment. Cross-tabulation of primary outcome by telephone versus print group, stratified by PRE survey status, revealed no evidence that completing the PRE survey affected the probability of having a DFE examination, nor that completing the PRE survey modified the effect of the telephone intervention. Therefore, in subsequent analyses, the data were grouped exclusively by intervention-group assignment, ignoring assignment to PRE survey or not. The principal hypothesis that the telephone intervention would result in a higher probability of having DFE examinations was tested applying a χ2-test to a 2 × 2 contingency table.
Four facets of the relationships among the intervention, the DFE outcome, and the RPS-DM scale scores were examined. First, the pre-intervention scores in each group were compared to verify that the randomization had successfully balanced them. Second, the scale scores were examined to assess for changes in different ways over time in the intervention and control groups. These two analyses were accomplished using generalized estimating equations (GEE) to estimate linear models assuming exchangeable correlation. The scale scores were the response variables; study group, time (pre- or post-intervention), and their interaction were predictors. Study-group coefficients estimated the difference in pre-test scores between groups, and the interaction term coefficients estimated the difference between the groups of the pre- and post-test score differences.19 The third analysis examined whether changes in scores were predictive of DFE outcome within the intervention groups by using a logistic regression model with the DFE outcome as response variable, and the pre- and post-test changes in scale scores and treatment group as predictor variables. The fourth analysis examined whether the pre-intervention survey scores modified the effect of the intervention on DFE outcome. Logistic regression was used to estimate models with the DFE outcome as response variable, and the pre-intervention scale scores, treatment group, and their interaction as predictors. A statistically significant coefficient for the interaction term indicated the differential effectiveness of the intervention depending on the initial scale score.
To explore in-depth the impact of the telephone calls on obtaining a DFE within 6 months of randomization, a time-to-event analysis was carried out with “time” measured by the number of calls successfully placed by the interventionist. Only subjects randomized to the telephone intervention were included in this analysis. The hazard function of the likelihood of obtaining a DFE following each telephone call was estimated.
Results
The results of this behavioral intervention study included: the flow of subjects through this study to the analysis of data, demographic characteristics of the sample, the primary outcome results, association of risk perceptions and other variables at pre- and post-intervention with the primary outcome, and the impact of each telephone call on obtaining a DFE.
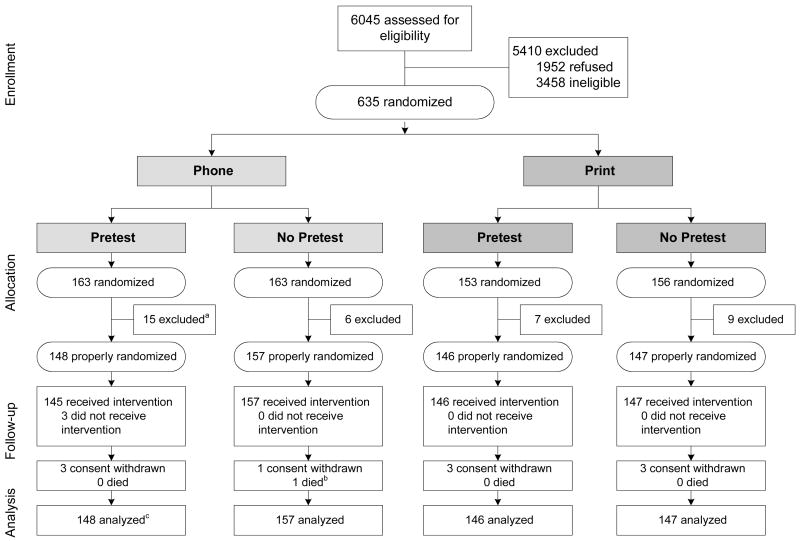
Figure 1 portrays the flow of participants from the available pool of subjects for screening through study analyses. Of the 10,273 names of adults with diabetes from three healthcare systems in the Bronx, a total of 6045 were contacted and assessed for eligibility by telephone. Of those, 3458 individuals were ineligible, the most frequent reasons were: self-report of having had a DFE during the previous 12 months (60%); self-report of no diabetes (16%); and reported as having died (7%). There were 38 additional subjects (21 in the telephone group and 16 in the print group) who were excluded from the final analyses due to belated discovery of failure to meet an eligibility criterion. That occurred when documentation of a DFE less than 12 months before their randomization was found, which had not been reported during the eligibility assessment. Subjects who withdrew consent or died during the 6-month study timeframe were not excluded from the analysis; rather, their DFE status as of the time of withdrawal or death was used as their outcome result.
