Abstract
Multidrug resistance mediated by the drug efflux protein, P-glycoprotein (P-gp), is one mechanism that tumor cells use to escape death induced by chemotherapeutic agents. However, the mechanism by which P-gp confers resistance to a large variety of structurally diverse molecules has remained elusive. In this study, classical multidrug resistant human CEM and K562 tumor cell lines expressing high levels of P-gp were less sensitive to multiple forms of caspase-dependent cell death, including that mediated by cytotoxic drugs and ligation of Fas. The DNA fragmentation and membrane damage inflicted by these stimuli were defined as caspase dependent by various soluble peptide fluoromethylketone caspase inhibitors. Inhibition of P-gp function by the anti-P-gp mAb MRK-16 or verapamil could reverse resistance to these forms of cell death. Inhibition of P-gp function also enhanced drug or Fas-mediated activation of caspase-3 in drug-resistant CEM cells. By contrast, caspase-independent cell death events in the same cells, including those mediated by pore-forming proteins or intact NK cells, were not affected by P-gp expression. These observations suggest that, in addition to effluxing drugs, P-gp may play a specific role in regulating some caspase-dependent apoptotic pathways.
Multidrug resistance (MDR) is a major obstacle to treating patients with cancer (1) and is often the result of overexpression of a 170- to 180-kDa plasma membrane glycoprotein known as P-glycoprotein (P-gp) (2–4). Drug transporter human P-gp is encoded by MDR1 and rodent P-gps by Mdr1a and Mdr1b (5). P-gps belong to the superfamily of ATP-binding cassette transporters and actively efflux a wide range of structurally diverse amphipathic drugs used to treat cancer (6, 7). Experiments designed to define the structure of P-gp suggest that there is no simple single drug-binding site or pore in P-gp (8). Amino acid substitutions in, or near, most of the transmembrane segments affect substrate specificity or transport efficiency, and ATP hydrolysis is required for transport (9, 10). How ATP hydrolysis is coupled to vectorial transport is not clear and remains a weak point of the proposed drug pore model for P-gp. More recently, following the revelation that a related mouse P-gp (MDR2) (≈75% identical in amino acid sequence to MDR1) was a phosphatidylcholine flippase (11, 12), a role for P-gp (MDR1) as a drug flippase or phospholipid translocator has been strengthened (13).
The major physiological role of P-gp in protecting vital cells against toxins has been postulated on the basis of P-gp expression in the apical membranes of gut epithelia, in the canicular membrane of liver cells, in kidney tubules, and at blood-tissue barriers (8). This function is illustrated elegantly in mice that lack one [Mdr1a (−/−) or Mdr1b(−/−)] or both drug-transporting P-gps, because these mice have profound defects in drug distribution (5, 14, 15). High P-gp expression also is found in hematopoietic pluripotent stem cells and specific lymphocyte lineages, including NK cells and mature single positive thymocytes (16–18). Significantly, P-gp is expressed in developing organs of the early fetus where the ordered process of cell differentiation and death is necessary for correct organogenesis. Although many additional functions for P-gp have been proposed based on expression in these lineages, to date immunological or developmental defects in mice deficient in both P-gp have not been reported (15).
Given that P-gp can confer resistance to a wide range of insults, including complement-mediated cytotoxicity (19), we have considered the possibility that, in addition to drug transport function, P-gp function may generally inhibit cell death. The majority of physiological cell death pathways appear to involve a family of cysteine aspases known as caspases (20). Herein, we used drug-resistant tumor cell lines to examine the relationship between P-gp function and cellular sensitivity to various forms of apoptosis. Overall, our data support the idea that P-gp may function to specifically inhibit caspase-dependent tumor cell apoptosis.
MATERIALS AND METHODS
Cell Culture.
The acute T cell leukemia cell line, CEM-CCRF, its doxorubicin (DOX)-selected and resistant P-gphigh derived line CEM-A7+, and various hybrids of CCRF and A7+ expressing high (IC10, 2G10) or very low (2H6 and 4G9) levels of P-gp have been previously described (21). Parental erythroblastoid leukemia K562 and K562 resistant to vincristine (VIN)-mediated death (KVIN2000) were a gift from Greg Woods (University of Tasmania, Hobart, Australia). All cells were grown in RPMI medium 1640 supplemented with 10% (vol/vol) fetal calf serum, 2 mM glutamine, 100 units/ml of penicillin, and 100 μg/ml of streptomycin (GIBCO).
Cytotoxicity Assays.
Effector cells and various soluble death stimuli were assessed by 51Cr release (22), [125] iododeoxyuridine (125IUdR) release (23), or terminal deoxynucleotidyltransferase-mediated UTP end labeling (TUNEL) assay (2 h) (24) assays as described. DOX and VIN were obtained from Phillip Kantharidis, Peter MacCallum Cancer Institute, East Melbourne, Australia. CH-11 (anti-human Fas IgM, Upstate Biotechnology, Lake Placid, NY) was purchased. Pneumolysin was a gift of James Paton, University of Adelaide, Adelaide, Australia. Rat perforin (pfp) and human granzyme B (gB) were purified as previously described (23, 25) and were used in combination as described (24). The cytotoxicity of effector cells was assessed at three effector/target ratios in a 4-h cytotoxicity assay. The spontaneous release of 51Cr or 125IUdR was determined by incubating the target cells with medium alone [or in the presence of anti-P-gp mAb, verapamil, or caspase fluoromethylketone (fmk) inhibitor where applicable]. It should be noted at the concentrations used, inhibitors alone did not cause release nor did they affect the long-term survival of cell lines. The maximum release was determined by adding SDS to a final concentration of 5%. The percent specific lysis was calculated as follows: 100 × [(experimental release − spontaneous release)/(maximum release − spontaneous release)]. To inhibit P-gp function, the labeled targets were preincubated for 30 min with MRK-16 (IgG2a mAb, final 1–100 μg/ml) (Kamiya Biochemical, Thousand Oaks, CA) or UIC2 (IgG2a mAb, final 0.1–5 μg/ml) (Coulter), isotype control W6/32 (IgG2a) mAb, or verapamil (0.5–10 μM) (Knoll Australia, Lane Cove, Australia) before the cytotoxicity assay. To inhibit caspase activity, labeled target cells were preincubated for an additional 30 min with peptidyl fmks (ZFA-fmk, BD-fmk, ZVAD-fmk, ZDEVD-fmk, and ZYVAD-fmk) (Enzyme System Products, Dublin, CA) (final 0–50 μM). Target cells were added to wells and then coincubated for the appropriate period with effector cells or increasing concentrations of other death stimuli.
