Abstract
Cocaine and methylphenidate block uptake by neuronal plasma membrane transporters for dopamine, serotonin, and norepinephrine. Cocaine also blocks voltage-gated sodium channels, a property not shared by methylphenidate. Several lines of evidence have suggested that cocaine blockade of the dopamine transporter (DAT), perhaps with additional contributions from serotonin transporter (5-HTT) recognition, was key to its rewarding actions. We now report that knockout mice without DAT and mice without 5-HTT establish cocaine-conditioned place preferences. Each strain displays cocaine-conditioned place preference in this major mouse model for assessing drug reward, while methylphenidate-conditioned place preference is also maintained in DAT knockout mice. These results have substantial implications for understanding cocaine actions and for strategies to produce anticocaine medications.
Cocaine use is a principal drug abuse problem in the United States and other countries, contributing to substantial morbidity and mortality among the millions of individuals who use it each year (1). No current medication provides effective treatment for cocaine dependence (2). These facts give particular importance to defining the sites for cocaine reward in the brain so that they can be more accurately targeted by potential therapeutic agents.
Several lines of evidence have provided support for a role of the dopamine transporter (DAT) as a primary site for cocaine reward. Structure–activity studies document good correlations between psychostimulant properties in tests of reward and their abilities to block DAT; poorer correlations are noted with their potencies in blocking other transporters (3, 4). Dopaminergic lesions blunt cocaine influences in model systems that test reward (5–7). Psychostimulants enhance dopamine release from dopaminergic circuits (8). Transgenic mice that overexpress DAT display enhanced cocaine-conditioned place preference (G.R.U., et al., unpublished observations). Finally, “indifference” to cocaine has been inferred from the reduced cocaine-stimulated locomotion recently described in mice that lack DAT (9, 10).
There are also limitations to postulated direct relationships between DAT blockade and psychostimulant-induced reward. Among these are the failure of several compounds that potently inhibit dopamine uptake, including mazindol, to display substantial abuse liability in humans or animal model studies (11–13). Because mazindol potently inhibits dopamine and norepinephrine transport, but only weakly inhibits serotonin transport, this difference from cocaine could conceivably contribute to a distinct profile on tests of reward (14–16). These and other more indirect lines of evidence support the idea that cocaine’s inhibition of serotonin uptake could also provide an alternative and plausible molecular site for contributions to cocaine reward (17–19).
To test the dopamine- or serotonin-transporter dependence of cocaine reward, we have constructed DAT knockout mice and assessed cocaine-conditioned place preferences in these DAT knockout mice and in previously described serotonin transporter (5-HTT) knockout mice (20). We have focused on conditioned place preference because it provides a technically tractable and robust measure of drug reward in mice, although other drug features occasionally contaminate this test (21, 22). This assay is able to detect the rewarding properties of virtually every class of abused substance. Mice express their drug preference 24 hr after the last drug administration, when they are likely to be free from acute cocaine effects on motor performance (5–7).
MATERIALS AND METHODS
Targeted Disruption of the Murine DAT Gene.
DAT knockout mice were produced by standard techniques (23). Thirteen- and 15-kb DAT genomic fragments were isolated from a λ-FIX II genomic library (Stratagene) prepared from the 129SvEv mouse strain (24), and a targeting vector constructed by using a 5.0-kb BamHI–SmaI 5′ fragment and a 5.5-kb SpeI–BamHI 3′ fragment subcloned into pPGKneo. The final construct, designated pDATKO, contains the herpes simplex virus thymidine kinase (TK) gene driven by the MC1 polyoma enhancer at the 3′ end of a SpeI–BamHI 3′ fragment. The first and second exons of the murine DAT gene are flanked by 5.0 kb of 5′ and 5.5 kb of 3′ sequence. Twenty-five micrograms of pDATKO DNA was linearized with NotI and transfected by electroporation into 107 J1 embryonic stem (ES) cells derived from 129/Sv mice [a generous gift from R. Jaenisch (25)]. J1 cells in which homologous recombination had occurred were selected by 8 days of growth in Dulbecco’s modified Eagle’s medium (DMEM) containing 15% fetal bovine serum (HyClone), 0.1 mM 2-mercaptoethanol, 500 μg/ml G418, and 2 μM ganciclovir (a generous gift from Syntex). EcoRI digests of DNA prepared from 400 G418- and ganciclovir-resistant clones were screened by Southern blot analyses using a 371-bp EcoRI–BamHI fragment. Four positive cell lines from the doubly resistant colonies displayed the 5-kb EcoRI fragment anticipated of homologous recombinants, which was readily distinguishable from the 12-kb fragment obtained from wild-type DNA. Two ES clones from the positive cell lines were used to establish mutant mice. Chimeric mice were generated by injecting 15–20 homologous recombinant ES cells into blastocysts harvested from C57BL/6J mice (The Jackson Laboratory) and implanting the blastocysts into uteri of pseudopregnant CD-1 mice (Charles River) 2.5 days after coitus. Chimeric mice were mated with wild-type C57BL/6J mice to produce F1 offspring. Southern analyses of DNA extracted from tail-tip specimens of F1 mice revealed that germ-line transmission was achieved from crosses between 4 chimeric animals. F2 homozygous, heterozygous, and wild-type offspring of F1 × F1 intercrosses were used for further biochemical and behavioral testing.