Figure 1. Flow of subjects through the “Vision is Precious” study, from eligibility assessment to analysis.
a All exclusions after randomization were due to belated discovery of failure to meet eligibility requirements, specifically these excluded subjects turned out to have had a dilated fundus exam (DFE) within the year preceding randomization, though they did not report this at the time of enrollment.
b One subject died within the 6-month follow-up period for the primary study outcome.
c Subjects who withdrew consent or died were not excluded from analysis; rather, the DFE status as of the time of withdrawal/death was used as their study outcome.
Selected Characteristicsacteristics of the Study Sample
These 598 subjects were 60.5% women with a mean age of 56.6 (SD=12.5) years. Forty-five percent reported their race as black and only 17% white; another 27% reported their race as unknown or refused to report it, partly because there was no option to report Hispanic/Latino as a race, as opposed to an ethnic category. Hispanic/Latino ethnicity was reported by 42.5%, with 22.7% requesting Spanish as their preferred language for the study. The annual household income was reported as <$30,000 by 60.4% of the sample, with 38.5% reporting an income <$15,000. There were no significant differences between the two study groups on any characteristics.
In carrying out the telephone intervention, there were 930 completed telephone calls to the intervention group subjects, averaging 8.8 minutes. Subjects received, on average, 3.2 phone calls and spoke with a health educator for 28.1 minutes over the 6-month intervention. In order to accomplish this, it was necessary to attempt an additional 4147 calls that did not connect (e.g., busy signal, wrong number). A full accounting of the costs of the intervention is beyond the scope of this report and is in preparation separately.
The intention-to-treat analysis for the primary outcome of documented receipt of a DFE within 6 months of randomization is shown in Table 2 with the results of the Solomon 4-group analysis at the top of the table and the combined group results at the bottom. The relative risk (telephone group/print group) of having a DFE within 6 months was 1.73 among those randomized to PRE survey, and 1.74 among those not randomized to PRE survey; these were not significantly different (p=0.97). Therefore, having concluded that completing the PRE survey affected neither the overall probability of having a DFE, nor the effectiveness of the telephone intervention, the main results showed a 74% increase in the probability of screening for diabetic retinopathy in the telephone group compared to the print group (RR=1.74 [95% CI=1.31–2.30], χ2=15.63, p<0.0005} (see Table 2).
Table 2.
Primary outcome results by randomized group in Solomon 4-group design and total combined groups
| Dilated fundus exam within 6 months Number (%)
|
|||
|---|---|---|---|
| Study group | No | Yes | Total combined |
| Telephone/pretest | 99 (66.9) | 49 (33.1) | 148 (100) |
| Telephone/no pretest | 103 (65.6) | 54 (34.4) | 157 (100) |
| Print/pretest | 118 (80.9) | 28 (19.2) | 146 (100) |
| Print/no pretest | 118 (80.3) | 29 (19.7) | 147 (100) |
| Total | 438 (73.2) | 160 (26.8) | 598 (100) |
| Combined groupsa | |||
| Telephone | 202 (66.2) | 103 (33.8) | 305 (100) |
| 236 (80.6) | 57 (19.5) | 293 (100) | |
RR=1.74, χ2=15.63, p<0.0005
There were no significant differences in the effect of the intervention by ethnicity (Hispanic, non-Hispanic, and not reported), by language (English or Spanish), or by healthcare site.
Four aspects of the relationships among RPS-DM scale scores, intervention, and study outcome were examined among those 226 subjects who were randomized to the PRE and POST survey group and completed these surveys. Of those randomized to both PRE and POST surveys, 75% completed both. Table 3 shows that each RPS-DM scale had similar pre-intervention distributions in the telephone and print groups, with no statistically significant differences. The composite risk, comparative disease risk, environmental risk, and worry scales all exhibited statistically significant (p<0.05) changes from pre- to post-intervention in the telephone group. In the print group, worry and risk-knowledge scores changed significantly during the course of the study. The difference between telephone and print groups in the amount of change over time in a scale score was significant at the 0.05 level for the composite risk, personal disease risk, environmental risk, and risk-knowledge scales. The change in worry scores from pre- to post-intervention was essentially the same in both study groups.