P-gp and Fas Detection.
CEM and K562 subclones were washed and incubated with saturating concentrations of mAb for 1 h on ice. Antibodies used were MRK-16 (0.1 μg/ml), CH-11, and appropriate isotype control mAbs. Bound mAb was detected by using fluorescein isothiocyanate-conjugated goat anti-mouse Ig (Silenus Laboratories, Hawthorn, Australia) for 1 h on ice, washed, and fixed in 2% paraformaldehyde/PBS. Cells were analyzed by using a FACScan flow cytometer (Becton Dickenson).
Anti-Caspase-3 Immunoblotting.
Cells (2 × 105) were lysed in 50 μl of ice-cold Nonidet P-40 lysis buffer (25 mM Hepes, pH7/250 mM NaCl/2.5 mM EDTA/0.1% Nonidet P-40/0.5 mM DTT/2 mM phenylmethylsulfonyl) at 4°C for 30 min. Insoluble material was removed by centrifugation at 4°C. Proteins (≈25 μg) were separated on SDS-15% polyacrylamide gels and electroblotted onto nylon membranes. Blots were probed with anti-human caspase-3 mAb (Transduction Laboratories, Lexington, KY) and visualized by enhanced chemiluminescence.
Mice.
C57BL/6 mice were maintained and bred at the Austin Research Institute Biological Research Laboratories. Mouse interleukin 2 activated adherent-spleen NK cells were generated as previously described (26).
RESULTS
P-gp Confers Resistance to DOX-Mediated Caspase-Dependent DNA Fragmentation.
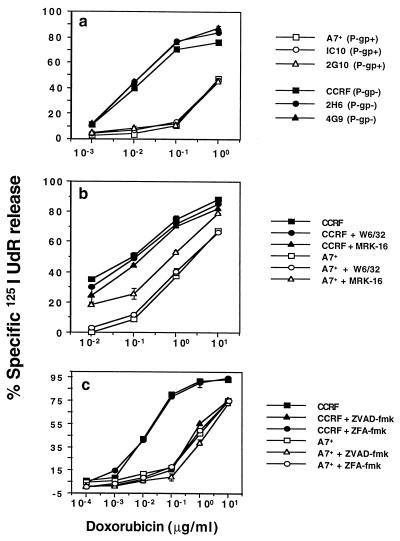
Multidrug-resistant and multidrug-sensitive CEM cell lines (21) were used to investigate a correlation between P-gp function and sensitivity to caspase-dependent and -independent apoptosis. CEM cell lines were phenotyped for P-gp (Table 1), and P-gphigh CEM cell lines were resistant to DOX-induced DNA fragmentation in comparison with P-gplow CEM cell lines (Fig. 1a). The anti-P-gp mAb, MRK-16, previously has been demonstrated to partially reverse DOX resistance by reducing drug efflux (19, 27). Increasing concentrations of the MRK-16 mAb (up to 100 μg/ml) reversed P-gphigh A7+ and IC10 cell line resistance to DOX (data not shown). An optimal concentration of MRK-16 mAb (50 μg/ml) was chosen for further experiments and by contrast, the same concentration of an isotype control mAb, W 6/32 that binds class I on CEM cell lines, did not reverse A7+ resistance to DOX (Fig. 1b).
Table 1.
P-gp and Fas expression as determined by flow cytometry
| Cell line | Mean fluorescence
|
|||
|---|---|---|---|---|
| P-gp | Fas | IgG control | IgM control | |
| CCRF | 7.5 | 38.8 | 5.4 | 3.9 |
| A7+ | 492.6 | 39.5 | 8.1 | 5.3 |
| 4G9 | 12.3 | 40.7 | 6.2 | 4.2 |
| 1C10 | 260.2 | 49.5 | 7.5 | 5.6 |
| 2H6 | 9.1 | 41.0 | 6.3 | 5.0 |
| 2G10 | 350.9 | 43.5 | 9.0 | 7.0 |
| K562 | 6.1 | 13.8 | 6.2 | 6.3 |
| KVIN | 166.7 | 18.0 | 7.7 | 11.7 |
CEM and K562 subclones were incubated with mAbs against P-gp (MRK-16, IgG), Fas (CH-11, IgM), or appropriate isotype control mAbs. Bound antibodies were analyzed by flow cytometry.
Figure 1.
P-gp confers resistance to DOX-mediated caspase-dependent DNA fragmentation. (a) CEM cell lines (P-gplow CCRF, 2H6 and 4G9, P-gphigh A7+, 2G10, and IC10) were labeled with 125IUdR for 1 h, washed in growth media, and incubated for 48 h in 96-well plates (2 × 104 cells/well) with DOX. (b) In some wells CCRF or A7+ cells also were preincubated with anti-P-gp mAb (MRK-16) or isotype control W6/32 mAb (50 μg/ml, final concentration) for 30 min, and DOX was added. Neither mAb caused more than 5% 125IUdR release above background in the absence of DOX. (c) In some wells CCRF or A7+ cells were preincubated for 30 min with 20 μM (final concentration) ZVAD-fmk or control ZFA-fmk inhibitor, and DOX was added. These data are calculated as the mean ± SE of triplicate samples and are representative of at least two different experiments. DOX concentrations (μg/ml) are shown on the x-axes of each part.