Radioligand Binding Studies.
Washed membranes prepared from rapidly dissected striatal specimens were incubated with [3H]CFT [(−)-2-β-carbomethoxy-3-β-(4-fluorophenyl)tropane-1,5-naphthalenedisulfonate] (WIN35,428) (83.5 Ci/mmol; NEN/DuPont; 1 Ci = 37 GBq) in ice-cold 0.32 M sucrose containing 10 mM sodium phosphate, pH 7.4, as described (3). Results from parallel incubations with 0.1 mM cocaine hydrochloride provided estimates of nonspecific binding. Reactions were terminated by the addition of ice-cold buffered solutions. Membrane-associated ligand was assessed after rapid filtration using Whatman GF/B filters and a Brandel apparatus (26). Protein concentrations were determined by the Bradford method (Bio-Rad) and results analyzed by using macligand.
Immunohistochemical Analyses.
DAT and tyrosine hydroxylase (TH) immunohistochemistry were performed as previously described (27). Specificity of primary antisera was evident in ELISAs, preadsorption tests, and the anatomic distribution of immunoreactivity (data not shown).
Behavioral Tests.
Mice were housed at 24°C in 50% relative humidity with a 12/12 hr light/dark cycle with lights on at 7:00 a.m. and off at 7:00 p.m. and ad libitum access to food and water under American Association for Laboratory Animal Care guidelines, as described (28). Mice tested for each experiment were compared with littermate controls to maintain near-identity of average genetic background. Locomotor activity was assessed as total distance traveled, which was calculated from measurement of the number of beam breaks when mice were placed individually in 46 × 25 × 19 cm clear plastic cages inside Optovarimex activity monitors (Columbus Instruments, Columbus, OH), to which the mice had not been previously exposed, under dim-light sound-attenuated conditions (29). Distance traveled was monitored for 3 hr. Immediately after baseline activity testing, the mice were removed from the monitors, injected with 10 mg/kg cocaine hydrochloride, and returned to the same chamber, where distance traveled was monitored for an additional 1 hr (28). Reward was assessed by conditioned place preference testing using a two-compartment Plexiglas chamber (22). One compartment (18 × 18 × 18 cm) had a wire mesh floor (1.3-cm grids) mounted over Plexiglas. The other compartment (18 × 18 × 18 cm) had corncob bedding on a smooth Plexiglas floor. An 18 × 5 × 1.3 cm platform was flush with the wall dividing two sides, which were separated by a removable Plexiglas wall. For pre- and post-conditioning test sessions, a 5-cm opening in the center wall allowed access to both compartments. During the conditioning sessions the opening was occluded to restrict animals to a single compartment. Locomotion and time spent in each compartment were recorded by using an Optivarimax animal activity monitoring apparatus. Initial preference, usually for the bedding-floored compartment, was determined as the side in which a mouse spent more than 600 sec of a 20-min trial. During two conditioning session days, animals were restricted for 20 min after injection with cocaine, methylphenidate, or saline to one side of the two-sided compartment, removed to their home cages for 4 hr, and then subjected to another 20-min conditioning trial. A single conditioned place preference assessment session followed the last conditioning session experienced by each mouse by 24 hr. In these sessions, mice had access to both compartments. The proportion of the 20-min session spent on each side was recorded. Results were compared with the proportion of time spent on that side in preconditioning sessions. Conditioned responses to saline, 5 and 10 mg/kg cocaine hydrochloride, and 5 mg/kg methylphenidate hydrochloride doses (1 ml/100 g weight, s.c.) were assessed (22). Statistical comparisons were made by using the Statistical Package for Social Science (SPSS, Chicago). Behavioral data were analyzed by Student’s t test and analyses of variance (ANOVA) followed by Scheffe post-hoc analyses. Data are presented as mean ± SEM for each experimental group.
