Abstract
The dynamic characteristics of reflex eye movements were measured in two strains of chronically prepared mice by using an infrared television camera system. The horizontal vestibulo-ocular reflex (HVOR) and horizontal optokinetic response (HOKR) were induced by sinusoidal oscillations of a turntable, in darkness, by 10° (peak to peak) at 0.11–0.50 Hz and of a checked-pattern screen, in light, by 5–20°at 0.11–0.17 Hz, respectively. The gains and phases of the HVOR and HOKR of the C57BL/6 mice were nearly equivalent to those of rabbits and rats, whereas the 129/Sv mice exhibited very low gains in the HVOR and moderate phase lags in the HOKR, suggesting an inherent sensory-motor anomaly. Adaptability of the HOKR was examined in C57BL/6 mice by sustained screen oscillation. When the screen was oscillated by 10° at 0.17 Hz, which induced sufficient retinal slips, the gain of the HOKR increased by 0.08 in 1 h on average, whereas the stimuli that induced relatively small or no retinal slips affected the gain very little. Lesions of the flocculi induced by local applications of 0.1% ibotenic acid and lesions of the inferior olivary nuclei induced by i.p. injection of 3-acetylpyridine in C57BL/6 mice little affected the dynamic characteristics of the HVOR and HOKR, but abolished the adaptation of the HOKR. These results indicate that the olivo-floccular system plays an essential role in the adaptive control of the ocular reflex in mice, as suggested in other animal species. The data presented provide the basis for analyzing the reflex eye movements of genetically engineered mice.
Mutant mice generated by gene-targeting methods are useful for the study of the molecular and cellular bases of animal behaviors, including learning and memory (1–3). The cerebellum is supposed to be responsible for some forms of motor learning. Since the impaired motor functions and deficient cerebellar long-term depression (LTD) of type 1 metabotropic glutamate receptor (mGluR1)-deficient mice were reported (4), several groups have tried to establish a possible link between the modification of synaptic transmission in the cerebellum and motor functions by using other types of mutant mice (5–8). Those studies supported the hypothesis that LTD is closely associated with a form of motor learning (eyeblink conditioning) (4, 7, 8), and at the same time raised an argument against the role of LTD in motor coordination (7, 8), although one can claim that the assays used may not have been sensitive enough to assess motor coordination. It also was proposed that the elimination of multiply innervated climbing fibers to a Purkinje cell plays a crucial role in motor coordination (6, 7).
To gain further insight into the cellular and molecular bases for motor learning, it is necessary to introduce more sensitive and quantitative paradigms to analyze the motor behaviors of mice. In this study, we quantitatively evaluated the dynamic characteristics of two forms of representative reflex eye movements, i.e., the horizontal vestibulo-ocular reflex (HVOR) and horizontal optokinetic response eye movement (HOKR), which are eye movements that compensate for the movements of the head and visual fields, and determined the adaptation paradigm for the HOKR in mice. We compared the dynamic characteristics of these two eye movements in two strains, C57BL/6 and 129/Sv, which usually are used to generate gene-knockout mice. The importance of genetic background has been pointed out in the evaluation of some forms of cognitive behaviors (9). We show here that the 129/Sv strain possesses an inherent severe sensory-motor anomaly, at least in the sensory-driven reflex eye movement systems. The HVOR and HOKR are seen in many animal species, and simple manipulation of visual environments induces immediate adaptations in their dynamic characteristics. The neural circuitry for these reflex eye movements and their adaptive mechanisms are assumed to be common irrespective of animal species (10). We examined the neural areas responsible for the adaptation of the HOKR by lesion studies and found that the flocculo-olivary areas are essential for the adaptation of the HOKR in mice.
MATERIALS AND METHODS
C57BL/6 and 129/Sv mice with black eyes (body weight 20–25 g, Japan Crea, Hamamatsu, Japan) were used. Under pentobarbital anesthesia (Nakalai, Kyoto, Japan; 60 mg/kg body weight) in aseptic conditions, a platform for fixation of the head was built on the cranial bone by using four small screws and one long bolt fixed in place by synthetic resin. No less than 48 h after surgery, a mouse was mounted on the turntable with its head fixed and its body loosely restrained in a plastic cylinder. The turntable was surrounded by a cylindrical (30 cm diameter) screen with a checked pattern (square size, 4°). The mouse’s head was tilted so that the lateral semicircular canals were positioned approximately parallel to the horizontal plane.