Soluble peptide inhibitors of caspases, including ZVAD-fmk [Cbz-Val-Ala-Asp(OMe)-fmk] (28) have been shown to block nuclear damage and cell lysis in intact cells (29, 30), including peripheral T cell blasts and T cell lines (28, 31). To determine the optimal caspase inhibitor concentration in CEM cell lines, ZVAD-fmk and a control ZFA-fmk inhibitor initially were titrated (1–50 μM) for activity in P-gplow CCRF target cells against a wide range of DOX concentrations (0.001–10 μg/ml). As little as 1 μM of ZVAD-fmk inhibited DOX-mediated 125IUdR release, with optimal inhibition of DOX activity in CCRF cells at 20 μM or greater (data not shown). ZFA-fmk did not block DOX-mediated apoptosis at all concentrations tested. DNA fragmentation mediated by low concentrations of DOX (<0.1 μg/ml) was almost completely inhibited in CCRF by ZVAD-fmk, but DNA fragmentation of A7+ was not greatly inhibited at any concentration of DOX (Fig. 1c). Previously, induction of CEM cell apoptosis by DOX has been found to be in the concentration range of 0.001 to 0.1 μg/ml, and higher concentrations were not associated with typical DNA fragmentation (32). Consistent with those observations, the highest concentrations of DOX (>0.1 μg/ml) were not completely blocked by ZVAD-fmk in CCRF cells (Fig. 1c), suggesting that at these concentrations, death mediated by DOX may be partially caspase independent.
The effect of caspase inhibitors on DOX-mediated cell lysis and DNA fragmentation were further investigated in the presence of MRK-16 mAb. The resistance of A7+ to DOX was reversed by MRK-16 (Fig. 2 a and b) mAb. Both the DNA fragmentation and membrane damage induced in the presence of MRK-16 mAb was significantly inhibited by ZVAD-fmk (Fig. 2 a and b). Similar data were obtained by using another P-gp specific mAb, UIC2, or verapamil, a pharmacological inhibitor of P-gp, to inhibit P-gp function (data not shown). Other P-gphigh and P-gplow CEM cell lines tested displayed an equivalent pattern of sensitivity to DOX, MRK-16 mAb, and caspase inhibition (data not shown).
Figure 2.
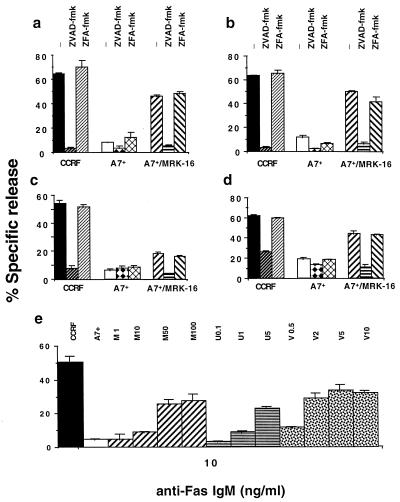
DOX- and Fas-mediated DNA fragmentation and membrane lysis is caspase dependent and reduced by P-gp function. CEM cell lines, CCRF (P-gplow) or A7+ (P-gphigh) were labeled with 51Cr (lysis; a and c) and 125IUdR (DNA fragmentation; b, d, and e) for 1 h, washed in growth media, and incubated for 16–48 h in 96-well plates (2 × 104 cells/well) with cell death stimuli at final concentrations as follows: (a and b) DOX (0.1 μg/ml, 48 h); (c and d) anti-human Fas IgM mAb, CH-11 (0.01 μg/ml, 16 h). (a–d) Some A7+ cells were preincubated for 30 min with anti-P-gp mAb (MRK-16) (50 μg/ml, final concentration) and/or followed by 20 μM (final concentration) ZVAD-fmk or control ZFA-fmk inhibitor for 30 min. Cell death stimuli then were added as indicated. (e) A7+ cells were preincubated with increasing concentrations of MRK-16 mAb (1–100 μg/ml, M1 to M100), UIC2 mAb (0.1–5 μg/ml, U0.1 to U5) or verapamil (0.5–10 μM, V0.5 to V10), then treated with 1 or 10 ng/ml anti-Fas mAb for 16 h. These data are calculated as the mean ± SE of duplicate samples and are representative of at least two different experiments.
P-gp Confers Resistance to Fas and Other Caspase-Dependent Apoptotic Stimuli.
In lymphoid cells the Fas/Fas ligand (FasL) system is a key regulator of apoptosis, and its primary function appears to be homeostatic regulation of T cells within the peripheral immune system (33–35). Cell death of T cell lines in response to Fas ligation appears to be caspase dependent (31), and several key caspases in this pathway have been defined (36). Despite expressing equivalent levels of Fas (Table 1), P-gphigh CEM cell lines were at least 20-fold more resistant to anti-Fas mAb than P-gplow cell lines over a concentration range of 1–1,000 ng/ml (10 ng/ml vs. CCRF or A7+ shown) (Fig. 2 c and d). Fas-mediated cell membrane damage and DNA fragmentation of sensitive CCRF was inhibited by caspase-specific ZVAD-fmk. Surprisingly, A7+ DNA fragmentation by anti-Fas mAb was significantly increased by MRK-16 mAb, and this DNA fragmentation was almost completely inhibited by ZVAD-fmk, but not ZFA-fmk (Fig. 2 c and d). A dose-response increase in Fas-mediated DNA fragmentation (10 ng/ml anti-Fas mAb, Fig. 2e) and membrane damage (data not shown) in A7+ cells was demonstrated with increasing concentrations of MRK-16 mAb, UIC2 mAb, or verapamil. Similar data were obtained at 1 ng/ml anti-Fas mAb (data not shown). P-gphigh cells were also less sensitive to whole effector cells (mediating death via FasL) or soluble recombinant FasL (data not shown); 10–20 times more resistant than P-gplow cell lines to vinblastine and etoposide (over a concentration range of 0.01–10 μg/ml); and five times more resistant to the glucocorticoid, dexamethasone (5–500 μg/ml). All of these drugs mediated DNA fragmentation via caspase activation (data not shown).