RESULTS
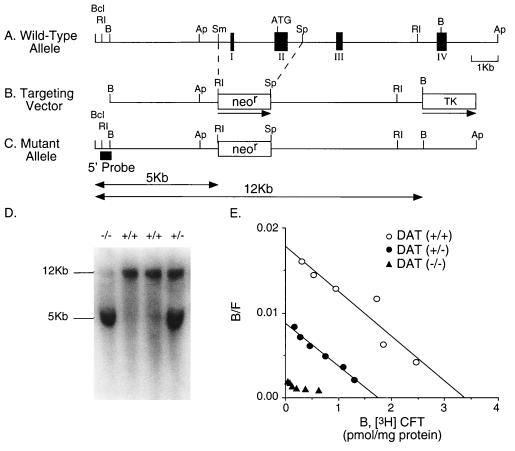
DAT knockout mice examined in this study (Fig. 1) display several features reported previously for another DAT knockout strain (9). Animals without functional DAT alleles are viable. Histologic evaluation of sections from several levels of the brain and spinal cord revealed no obvious differences between animals of each genotype (Fig. 2 and data not shown). DAT knockout mice display gene-dose-dependent reductions of DAT expression. There is negligible binding of the radiolabeled cocaine analog [3H]CFT (WIN35,428) to striatal membranes prepared from homozygotes. Bmax values of DAT heterozygote mice are 55% of wild-type values (1.45 ± 0.18 vs. 2.62 ± 0.5 pmol/mg of protein, n = 3; Fig. 1E). Striatal DAT immunoreactivity is substantially reduced in heterozygotes, and virtually eliminated in homozygote knockout animals (Fig. 2A). Immunoreactivity for TH, the rate-limiting enzyme for dopamine synthesis, is also modestly reduced in heterozygous mice and dramatically reduced in homozygous knockout mice (Fig. 2B). Our results fit with the results of reduced expression of other dopaminergic markers previously reported in another DAT knockout strain (9).
Figure 1.
Disruption of the DAT gene. The DAT gene was inactivated by replacement of exon I and II with a neomycin-resistance (neor) cassette. (A) Schematic representation of 5′ portions of the murine DAT gene, with positions of exons I–IV (filled boxes), the start codon (ATG), and BclI (Bcl), EcoRI (RI), BamHI (B), ApaI (Ap), SmaI (Sm), and SpeI (Sp) restriction endonuclease sites noted. Scale bar = 1 kb. (B) The pDATKO targeting vector, indicating neomycin resistance gene (neor) and MC1 thymidine kinase (TK) sequences. The direction of gene transcription is marked by the horizontal arrows. Abbreviations and scale as in A. (C) Predicted mutant allele resulting in the disrupted DAT gene. The locations of the 5′ probe used in the Southern blot are indicated. The first and second exons are missing from the mutant allele. Abbreviations and scale as in A and B. The sizes of the EcoRI fragment from wild-type (12 kb) and mutated alleles (5 kb) are indicated. (D) Genomic Southern blot analysis of hybridization of the 5′ DAT genomic probe to EcoRI-digested DNA extracted from wild-type (+/+), heterozygote (+/−), and homozygote (−/−) mouse tails. The presence of a 5-kb fragment indicates a homozygous mutant genotype, whereas wild-type fragments are 12 kb. (E) Scatchard analyses of saturation radioligand binding of [3H]CFT (WIN35,428) to striatal membranes from DAT knockout mice. Mean (± SEM, n = 3) values for Bmax were 2.62 ± 0.5 pmol/mg of protein, 1.45 ± 0.18, and undetectable for wild-type (+/+, ○), heterozygous (+/−, •), and homozygous (−/−, ▴) DAT knockout mice, respectively. Kd values were 20.1 ± 1.6 nM, 27.0 ± 4.3 nM, and undetectable, respectively.
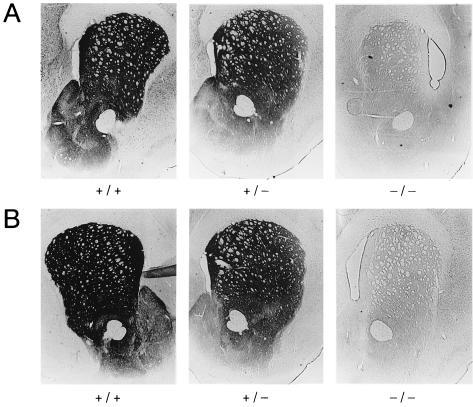
Figure 2.