Table 3.
Risk perception scale scores at pre- and post-intervention by study group
| Risk perception scales | Study group and time Mean score over standard deviation
|
|||
|---|---|---|---|---|
| Telephone | ||||
| Pre | Post | Pre | Post | |
| Composite risk | 2.47 | 2.64** | 2.41 | 2.40 |
| 0.47 | 0.48 | 0.47 | 0.51 | |
| Comparative disease risk | 2.86 | 3.03** | 2.68 | 2.63 |
| 0.80 | 0.79 | 0.86 | 0.93 | |
| Environmental risk | 2.15 | 2.47** | 2.12 | 2.23 |
| 0.74 | 0.79 | 0.71 | 0.78 | |
| Optimistic bias | 2.35 | 2.43 | 2.40 | 2.39 |
| 0.57 | 0.62 | 0.63 | 0.50 | |
| Personal control | 2.93 | 2.88 | 2.95 | 3.00 |
| 0.50 | 0.40 | 0.46 | 0.41 | |
| Worry | 3.10 | 3.00** | 3.07 | 2.93** |
| 0.55 | 0.54 | 0.58 | 0.61 | |
| Risk knowledge | 3.65 | 3.72 | 3.84 | 3.48** |
| 1.43 | 1.53 | 1.23 | 1.60 | |
p≤0.05;
p≤0.01
There were no associations between the change in any of the scale scores and the probability of getting a DFE. The inclusion of these variables in a model did not alter materially the estimate of the treatment effect on DFE outcome. The same conclusions hold if the model is further adjusted for demographic variables.
The only RPS-DM scale whose baseline value mediated the effect of the telephone intervention was the worry scale. The model estimates of the OR for having a DFE within 6 months of randomization (telephone/print) was 3.47 (95% CI=1.78–6.77) for subjects with initial worry score at the group mean of 3.08, whereas it was 1.04 (95% CI=0.31–3.48) for subjects whose pre-intervention worry score was one unit lower (2.08). Thus the telephone intervention was more effective with subjects whose initial level of worry about diabetes was higher. The pre-intervention optimistic bias score, itself, independently predicted DFE outcome, with each unit increase in the score being associated with reducing the odds of obtaining a DFE by a factor of 0.40 (95% CI=0.23–0.73). However, the effectiveness of the telephone intervention was not altered by the optimistic bias score. These results also remain essentially unchanged if adjusted for demographics.
The association of the HbA1c value with the primary study outcome was studied from the perspective of level of metabolic control. Those who had an HbA1c value available during the 12-month timeframe (49.5% [n=145] of the print group and 50.8% [n=155] in the telephone group [p=0.75]) were classified as poorly controlled (HbA1c >9%, n=106), moderately controlled (7%–9%, n=107), or well-controlled (≤7%, n=87). Among those with poor glycemic control, the probability of DFE within 6 months of randomization was only 16.4% in the print group, but 43.1% in the telephone group. For those with moderate glycemic control, the corresponding probabilities were 31.4% and 41.1%, respectively; and for those with good control, 28.2% and 41.7%. The relative risks for having a DFE in the poor, moderate, and good glycemic control groups did not differ.
The effectiveness of each telephone call was estimated by conceptualizing the number of calls as a “time” variable, the occurrence of a DFE as a “death,” and carrying out a “survival” analysis. Subjects who never obtained a DFE were analyzed as requiring an indeterminate, possibly infinite, number of calls exceeding the number actually received. Before the first call, 3.1% (95% CI=1.1–5.1) of subjects obtained a DFE. The initial call propelled 10.4% (95% CI=6.4–14.4) into action. After the fourth, apparently most effective call, 18.1% (95% CI=9.8–26.4) of those who had not yet obtained a DFE did so. Only 9.1% (95% CI=1.1–17.1) did following the fifth. There was then a sharp decline, with the sixth and seventh calls being ineffective.
Conclusion
A limited telephone intervention delivered to a low-income, urban, minority population can significantly improve participation in diabetic retinopathy screening. A sample of 598 adults with diabetes was recruited to evaluate a tailored telephone intervention compared to a standard print intervention in both Spanish and English. These subjects are similar in their initial eligibility response to the almost 30% of respondents to the Behavioral Risk Factor Surveillance System survey for 2001 who reported that they had not had a dilated eye exam within the last year.7 Self-report generally leads to an overestimation of eye exams, not underestimation.20,21 Of those initially randomized into our study, only 6% were later found to be ineligible and were excluded, because a report of an eye examination was found within that 1-year, pre-randomization timeframe.