Caspase-Independent Forms of Membrane Lysis Unaffected by P-gp Overexpression.
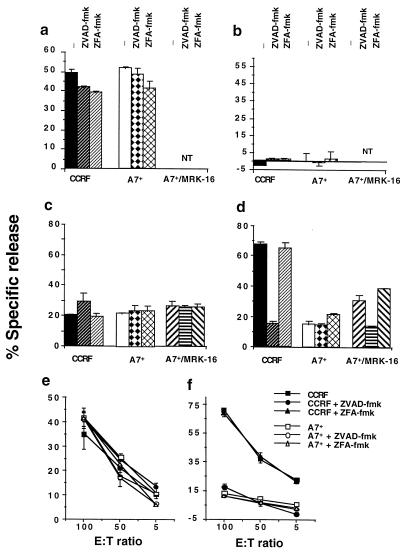
Pfp and serine protease, gB, are the major cytolytic granule proteins that act in synergy to deliver the lethal hit of cytolytic lymphocyte granule exocytosis (37). All CEM cell lines were equivalently lysed by pfp alone over a large concentration range (30–600 units/ml), and this lysis (300 units/ml shown) was not affected by caspase-specific ZVAD-fmk inhibitor (CCRF and A7+ shown, Fig. 3a). Consistent with previous reports (38), pfp alone did not cause target cell DNA fragmentation (Fig. 3b). Similar data was obtained when CEM cell lines were exposed to increasing concentrations of another pore-forming protein, pneumolysin (data not shown). A combination of a sublytic concentration of pfp (30 units/ml) and increasing concentrations of gB (0.5 μg/ml shown) did mediate target cell lysis and DNA fragmentation in CEM cell lines (Fig. 3 c and d). CCRF and A7+ were equivalently lysed by pfp and gB, and this lysis was not affected by caspase-specific ZVAD-fmk inhibitor (Fig. 3c). Only CCRF was sensitive to pfp and gB-mediated DNA fragmentation, and this fragmentation could be blocked by ZVAD-fmk (Fig. 3d). The resistance of A7+ cells could be partially reversed by the MRK-16 mAb and the resultant DNA fragmentation blocked by ZVAD-fmk. The relative resistance of several P-gphigh CEM cell lines also was supported by determining the percentage of apoptotic cells by terminal deoxynucleotidyltransferase-mediated UTP end labeling (TUNEL) staining (data not shown).
Figure 3.
Caspase-independent forms of membrane lysis unaffected by P-gp overexpression. CEM cell lines, CCRF (P-gplow) or A7+ (P-gphigh) were labeled with 51Cr (lysis; a, c, and e) and 125IUdR (DNA fragmentation; b, d, and f) for 1 h, washed in growth media, and incubated for 4 h in 96-well plates (2 × 104 cells/well) with cell death stimuli at final concentrations as follows: (a and b) pfp (300 units); (c and d) pfp (30 units) and gB (0.5 μg/ml); and (e and f) NK cells at effector/target ratios as shown. In some wells CCRF or A7+ cells were preincubated for 30 min with anti-P-gp mAb (MRK-16) (50 μg/ml, final concentration) and/or followed by 20 μM (final concentration) ZVAD-fmk or control ZFA-fmk inhibitor for 30 min. Cell death stimuli then were added as indicated for 4 h (NT = not tested). gB alone at these concentrations was without effect. These data are calculated as the mean ± SE of duplicate samples and are representative of at least two different experiments.
Mouse adherent-NK cells (A-NK) display pfp-dependent xenolysis of human target cells (26). A-NK cells induced significant and equivalent levels of lysis in CCRF and A7+ cell lines, and this lysis was not inhibited by ZVAD-fmk (Fig. 3e). Thus target cell susceptibility to NK-mediated cell lysis was not influenced by P-gp expression. In contrast, A-NK cell-mediated DNA fragmentation of CCRF was considerably greater than that in A7+ cells, and this DNA fragmentation was specifically inhibited by ZVAD-fmk (Fig. 3f). Thus DNA fragmentation mediated by A-NK cells was caspase dependent and was affected by target cell P-gp expression. Other P-gphigh and P-gplow CEM cell lines tested displayed an equivalent pattern of sensitivity to A-NK cells and caspase inhibition (data not shown).
P-gp Affects Caspase-3-Mediated DNA Fragmentation in CEM Cell Lines.