Reduction of DAT and TH immunostaining in striatum of DAT knockout mice. (A) DAT immunoreactivity in sections through striatum and nucleus accumbens from wild-type (+/+), heterozygous (+/−), and homozygous (−/−) DAT knockout mice, as indicated. (B) TH immunoreactivity in striata and nucleus accumbens from wild-type (+/+), heterozygous (+/−), and homozygous (−/−) DAT knockout mice, as indicated.
The homozygous DAT knockout mice produced for the present study display locomotor features similar to those previously described in another DAT knockout strain (9). Homozygous mice tested here display enhanced exploratory behavior when placed in a novel environment; they are 3 and 6 times more active than wild-type mice during the first and second hours of experiment after being placed into a novel environment (Fig. 3; ANOVA followed by Scheffe post-hoc analysis: F(2,24) = 9.0, P < 0.05 for the first and F(2,24) = 9.4, P < 0.05 for the second hour). Although cocaine (10 mg/kg) increases locomotion in well habituated heterozygous or wild-type mice (F(3,44) = 12.9, P < 0.05 and F(3,28) = 8.2, P < 0.05 compared with pre-injection habituated activity, respectively), the homozygous DAT knockout mice tested here did not show any significant cocaine-induced increase in locomotion when compared with pre-injection habituated activity (Fig. 3). These mice are thus “indifferent” to these locomotor-stimulating cocaine properties, as previously described (9).
Figure 3.
Spontaneous hyperlocomotion and attenuated cocaine-induced locomotion in DAT knockout mice. Locomotor activities were recorded for 1-hr periods in wild type (+/+, open bars) and heterozygous (+/−, gray bars) and homozygous (−/−, black bars) DAT knockout mice placed into an activity monitor cage to which they had not been previously exposed at the time 3 hr before injection with cocaine (10 mg/kg s.c.) at the arrow (time 0). Homozygous knockout mice show greater locomotor activity when placed in a novel environment, but less locomotor response to cocaine. Values represent mean ± SEM; ∗, P < 0.05 versus wild-type group; #, P < 0.05 versus the locomotor activity before injection; n = 7–14 mice per genotype.
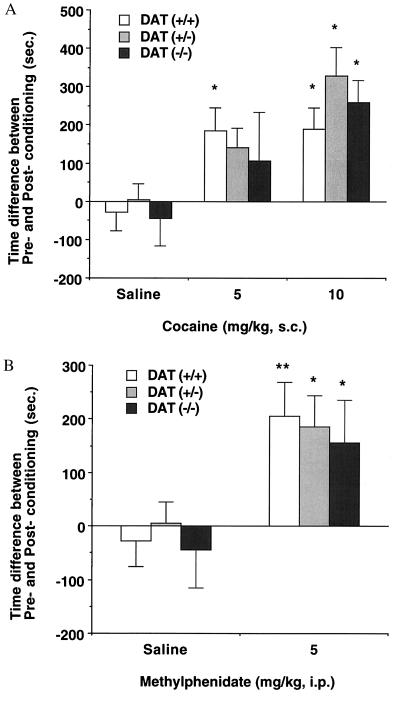
To investigate the effects of DAT gene deletions on the cocaine’s rewarding properties, cocaine-conditioned place preference was studied in wild-type, heterozygous, and homozygous DAT knockout mice (21, 22). Data from conditioned place preference assessments in our mice differ substantially from those obtained for cocaine-induced locomotion. Wild-type mice displaying full DAT expression showed robust cocaine conditioning that is significant at the doses of 5 and 10 mg/kg of body weight (F(2,54) = 5.1, P < 0.05 vs. saline control; Fig. 4A). Heterozygous mice displaying half of wild-type levels of DAT expression also increase preference for the compartment paired with 10 mg/kg cocaine (F(2,49) = 9.2, P < 0.05 vs. saline controls). Strikingly, significant conditioning is also observed in homozygous mice administered 10 mg/kg cocaine doses (F(2,36) = 4.2, P < 0.05 vs. saline controls). These cocaine-induced preferences are found in studies of each of two cohorts of knockout mice tested on separate occasions, with genotypes confirmed twice. We also tested place preferences induced by methylphenidate, which lacks the sodium channel blockade displayed by cocaine (30, 31). Wild-type mice showed robust preference for the side paired with 5 mg/kg methylphenidate (P < 0.01 vs. saline controls by t test; Fig. 4B). Both heterozygous and homozygous DAT knockout mice also displayed increased preferences for the compartments paired with 5 mg/kg methylphenidate (P < 0.05 vs. saline controls by t test) that were indistinguishable from the preference induced in wild-type mice. Methylphenidate-induced preferences were found in studies of each of two cohorts of knockout mice tested on separate occasions, with genotypes confirmed three times. None of the DAT knockout mice that received saline in conditioning sessions exhibited significantly altered preferences for either test compartment.