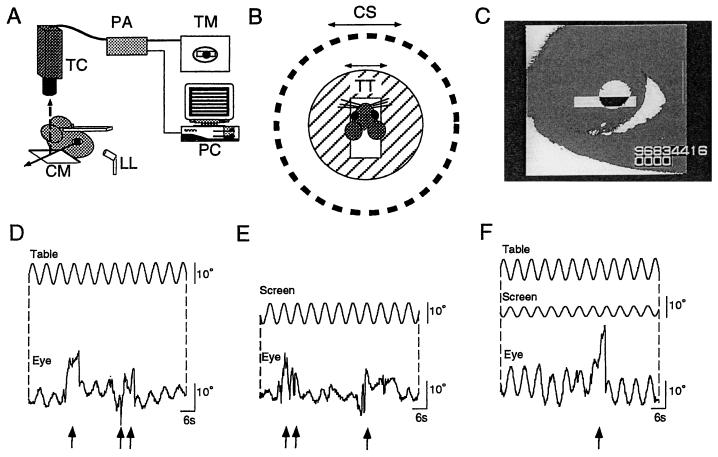
The HVOR was induced by sinusoidal oscillation of the turntable in the horizontal plane by 10° (peak to peak) at 0.11–0.50 Hz in darkness. The HOKR was induced by sinusoidal oscillation of the screen by 5–20° (peak to peak) at 0.11–0.17 Hz (maximum screen velocity, 2.6–10.5°/s) in the light. The evoked eye movements were recorded by using an infrared television camera system (11). As shown in Fig. 1A, the frontal view of the right eye was monitored by the infrared charge-coupled device television camera (SSC-M350, Sony, Tokyo) through a cold mirror. The pupil of the right eye was illuminated by an infrared light (wavelength, 900 nm)-emitting diode and displayed on a 12-inch television monitor, magnified ×32. The area of the pupil was determined by the difference in brightness between the pupil and the iris. The real-time position of the eye was measured by calculating the central position of the left and right margin of the pupil within the window (Fig. 1C) at 50 Hz using a position-analyzing system (C-1170, Hamamatsu Photonics, Hamamatsu City, Japan) and stored in the personal computer system. More than 10 cycles of the evoked eye movements free from artifacts caused by blinking or body movements (Fig. 1 D-F) were averaged, and the mean amplitude and phase were calculated by a modified Fourier analysis (12, 13). The mean effective diameter of the mouse eyeball was 3.2 mm (n = 5) in this study. The gain was defined as the ratio of the peak-to-peak amplitude of eye movement vs. the peak-to-peak amplitude of the turntable or screen oscillation. The phase was defined as 0° when the peak of eye movement matched the peak of the screen oscillation in the HOKR, and when the peak of eye movement was completely opposite to the peak of the turntable oscillation in the HVOR.
Figure 1.
(A) Experimental set-up for measuring mouse eye movements. LL, infrared light-emitting laser diode; CM, cold mirror; TC, charge-coupled device television camera; PA, position analyzer; TM, television monitor; PC, personal computer. Solid and broken arrows represent daylight passing through the CM and infrared light reflected by the CM, respectively. (B) Top view of stimulating apparatuses. TT, turntable; CS, checked-pattern screen (radius 30 cm). (C) View of the right mouse eye on the television monitor. Eye movements under turntable oscillation (D: peak-to-peak 10° at 0.17 Hz) in darkness (HVOR), screen oscillation (E: 10° at 0.17 Hz) in light (HOKR), and outphase combination of turntable and screen oscillations (F: 10° at 0.17 Hz and 5° at 0.17 Hz, respectively) in light. Arrows indicate artifacts caused by blinking or body movements.
The adaptability of the HOKR in C57BL/6 mice was investigated by 1 h of sustained oscillation of the screen by 10° at 0.17 Hz (5.2°/s) in the light. This condition was chosen as the one that effectively induced large retinal slips in these mice. The HOKR was measured every 30 min. As a control experiment, sustained oscillation of the screen at slower velocity (5° at 0.11 Hz, 1.7°/s), which induced relatively small retinal slips, and sustained oscillation of the turntable in darkness by 10° at 0.17 Hz, which induced no retinal slips, for 1 h were used.