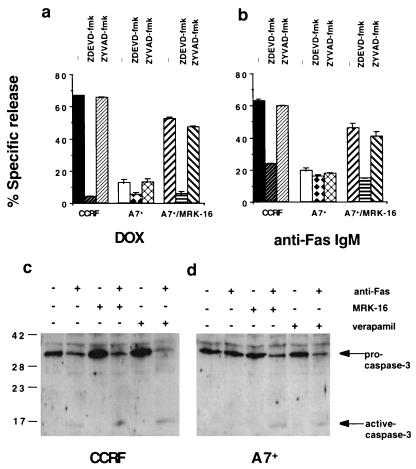
To more specifically evaluate those caspases that P-gp may regulate, caspase-1-like- (ZYVAD-fmk) and caspase-3-like- (ZDEVD-fmk) protease-specific inhibitors were used. Both DOX- and anti-Fas IgM-mediated DNA fragmentation in CCRF were inhibited by 20 μM ZDEVD-fmk, but not ZYVAD-fmk, suggesting that a caspase-3-like activity was required for DNA fragmentation (Fig. 4 a and b). The increased sensitivity of A7+ to DOX or anti-Fas IgM on MRK-16 mAb pretreatment also was significantly inhibited by ZDEVD-fmk (Fig. 4 a and b). The ZDEVD-fmk inhibitor similarly blocked membrane damage induced in these circumstances (data not shown). It should be noted that ZYVAD-fmk previously has been demonstrated to inhibit Fas-mediated apoptosis in CEM-C7 T cells, but only at concentrations greater than or equal to 500 μM (39).
Figure 4.
P-gp affects caspase-3-dependent DNA fragmentation in drug-resistant cell lines. CEM cell lines [CCRF (P-gplow) and A7+ (P-gphigh)] were labeled with 125IUdR for 1 h, washed in growth media, and incubated for 16–48 h in 96-well plates (2 × 104 cells/well) with cell death stimuli at final concentrations as follows: (a) DOX (0.1 μg/ml, 48 h); (b) anti-human Fas IgM mAb CH-11 (0.01 μg/ml, 16 h). In some wells cells were preincubated for 30 min with anti-P-gp mAb (MRK-16) (50 μg/ml, final concentration) and/or followed by 20 μM (final concentration) ZDEVD-fmk or ZYVAD-fmk inhibitor for 30 min. Cell death stimuli then were added as indicated for 16–48 h. These data are calculated as the mean ± SE of duplicate samples and are representative of at least two different experiments. Protein lysates from CCRF (c) or A7+ (d) cells treated with anti-Fas IgM (0.01 μg/ml, 4 h) in the presence or absence of MRK-16 (50 μg/ml, final concentration) or verapamil (5 μM) were separated by SDS/PAGE, and Western blots were performed by using an anti-caspase-3 mAb. The addition of anti-Fas IgM, MRK-16, and verapamil is indicated by the table above the blots, and the position of molecular weight markers in kDa is indicated on the left. The expression of pro-caspase-3 and an active cleaved product is indicated by arrows.
Activation of caspase-3 results in proteolytic cleavage of the ≈32-kDa pro-caspase-3 into active p17 and p12 polypeptides (40). Cell lysates from CCRF and A7+ cells treated with anti-Fas mAb for 4 h, with or without pretreatment with MRK-16 or verapamil, were tested by Western blot for caspase-3 activation. The anti-Fas IgM induced caspase-3 cleavage and activation in CCRF cells in the presence or absence of MRK-16 or verapamil as shown by the decrease in the levels of pro-caspase 3 and the appearance of a 17-kDa processed form (Fig. 4c). By contrast, caspase-3 activation in A7+ cells treated with anti-Fas mAb was observed only after pretreatment with MRK-16 or verapamil (Fig. 4d). Complete activation of pro-caspase 3 was observed in CCRF and A7+ cells treated with anti-Fas mAb for 24 h or DOX for 48 h in the presence or absence of MRK-16 or verapamil (data not shown).
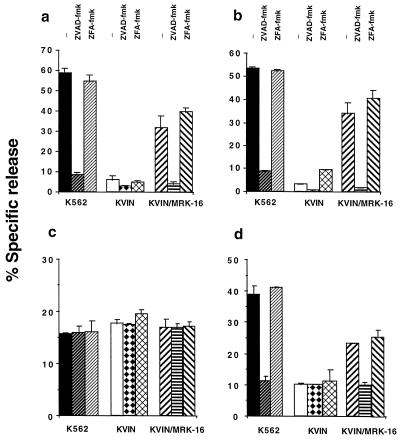
P-gp Inhibits DNA Fragmentation in a K562 Drug-Resistant Cell Line.
K562 (P-gplow, Table 1) and its VIN selected partner, K562VIN (P-gphigh), were used to demonstrate that P-gp function could inhibit caspase-dependent apoptosis in non-T cells. VIN-mediated membrane damage and DNA fragmentation was significantly inhibited by ZVAD-fmk (Fig. 5 a and b) and ZDEVD-fmk (data not shown) in K562 cells, but not K562VIN cells. Inhibition of P-gp function in K562VIN by MRK-16 mAb reversed resistance to VIN, and subsequent cell death was inhibited by ZVAD-fmk (Fig. 5 a and b) and ZDEVD-fmk (data not shown). A sublytic concentration of pfp (60 units/ml) and gB (0.5 μg/ml shown) mediated equivalent membrane lysis in K562 and K562VIN, and this lysis was not affected by caspase-specific fmk inhibitors (Fig. 5c). In contrast, K562 were comparatively sensitive to pfp and gB-mediated DNA fragmentation and this fragmentation could be blocked by ZVAD-fmk (Fig. 4f) or ZDEVD-fmk (data not shown). The resistance of K562VIN cells could be partially reversed by the MRK-16 mAb, and the subsequent DNA fragmentation was blocked by ZVAD-fmk. The data imply that in at least two different cell lineages caspase-mediated apoptosis is impinged on by functional P-gp, but caspase-independent cell membrane lysis is not affected.
Figure 5.