Figure 4.
Cocaine- and methylphenidate-conditioned place preferences in DAT knockout mice. (A) Conditioned place preference induced by cocaine in wild type (+/+, open bars) and heterozygous (+/−, gray bars) and homozygous (−/−, black bars) DAT knockout mice. Time scores shown represent differences between post-conditioning (Post) and pre-conditioning (Pre) time spent in the cocaine-paired environment. Wild-type mice displayed significant place preference associated with 5 and 10 mg/kg cocaine, whereas heterozygous and homozygous animals showed significant place preference associated with 10 mg/kg cocaine. ∗ P < 0.05 vs. saline-injected group by ANOVA; n = 8–23 mice per genotype. The following data show the real mean time scores ± SEM (sec) of pre-conditioning (Pre) and post-conditioning (Post) sessions on initially nonpreferred compartment in each genotype. Wild-type [Pre/Post], heterozygote [Pre/Post], homozygote [Pre/Post]: saline ([411.4 ± 30.0/382.8 ± 50.4], [387.0 ± 36.0/392.6 ± 47.2], [408.6 ± 29.1/364.6 ± 65.0]), cocaine 5 mg/kg ([304.1 ± 35.0/489.0 ± 67.8], [354.4 ± 37.7/495.9 ± 70.7], [348.2 ± 29.1/455.0 ± 114.8]), cocaine 10 mg/kg ([336.7 ± 25.6/526.0 ± 59.2], [327.9 ± 26.0/655.9 ± 64.9], [328.7 ± 26.9/597.5 ± 50.5]). (B) Conditioned place preference induced by methylphenidate in wild type (+/+, open bars) and heterozygous (+/−, gray bars) and homozygous (−/−, black bars) DAT knockout mice. Time scores shown represent differences between post-conditioning (Post) and pre-conditioning (Pre) time spent in the cocaine-paired environment. All three genotypes displayed significant place preference associated with 5 mg/kg methylphenidate. ∗∗, P < 0.01; ∗, P < 0.05 vs. saline-injected group by ANOVA; n = 12–21 mice per genotype. The following data show the real mean time scores ± SEM (sec) of pre-conditioning (Pre) and post-conditioning (Post) sessions on initially nonpreferred compartment in each genotype. Wild-type [Pre/Post], heterozygote [Pre/Post], homozygote [Pre/Post]: saline ([411.4 ± 30.0/382.8 ± 50.4], [387.0 ± 36.0/392.6 ± 47.2], [408.6 ± 29.1/364.6 ± 65.0]), methylphenidate 5 mg/kg ([356.6 ± 34.6/562.5 ± 68.3], [367.4 ± 22.1/552.6 ± 56.0], [376.6 ± 38.7/533.0 ± 77.2]).
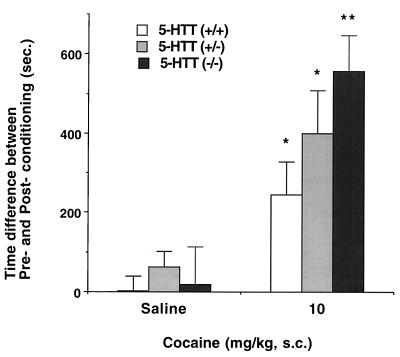
To explore the possibility that cocaine’s 5-HTT blockade could be responsible for cocaine reward, homozygous and heterozygous 5-HTT-deleted mice and wild-type control mice were also tested. The homozygous 5-HTT knockout mice, whose absence of 5-HTT expression has been confirmed in uptake and receptor autoradiographic studies (20), also display significant cocaine-conditioned place preferences (Fig. 5; t test, P < 0.001 vs. saline controls). Heterozygous mice displaying half of wild-type levels of 5-HTT expression and wild-type mice with full 5-HTT expression display significant place preference for the drug-paired compartment at 10 mg/kg cocaine when compared with saline controls (Student’s t test: P < 0.01 vs. saline controls). The place preference for 10 mg/kg cocaine differs significantly among the three genotypes. Homozygous 5-HTT knockout mice even display significantly enhanced cocaine-conditioned place preferences when compared with wild-type controls (F(2,38) = 3.7, P < 0.05 vs. wild-type controls). Methylphenidate administration (5 mg/kg) yielded a robust conditioned place preference in wild-type mice and an intact or even enhanced preference in heterozygous and homozygous 5-HTT knockout mice (data not shown). None of the 5-HTT knockout mice that received saline in conditioning sessions exhibited significantly altered preferences for either test compartment.