In order to evaluate a reasonable intervention, the telephone intervention was limited to a maximum of seven phone calls in the 6-month window based on our previous research.9 Table 2 shows the success of the telephone intervention, with a 74% increase in the probability of screening compared to the print intervention. On the one hand, the proportions of subjects who had a documented DFE during that 6-month period seem low (33.8% in the telephone group and 18.5% in the print group). On the other hand, the characteristics of this sample portray disadvantaged individuals who had not had the routine behavior of a DFE in the previous year. The fact that the interventions were as effective in English as in Spanish is a credit to the talented bilingual interventionists who tailored the intervention to the individual, while adhering to the study protocol.
Several of the Risk Perception Survey scores changed significantly over time. The post-intervention composite risk perception score and subscale scores for comparative disease risk and environmental risk were significantly increased in the telephone group only; these may have been influenced by the tailored health information. The worry subscale score decreased significantly in both groups, perhaps an artifact of being in a research study and receiving more than usual care. Logistic regression results demonstrate the strong effect of the intervention per se. It also shows that those with less optimistic bias (i.e., more realism or pessimism) about getting diabetes complications and those with less worry than others about getting diabetes complications are more likely to have a DFE within the 6-month window. The telephone intervention’s effectiveness is not modified by the optimistic bias score, but was stronger in those with higher worry scores, as seen with the significant interaction of the worry score and the telephone intervention. This may indicate that some worry helps individuals to focus on problems and, with the tailored intervention, makes them better equipped to solve problems related to getting a DFE.
The important clinical implication of the VIP study is that telephone calls by bilingual health educators can improve diabetic retinopathy screening by 74%, thereby reducing the risk of eye complications in a poor urban population. The intervention followed a protocol of talking points to educate individuals about risk of eye complications, and elicit and problem-solve barriers related to obtaining a DFE. Risk perceptions related to diabetes complications, such as retinopathy, were influenced by the intervention, and it was equally successful in English and Spanish and in both men and women. Future research is needed to determine if these findings generalize to other low-income populations.
Acknowledgments
This research was supported by National Institutes of Health grant EY13497 and partially by DK 20541, and the Rockefeller Foundation. We thank Drs. Steven Martin, Michael Camardi, and David Bernard for assistance with recruitment and Dr. Ronald Klein for consultation. We appreciate our talented health educators and staff: E. Blanco, M. Kalten, Dr. J. Usher, G. Mojica, M. Mera, T. DeWitt, and T. Johnson for the success of this study. Northwest Survey and Data Services of Eugene OR performed the telephone surveys for this study.
Footnotes
No financial disclosures were reported by the authors of this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Frank RN. Diabetic retinopathy. N Engl J Med. 2004;350:48–58. doi: 10.1056/NEJMra021678. [DOI] [PubMed] [Google Scholar]
- 2.The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;320:977–86. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 3.Diabetes Control and Complications Trial/Epidemiology of Diabetes Intervention and Complications Research Group. Effect of intensive therapy on the microvascular complications of type 1 diabetes mellitus. JAMA. 2002;287:2653–9. [Google Scholar]
- 4.UK prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 Diabetes (UKPDS) Lancet. 1998;352:837–53. [PubMed] [Google Scholar]