P-gp affects caspase-dependent DNA fragmentation in drug-resistant K562 cell lines. K562 cell lines [K562 (P-gplow) or KVIN (P-gphigh)] were labeled with 51Cr (a and c) and/or 125IUdR (b and d) for 1 h, washed in growth media, and incubated for 4–48 h in 96-well plates (2 × 104 cells/well) with cell death stimuli at final concentrations as follows: (a and b) VIN (0.1 μg/ml, 48 h) and (c and d) pfp (60 units) and gB (0.5 μg/ml, 4 h). In some wells cells were preincubated for 30 min with anti-P-gp mAb (MRK-16) (50 μg/ml, final concentration) and/or followed by 20 μM (final concentration) ZVAD-fmk or control ZFA-fmk inhibitor for 30 min. Cell death stimuli then were added as indicated for 4–48 h. These data are calculated as the mean ± SE of duplicate samples and are representative of at least two different experiments.
DISCUSSION
P-gp, responsible for MDR, has been demonstrated to protect human tumor cells from multiple forms of caspase-mediated apoptosis. CEM and K562 cell lines overexpressing P-gp were considerably less sensitive to a spectrum of cell death stimuli that mediate DNA fragmentation in non-P-gp expressing tumor cell lines via a caspase-dependent pathway. Importantly, in two different drug-selected cell lines (selected with different drugs), the resistance to several death stimuli could be reversed by inhibition of P-gp function by using two different P-gp-specific mAbs. In particular, drug-resistant cell lines were resistant to Fas-mediated apoptosis, and inhibition of P-gp function in drug-resistant cell lines restored sensitivity to anti-Fas mAb-induced cell death.
Currently, there is conflicting evidence regarding the relationship between drug resistance and resistance to Fas-mediated apoptosis. Some studies advocate a role for FasL in drug-mediated apoptosis (32) or coselection of drug and Fas resistance in CEM cells (41). Others clearly demonstrate that resistance to Fas-mediated apoptosis, which may be caused by defects in or absence of signaling proteins proximal to the Fas receptor, can be bypassed by cytotoxic drugs (42). Such studies have used cells that are resistant via non-P-gp mechanisms (41) or failed to evaluate the impact of P-gp activity on Fas-mediated apoptosis. Certainly, our P-gphigh cells expressed equivalent levels of surface Fas as determined by flow cytometry, and these cells were not routinely maintained in drug. Given that resistance to Fas-mediated apoptosis is reversed by inhibiting P-gp function, drug-resistant CEM cells do not appear to lack key upstream components of the Fas pathway; such as the adaptor protein FADD, or protease FLICE (caspase-8).
Many forms of apoptosis cause caspase-3 activation, resulting in autocatalysis and cleavage of other caspases and downstream substrates (43–46). Caspase-3 activation requires several key cytosolic components including: apoptotic protease activating factor-1 (Apaf-1), cytochrome c (Apaf-2), Apaf-3 (caspase-9), and dATP (43, 47). The sensitivity of most apoptotic programs to levels of cytosolic ATP, including Fas-induced apoptosis (48), raises the possibility that the level of cytosolic ATP, cytochrome c, or activated caspase-3 may regulate the initiation of apoptosis. Therefore, an alternate hypothesis might be that P-gp might be able to regulate the cytosolic levels of these proteins or upstream mediators (such as caspase-8 or FADD). Clearly the possibility of their extrusion by P-gp needs to be explored.
Importantly, the DNA of drug-resistant cells could be fragmented in a caspase-3-dependent manner when P-gp function was inhibited. Inhibition of P-gp function, with either the MRK-16 Ab or verapamil, resulted in cleavage and activation of pro-caspase-3 in drug-resistant cell lines on addition of an apoptotic stimuli. At present it is not known whether the effect of P-gp is on caspase-3 itself, or on upstream caspases such as caspase-1, -8, and/or -9. As specific inhibitors for individual caspases continue to be developed, their use will aid dissection of the caspases affected by P-gp function. Apoptotic mitochondrial damage also is blocked by caspase inhibitors (49), and hence evaluation of the effect of P-gp activity on mitochondrial events and clonogenic potential of MDR cells undergoing Fas-mediated apoptosis will be of interest. Other drugs that are not effluxed by P-gp, but that do require caspase activity for the execution of apoptosis, also may shed light on the specificity of the effect of P-gp on caspase pathways.
The ability of P-gp to specifically inhibit caspase-dependent apoptosis also was substantiated by the equivalent sensitivity of P-gphigh and P-gplow cell lines (in two different cell types) to several different forms of caspase-independent cell death. Aside from high concentrations of some chemotherapeutic drugs, membrane-disrupting agents (such as the pore-forming pneumolysin and pfp used herein) and CTL/NK cell granule exocytosis (31) are the only well-characterized forms of caspase-independent cell death. Importantly, whereas pore-formers trigger membrane disruption alone, lysis of cell lines caused by other death stimuli was caspase dependent and could be regulated by functional P-gp. Furthermore, we have shown that pfp in combination with grB (or intact NK cells) can lyse cells in a caspase-independent manner, but activate caspases leading to nuclear damage. Our data supports that of Sarin et al. (31), and thus granule exocytosis-mediated cell death has provided a unique form of cell death that well illustrates the differential effect of P-gp on events that are caspase dependent and caspase independent.
It is tempting to speculate that P-gp may have a dual physiological role in protecting cells from apoptosis. Clearly, P-gp can remove harmful toxins from the cell, thereby inhibiting their chemotoxic effect. The results described herein show that P-gp function also can influence caspase-3 activation, a necessary step for apoptosis induced by a variety of pharmaceutical and physiological apoptotic stimuli. Conceptually, these experiments might be better performed in cell lines that were not selected in chemotherapeutic drugs; however, cell lines transfected to express P-gp are routinely selected in drug, and only one pair of human nondrug-selected P-gp cell lines have been described previously (19). Importantly, in this study using drug-selected cell lines, resistance could be reversed by using specific anti-P-gp mAbs. The lowest concentrations at which apoptosis was induced by chemotherapeutic drugs in vitro are therapeutically relevant, and because P-gp is expressed on a variety of tumors in vivo, our data may have important implications for cancer therapy. Very few examples of stimuli that trigger caspase-independent cell death pathways currently exist, but clearly a greater understanding of these pathways may provide the key to better therapies for MDR tumors.