Figure 5.
Cocaine-conditioned place preferences in 5-HTT knockout mice. Conditioned place preference induced by cocaine in wild type (+/+, open bars) and heterozygous (+/−, gray bars) and homozygous (−/−, black bars) 5-HTT knockout mice. Time scores shown represent differences between post-conditioning (Post) and pre-conditioning (Pre) time spent in the cocaine-paired environment. Homozygous mice showed significantly more place preference than wild type (P < 0.05, ANOVA; n = 8–17 mice per genotype). 5-HTT knockout mice displayed significant place preference associated with 10 mg/kg cocaine in gene-dose-related fashion (∗, P < 0.01; ∗∗, P < 0.001 vs. saline- injected group by Student’s t test; n = 8–17 mice per genotype). 5-HTT knockout mice were constructed as in ref. 20. The following data show the real mean time scores ± SEM (sec) of pre-conditioning (Pre) and post-conditioning (Post) sessions on initially nonpreferred compartment in each genotype. Wild type [Pre/Post], heterozygote [Pre/Post], homozygote [Pre/Post]: saline ([324.7 ± 36.0/326.2 ± 51.7], [364.5 ± 52.5/426.3 ± 62.0], [296.5 ± 60.0/315.9 ± 76.7]), cocaine 10 mg/kg ([329.1 ± 33.6/575.3 ± 74.8], [221.5 ± 23.3/620.3 ± 106.0], [290.7 ± 44.1/848.1 ± 76.0].
DISCUSSION
Taken together, the current results suggest that animals with life-long deletions of either DAT or 5-HTT can readily manifest cocaine reward, as measured by conditioned place preference. These mice can thus receive cocaine-conditioned cues, retain information about this model of drug reward over at least 24 hr, and use this information to enhance time spent in the previously cocaine-associated environment 24 hr after the last drug dose in the absence of either DAT or 5-HTT. Neither DAT nor 5-HTT, by itself, is absolutely required for full cocaine-conditioned reward, as assessed in this test.
These striking data from cocaine studies suggest that if cocaine reward-like responses do not require DAT or 5-HTT in the knockout animals, several possible roles for DAT or 5-HTT in cocaine reward-like responses in wild-type mice nevertheless remain. These include the possibility that neither DAT nor 5-HTT plays a role in cocaine reward of wild-type mice, but that actions at other previously known primary cocaine targets such as the norepinephrine transporter (NET) or sodium channel mediate cocaine reward (32). The data from methylphenidate argue against a prominent role for sodium channel blockade in these processes. While the structure activity profiles of DAT and 5-HTT ligands in producing reward-like responses do argue against NET as a principal site for cocaine reward, this possibility does remain open.
3H radioligand-binding studies using cocaine as a ligand have noted elevated “background” binding levels to sites that cannot be blocked by blockers of DAT, 5-HTT, NET, or sodium channels (33, 34). Such results indicate the formal possibility that a previously unknown cocaine target could mediate cocaine reward responses, although reward can also be mediated by other compound classes with different pattern of “nonspecific” binding.
Another possibility also exists. Developmental adaptations in these animals could conceivably supervene and allow occupancy of another transporter, or previously unelucidated site, to substitute for occupancy of the deleted transporter. In this case, DAT or 5-HTT could still represent the major normal targets for cocaine reward responses. However, these normal central role(s) could be replaced when DAT or 5-HTT is absent throughout development. If double knockout mice with total deletions of both transporters were viable, cocaine reward could be tested in animals without both transporters to assess the possibility that DAT substitutes for 5-HTT and/or vice versa. However, the high premature lethality manifest by even DAT knockout homozygotes (I.S. and G.R.U., unpublished observations) limits the likelihood that double homozygotes missing both transporters will be both viable and sufficiently normal to provide unambiguous results from behavioral tests of drug reward.