- 5.Fong DS, Aiello L, Gardner TW, et al. Retinopathy in diabetes. Diabetes Care. 2004;27(Suppl 1):S84–7. doi: 10.2337/diacare.27.2007.s84. [DOI] [PubMed] [Google Scholar]
- 6.American Diabetes Association. Standards of medical care in diabetes: 2007. Diabetes Care. 2007;30:S4–41. doi: 10.2337/dc07-S004. [DOI] [PubMed] [Google Scholar]
- 7.Okoro CA, Mokdad AH, Ford ES, Bowman BA, Vinicor F, Giles WH. Are persons with diabetes practicing healthier behaviors in the year 2001? Results from the Behavioral Risk Factor Surveillance System. Prev Med. 2004;38:203–8. doi: 10.1016/j.ypmed.2003.09.039. [DOI] [PubMed] [Google Scholar]
- 8.Saadine JB, Engelgau MM, Beckles GL, Gregg EW, Thompson TJ, Venkat Narayan KM. A diabetes report card for the United States: Quality of care in the 1990s. Ann Int Med. 2002;136:565–74. doi: 10.7326/0003-4819-136-8-200204160-00005. [DOI] [PubMed] [Google Scholar]
- 9.Basch CE, Walker EA, Howard CJ, Shamoon H, Zybert P. The effect of health education on the rate of ophthalmic examinations among African Americans with diabetes mellitus. Am J Public Health. 1999;89:1878–82. doi: 10.2105/ajph.89.12.1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anderson RM, Musch DC, Nwankwo RB, et al. Personalized follow-up increases return rate at urban eye disease screening clinics for African Americans with diabetes: results of a randomized trial. Ethn Dis. 2003;13:40–46. [PubMed] [Google Scholar]
- 11.Pettitt DJ, Wollitzer AO, Javonovic L, He G, Ipp E the California Medi-Cal Type 2 Diabetes Study Group. Decreasing the risk of diabetic retinopathy in a study of case management. Diabetes Care. 2005;28:2819–22. doi: 10.2337/diacare.28.12.2819. [DOI] [PubMed] [Google Scholar]
- 12.Campbell DT, Stanley JC. Experimental and quasi-experimental designs for research. Boston: Houghton Mifflin; 1963. pp. 24–5. [Google Scholar]
- 13.Walker EA, Caban A, Schechter CB, et al. Measuring comparative risk perceptions in an urban minority population: The Risk Perception Survey for Diabetes. Diabetes Educ. 2007;33:103–10. doi: 10.1177/0145721706298198. [DOI] [PubMed] [Google Scholar]
- 14.Slovic P. Perception of risk. Science. 1987;236:280–85. doi: 10.1126/science.3563507. [DOI] [PubMed] [Google Scholar]
- 15.Weinstein ND. Optimistic biases about personal risks. Science. 1989;246:1232–3. doi: 10.1126/science.2686031. [DOI] [PubMed] [Google Scholar]
- 16.Ruggiero L. Helping people with behavior change: from theory to practice. Diabetes Spectrum. 2000;13:125–32. [Google Scholar]
- 17.Hill-Briggs F. Problem-solving in diabetes self management: A model of chronic illness self-management behavior. Ann Behav Med. 2003;25:182–93. doi: 10.1207/S15324796ABM2503_04. [DOI] [PubMed] [Google Scholar]
- 18.Mensing C, editor. The art and science of diabetes self-management education. Chicago: American Association of Diabetes Educators; 2006. [Google Scholar]
- 19.Vittinghoff E, Glidden DV, Shiboski SC, McCullouch C. Regression methods in biostatistics. New York: Springer; 2005. pp. 98–109. [Google Scholar]
- 20.Fowles JB, Rosheim K, Fowler EJ, Craft C, Arrichiello L. The validity of self-reported diabetes quality of care measures. Int J Qual Health Care. 1999;11:407–12. doi: 10.1093/intqhc/11.5.407. [DOI] [PubMed] [Google Scholar]
- 21.Walker EA, Zybert PA, Basch CE. What is the sensitivity and specificity of self-report for retinopathy screening? Diabetes Care. 2002;25:933. doi: 10.2337/diacare.25.5.933. [DOI] [PubMed] [Google Scholar]
- 22.Weinstein ND. Exploring the link between risk perceptions and preventive health behaviors. In: Suls J, Wallston KA, editors. Social psychological foundations of health and illness. Malden MA: Blackwell; 2003. pp. 22–53. [Google Scholar]
- 23.Brewer NT, Weinstein ND, Cuite CL, Herrington JE. Risk perceptions and their relation to risk behavior. Ann Behav Med. 2004;27:125–30. doi: 10.1207/s15324796abm2702_7. [DOI] [PubMed] [Google Scholar]
- 24.Fisher EB, Walker EA, Bostrum A, Fischhoff B, Haire-Joshu D, Bennett Johnson S. Behavioral science research in the prevention of diabetes. Diabetes Care. 2002;25:599–606. doi: 10.2337/diacare.25.3.599. [DOI] [PubMed] [Google Scholar]