Acknowledgments
We thank Joseph Trapani, Geoff Pietersz, and Sarah Russell for their critical review of this manuscript and John Zalcberg and Phillip Kantharidis for reagents and helpful discussions. M.J.S. is currently supported by Wellcome Trust Australasian Senior Research Fellowship, and R.W.J. is supported by a National Health and Medical Research Council of Australia C. J. Martin Fellowship. The work was supported by Project Grants from the National Health and Medical Research Council of Australia.
ABBREVIATIONS
- A-NK
adherent natural killer
- DOX
doxorubicin
- fmk
fluoromethylketone
- gB
granzyme B
- 125IUdR
[125]iododeoxyuridine
- MDR
multidrug resistance
- pfp
perforin
- P-gp
P-glycoprotein
- VIN
vincristine
References
- 1.Gottesman M M, Pastan I. Annu Rev Biochem. 1993;62:385–427. doi: 10.1146/annurev.bi.62.070193.002125. [DOI] [PubMed] [Google Scholar]
- 2.Juliano R L, Ling V. Biochim Biophys Acta. 1976;455:152–162. doi: 10.1016/0005-2736(76)90160-7. [DOI] [PubMed] [Google Scholar]
- 3.Gerlach J H, Endicott J A, Juranka P F, Henderson G, Sarangi F, Deuchars K L, Ling V. Nature (London) 1986;324:485–489. doi: 10.1038/324485a0. [DOI] [PubMed] [Google Scholar]
- 4.Ueda K, Cardarelli C, Gottesman M M, Pastan I. Proc Natl Acad Sci USA. 1987;84:3004–3008. doi: 10.1073/pnas.84.9.3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borst P, Schinkel A H. Trends Genet. 1997;13:217–222. doi: 10.1016/S0168-9525(97)01112-8. [DOI] [PubMed] [Google Scholar]
- 6.Higgins C F. Annu Rev Cell Biol. 1992;8:67–113. doi: 10.1146/annurev.cb.08.110192.000435. [DOI] [PubMed] [Google Scholar]
- 7.Doige C A, Ferro-Luzzi Ames G. Annu Rev Microbiol. 1993;47:291–319. doi: 10.1146/annurev.mi.47.100193.001451. [DOI] [PubMed] [Google Scholar]
- 8.Germann U A. Eur J Cancer. 1996;32A:927–944. doi: 10.1016/0959-8049(96)00057-3. [DOI] [PubMed] [Google Scholar]
- 9.Senior A E, Al-Shawi M K, Urbatsch I L. FEBS Lett. 1995;377:285–289. doi: 10.1016/0014-5793(95)01345-8. [DOI] [PubMed] [Google Scholar]
- 10.Urbatsch I L, Sankaran B, Bhagat S, Senior A E. J Biol Chem. 1995;270:26956–26961. doi: 10.1074/jbc.270.45.26956. [DOI] [PubMed] [Google Scholar]
- 11.Smit J J M, Schinkel A H, Oude Elferink R P, Groen A K, Wagenaar E, van Deemter L, Mol C A, Ottenhoff R, van der Lugt N M, van Roon M A. Cell. 1993;75:451–462. doi: 10.1016/0092-8674(93)90380-9. [DOI] [PubMed] [Google Scholar]
- 12.Ruetz S, Gros P. Cell. 1994;77:1071–1083. doi: 10.1016/0092-8674(94)90446-4. [DOI] [PubMed] [Google Scholar]
- 13.Van Helvoort A, Smith A J, Sprong H, Fritzsche I, Schinkel A H, Borst P, van Meer G. Cell. 1996;87:507–517. doi: 10.1016/s0092-8674(00)81370-7. [DOI] [PubMed] [Google Scholar]
- 14.Schinkel A H, Smit J J, van Tellingen O, Beijnen J H, Wagenaar E, van Deemter L, Mol C A, van der Valk M A, Robanus-Maandag E C, te Riele H P, et al. Cell. 1994;77:491–502. doi: 10.1016/0092-8674(94)90212-7. [DOI] [PubMed] [Google Scholar]
- 15.Schinkel A H, Mayer U, Wagenaar E, Mol C A, van Deemter L, Smit J J, van der Valk M A, Voordouw A C, Spits H, van Tellingen O, et al. Proc Natl Acad Sci USA. 1997;94:4028–4033. doi: 10.1073/pnas.94.8.4028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Borst P, Schinkel A H. Eur J Cancer. 1996;32:985–990. doi: 10.1016/0959-8049(96)00063-9. [DOI] [PubMed] [Google Scholar]
- 17.Gupta S, Gollapudi S. J Clin Immunol. 1993;13:289–301. doi: 10.1007/BF00920237. [DOI] [PubMed] [Google Scholar]
- 18.MacDonald H R, Bommhardt U, Cerottini J C. Eur J Immunol. 1995;25:1457–1460. doi: 10.1002/eji.1830250549. [DOI] [PubMed] [Google Scholar]
- 19.Weisburg J H, Curcio M, Caron P C, Raghu G, Mechetner E B, Roepe P D, Scheinberg D A. J Exp Med. 1996;183:2699–2704. doi: 10.1084/jem.183.6.2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Henkart P A. Immunity. 1996;4:195–201. doi: 10.1016/s1074-7613(00)80428-8. [DOI] [PubMed] [Google Scholar]
- 21.Zalcberg J R, Hu X F, Wall D M, Mirski S, Cole S, Nadalin G, De Luise M, Parkin J D, Vrazas V, Campbell L, Kantharidis P. Int J Cancer. 1994;57:522–528. doi: 10.1002/ijc.2910570414. [DOI] [PubMed] [Google Scholar]