The trend toward greater preference induced by the 5 mg/kg cocaine in wild-type than in heterozygous and homozygous DAT knockout mice could conceivably support a role for DAT in determining cocaine’s potency (e.g., Fig. 4A). Similarly, the trend toward greater cocaine-conditioned place preference in mice with 5-HTT deletion should argue that 5-HTT occupancies might be slightly aversive. However, the modest size of each of these observed differences suggests substantial caution in interpretation.
If the current data do suggest that actions at several different systems with substantial redundancy may normally allow cocaine to provide rewarding cues to humans, then therapeutic strategies aimed at a single transporter might be unlikely to be successful in blocking cocaine reward. Medication strategies utilizing “dirty drugs” active at multiple sites might be necessary to combat this important “dirty” abused drug, cocaine.
Acknowledgments
We thank R. Jaenisch (Massachusetts Institute of Technology, Cambridge, MA), who generously provided J1 ES cells; Yuji Mishina (Univ. of Texas, Houston) and Masahiko Funada (Daiichi College of Pharmaceutical Science, Fukuoka, Japan) for excellent consultation; Syntex, Inc. (Palo Alto, CA) for their generous gift of ganciclovir; Steven Kinsey, Hsin-Fei Liu, and Nancy Goodman for careful technical assistance; Mary Jane Robinson for assistance with manuscript preparation; and Lisa Naccarato and the Charles River/Triad animal care staff for important contributions to animal care and breeding. This work was supported by the intramural research programs of the National Institute on Drug Abuse and the National Institute for Mental Health and by the Bundesministerium für Bildung, Wissenschaft, Forschung, und Technologie, Germany.
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: ES cells, embryonic stem cells; DAT, dopamine transporter; 5-HTT, serotonin transporter; TH, tyrosine hydroxylase.
References
- 1.Substance Abuse and Mental Health Services Administration. National Household Survey on Drug Abuse: Population Estimates 1995. Rockville, MD: U.S. Dept. of Health and Human Services; 1996. [Google Scholar]
- 2.Caroll F I, Lewis A H, Biswas J. Pharm News. 1994;1:11–17. [Google Scholar]
- 3.Spealman R D, Madras B K, Bergman J. J Pharmacol Exp Ther. 1989;251:142–149. [PubMed] [Google Scholar]
- 4.Kuhar M J, Ritz M C, Boja J W. Trends Neurosci. 1991;14:299–302. doi: 10.1016/0166-2236(91)90141-g. [DOI] [PubMed] [Google Scholar]
- 5.Roberts D C, Corcoran M E, Fibiger H C. Pharmacol Biochem Behav. 1977;6:615–620. doi: 10.1016/0091-3057(77)90084-3. [DOI] [PubMed] [Google Scholar]
- 6.Roberts D C, Koob G F. Pharmacol Biochem Behav. 1982;17:901–904. doi: 10.1016/0091-3057(82)90469-5. [DOI] [PubMed] [Google Scholar]
- 7.Pettit H O, Ettenberg A, Bloom F E, Koob G F. Psychopharmacology (Berlin) 1984;84:167–173. doi: 10.1007/BF00427441. [DOI] [PubMed] [Google Scholar]
- 8.Di Chiara G, Imperato A. Proc Natl Acad Sci USA. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giros B, Jaber M, Jones S R, Wightman R M, Caron M G. Nature (London) 1996;379:606–612. doi: 10.1038/379606a0. [DOI] [PubMed] [Google Scholar]
- 10.Uhl G R, Vandenbergh D J, Miner L L. Curr Biol. 1996;6:935–936. doi: 10.1016/s0960-9822(02)00630-9. [DOI] [PubMed] [Google Scholar]
- 11.Wilson M C, Schuster C R. Pharmacol Biochem Behav. 1976;4:207–210. doi: 10.1016/0091-3057(76)90017-4. [DOI] [PubMed] [Google Scholar]