- 22.Rouvier E, Luciani M-F, Golstein P. J Exp Med. 1993;177:195–200. doi: 10.1084/jem.177.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shi L, Kraut R P, Aebersold R, Greenberg A H. J Exp Med. 1992;175:553–566. doi: 10.1084/jem.175.2.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sutton V R, Vaux D L, Trapani J A. J Immunol. 1997;158:5783–5790. [PubMed] [Google Scholar]
- 25.Trapani J A, Browne K A, Dawson M, Smyth M J. Biochem Biophys Res Commun. 1993;195:910–920. doi: 10.1006/bbrc.1993.2131. [DOI] [PubMed] [Google Scholar]
- 26.Smyth M J, Thia K Y T, Kershaw M H. Xenotransplantation. 1997;4:78–84. [Google Scholar]
- 27.Meyers M B, Rittman-Grauer L, O’Brien J P, Safa A R. Cancer Res. 1989;49:3209–3214. [PubMed] [Google Scholar]
- 28.Sarin A, Wu M-L, Henkart P A. J Exp Med. 1996;184:2445–2450. doi: 10.1084/jem.184.6.2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jacobson M D, Weil M, Raff M C. J Cell Biol. 1996;133:1041–1051. doi: 10.1083/jcb.133.5.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pronk G J, Ramer K, Amiri P, Williams L T. Science. 1996;271:808–810. doi: 10.1126/science.271.5250.808. [DOI] [PubMed] [Google Scholar]
- 31.Sarin A, Williams M S, Alexander-Miller M A, Berzofsky J A, Zacharchuk C M, Henkart P A. Immunity. 1997;6:209–215. doi: 10.1016/s1074-7613(00)80427-6. [DOI] [PubMed] [Google Scholar]
- 32.Friesen C, Herr I, Krammer P H, Debatin K-M. Nat Med. 1996;2:574–577. doi: 10.1038/nm0596-574. [DOI] [PubMed] [Google Scholar]
- 33.Dhein J, Walczak H, Baumler C, Debatin K-M, Krammer P H. Nature (London) 1995;373:438–441. doi: 10.1038/373438a0. [DOI] [PubMed] [Google Scholar]
- 34.Ju S-T, Panka D J, Cui H, Ettinger R, el-Khatib M, Sherr D H, Stanger B Z, Marshak-Rothstein A. Nature (London) 1995;373:444–448. doi: 10.1038/373444a0. [DOI] [PubMed] [Google Scholar]
- 35.Nagata S, Golstein P. Science. 1995;267:1449–1456. doi: 10.1126/science.7533326. [DOI] [PubMed] [Google Scholar]
- 36.Muzio M, Chinnaiyan A M, Kischkel F C, O’Rourke K, Shevchenko A, Ni J, Scaffidi C, Bretz J D, Zhang M, Gentz R, et al. Cell. 1996;85:817–827. doi: 10.1016/s0092-8674(00)81266-0. [DOI] [PubMed] [Google Scholar]
- 37.Smyth M J, Trapani J A. Immunol Today. 1995;16:202–206. doi: 10.1016/0167-5699(95)80122-7. [DOI] [PubMed] [Google Scholar]
- 38.Duke R C, Persechini P M, Chang S, Liu C C, Cohen J J, Young J D. J Exp Med. 1989;170:1451–1456. doi: 10.1084/jem.170.4.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Longthorne V L, Williams G T. EMBO J. 1997;16:3805–3812. doi: 10.1093/emboj/16.13.3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xue D, Shaham S, Horvitz H R. Genes Dev. 1996;10:1073–1083. doi: 10.1101/gad.10.9.1073. [DOI] [PubMed] [Google Scholar]
- 41.Landowski T, Gleason-Guzman M C, Dalton W S. Blood. 1997;89:1854–1861. [PubMed] [Google Scholar]
- 42.Eischen C M, Kottke T J, Martins L M, Basi G S, Tung J S, Earnshaw W C, Leibson P J, Kaufmann S H. Blood. 1997;90:935–943. [PubMed] [Google Scholar]
- 43.Liu X, Kim C N, Yang J, Jemmerson R, Wang X. Cell. 1996;86:147–157. doi: 10.1016/s0092-8674(00)80085-9. [DOI] [PubMed] [Google Scholar]
- 44.Srinivasula S M, Fernandes-Alnemri T, Zangrilli J, Robertson N, Armstrong R C, Wang L, Trapani J A, Tomaselli K J, Litwack G, Alnemri E S. J Biol Chem. 1996;271:27099–27106. doi: 10.1074/jbc.271.43.27099. [DOI] [PubMed] [Google Scholar]
- 45.Schlegel J, Peters I, Orrenius S, Miller D K, Thornberry N A, Yamin T T, Nicholson D W. J Biol Chem. 1996;271:1841–1844. doi: 10.1074/jbc.271.4.1841. [DOI] [PubMed] [Google Scholar]
- 46.Darmon A J, Nicholson D W, Bleackley R C. Nature (London) 1995;377:446–448. doi: 10.1038/377446a0. [DOI] [PubMed] [Google Scholar]
- 47.Li P, Nijhawan D, Budihardjo I, Srinivasula S M, Ahmad M, Alnemri E, Wang X. Cell. 1997;91:479–489. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- 48.Eguchi Y, Shimizu S, Tsujimoto Y. Cancer Res. 1997;57:1835–1840. [PubMed] [Google Scholar]
- 49.Krippner A, Matsuno-Yagi A, Gottlieb R A, Babior B M. J Biol Chem. 1996;271:21629–21636. doi: 10.1074/jbc.271.35.21629. [DOI] [PubMed] [Google Scholar]