- 12.Yanagita T, Wakasa Y, Oinuma N. Jitchuken Zenrinsho Kenkyuho. 1982;8:247–257. [Google Scholar]
- 13.Chait L D, Uhlenhuth E H, Johanson C E. J Pharmacol Exp Ther. 1987;242:777–783. [PubMed] [Google Scholar]
- 14.Javitch J A, Blaustein R O, Snyder S H. Mol Pharmacol. 1984;26:35–44. [PubMed] [Google Scholar]
- 15.Cooper J R, Bloom F E, Roth R H. The Biochemical Basis of Neuropharmacology. New York: Oxford Univ. Press; 1991. [Google Scholar]
- 16.Uhl G R, Hartig P R. Trends Pharmacol Sci. 1992;13:421–425. doi: 10.1016/0165-6147(92)90133-q. [DOI] [PubMed] [Google Scholar]
- 17.Kleven M S, Woolverton W L. Drug Alcohol Depend. 1993;31:149–158. doi: 10.1016/0376-8716(93)90067-z. [DOI] [PubMed] [Google Scholar]
- 18.Spealman R D. Psychopharmacology (Berlin) 1993;112:93–99. doi: 10.1007/BF02247368. [DOI] [PubMed] [Google Scholar]
- 19.Walsh S L, Preston K L, Sullivan J T, Fromme R, Bigelow G E. J Clin Psychopharmacol. 1994;14:396–407. [PubMed] [Google Scholar]
- 20.Bengel D, Murphy D L, Andrews A M, Wichems C H, Feltner D, Heils A, Mossner R, Westphal H, Lesch K. Mol Pharmacol. 1998;53:649–655. doi: 10.1124/mol.53.4.649. [DOI] [PubMed] [Google Scholar]
- 21.Martin I M, Ortmann R, Fibiger H C. Brain Res. 1985;332:59–67. doi: 10.1016/0006-8993(85)90389-0. [DOI] [PubMed] [Google Scholar]
- 22.Miner L L, Drago J, Chamberlain P M, Donovan D, Uhl G R. NeuroReport. 1995;6:2314–2316. doi: 10.1097/00001756-199511270-00011. [DOI] [PubMed] [Google Scholar]
- 23.Joyner A L. Gene Targeting, A Practical Approach. New York: Oxford Univ. Press; 1993. [Google Scholar]
- 24.Donovan D M, Vandenbergh D J, Perry M P, Bird G S, Ingersoll R, Nanthakumar E, Uhl G R. Brain Res Mol Brain Res. 1995;30:327–335. doi: 10.1016/0169-328x(95)00018-n. [DOI] [PubMed] [Google Scholar]
- 25.Li E, Bestor T H, Jaenisch R. Cell. 1992;69:915–926. doi: 10.1016/0092-8674(92)90611-f. [DOI] [PubMed] [Google Scholar]
- 26.Boja J W, Carroll F I, Rahman M A, Philip A, Lewin A H, Kuhar M J. Eur J Pharmacol. 1990;184:329–332. doi: 10.1016/0014-2999(90)90627-i. [DOI] [PubMed] [Google Scholar]
- 27.Freed C, Revay R, Vaughan R A, Kriek E, Grant S, Uhl G R, Kuhar M J. J Comp Neurol. 1995;359:340–349. doi: 10.1002/cne.903590211. [DOI] [PubMed] [Google Scholar]
- 28.Miner L L, Marley R J. Psychopharmacology (Berlin) 1995;122:209–214. doi: 10.1007/BF02246541. [DOI] [PubMed] [Google Scholar]
- 29.Sora I, Takahashi N, Funada M, Ujike H, Revay R S, Donovan D M, Miner L L, Uhl G R. Proc Natl Acad Sci USA. 1997;94:1544–1549. doi: 10.1073/pnas.94.4.1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Catterall W, Mackie K. In: Goodman & Gilman’s The Pharmacological Basis of Therapeutics. Hardman G G, Gilman A, Limbird L L, editors. New York: McGraw-Hill; 1996. pp. 331–347. [Google Scholar]
- 31.Hoffman B B, Lefkowitz R J. In: Goodman & Gilman’s The Pharmacological Basis of Therapeutics. Hardman G G, Gilman A, Limbird L L, editors. New York: McGraw-Hill; 1996. pp. 199–248. [Google Scholar]
- 32.Weidman S J. J Physiol. 1955;129:568–582. doi: 10.1113/jphysiol.1955.sp005379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Madras B K, Fahey M A, Bergman J, Canfield D R, Spealman R D. J Pharmacol Exp Ther. 1989;251:131–141. [PubMed] [Google Scholar]
- 34.Reith M E A. In: Cocaine: Pharmacology, Physiology, and Clinical Strategies. Lakoski J M, Galloway M P, White F J, editors. Boca Raton, FL: CRC; 1991. pp. 203–227. [Google Scholar]