The flocculi or inferior olivary nuclei were lesioned in C57BL/6 mice. Under sodium pentobarbital anesthesia, small holes were made bilaterally in the bones overlying the paraflocculus, and a microsyringe (80135, Hamilton) was inserted stereotaxically into the flocculus. Ibotenic acid (Sigma) (0.1%) dissolved in 0.1 M PBS was injected bilaterally (0.2 μl for each side) 4–10 days before the eye movement measurements. Inferior olivary nuclei were lesioned by i.p. injection of 3-acetylpyridine (3-AP, Nakalai; 500 mg/kg body weight) followed by 500 mg/kg of nicotinamide (Nakalai) with an interval of 3 h, according to a procedure described previously (14). The 3-AP was injected at least 17 days before eye movement measurements. After the entire series of eye movement measurements was complete, the lesioned mice were deeply anesthetized with sodium pentobarbital and perfused intracardially with PBS followed by a 10% formol-PBS solution. Excised brain tissues were stored in 10% formol-PBS solution overnight, and embedded in 30% gelatin and OCT compound (Sakura, Tokyo). Coronal sections were serially prepared at 40-μm thickness by using a conventional cryostat and stained with cresyl violet for microscopic inspection. The extensions of lesions were estimated by the loss of granule cells in the cerebellar cortex and the loss and the presence of degenerative neurons in the inferior olivary nuclei. These experimental protocols were approved by the management committee at the Laboratory of Experimental Medicine, based on the school’s guide for laboratory animals.
RESULTS
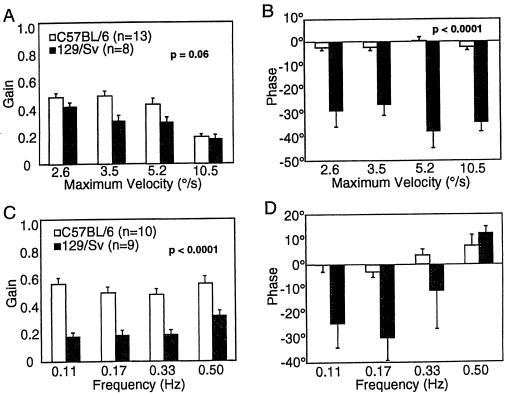
The HVOR was evoked by turntable oscillation in darkness, and the HOKR was evoked by screen oscillation in light, as shown in Fig. 1 D and E. The HOKR and HVOR interacted additively when the turntable and screen were simultaneously oscillated in light (Fig. 1F). The gain of the HOKR in C57BL/6 mice was nearly constant (0.5) at lower screen velocities and decreased at high screen velocity (20° at 0.17 Hz; 10.5°/s, Fig. 2A). The gains of the HVOR in the C57BL/6 mice were nearly constant (around 0.5) at 0.11–0.5 Hz (Fig. 2C). The phases were stable at around 0° irrespective of the gain of the HOKR or HVOR (Fig. 2 B and D). On the contrary, the gains of the HVOR were significantly lower (P < 0.0001 by two-way ANOVA) in the 129/Sv mice than in the C57BL/6 mice at 0.11–0.5 Hz (Fig. 2C), and the phases of the HOKR were significantly delayed (P < 0.0001 by two-way ANOVA) by 30° at any of the velocities used (Fig. 2B). The gains of the HOKR in the 129/Sv mice tended to be lower than those of the C57BL/6 mice (Fig. 2A), but statistical significance was not attached to these difference (P > 0.05 by two-way ANOVA). These results suggested that the 129/Sv mice possess an inherent profound anomaly in their reflex eye movement systems.
Figure 2.
Differences in dynamic characteristics of HVOR and HOKR between C57BL/6 and 129/Sv mice. (A) The gain of the HOKR and (B) the phase of the HOKR. The screen was sinusoidally oscillated by 5° peak to peak at 0.17 Hz (maximum velocity 2.6°/s), 10° at 0.11 Hz (3.5°/s), 10° at 0.17 Hz (5.2°/s), and 20° at 0.17 Hz (10.5°/s). (C) The gain and (D) the phase of the HVOR. The turntable was sinusoidally oscillated by 10° peak to peak at all frequencies. The P-values were obtained by the two-way ANOVA. The HVOR phases in 129/Sv mice at 0.11–0.33 Hz may include errors caused by too-low HVOR gains. Vertical bars indicate SE in all panels.
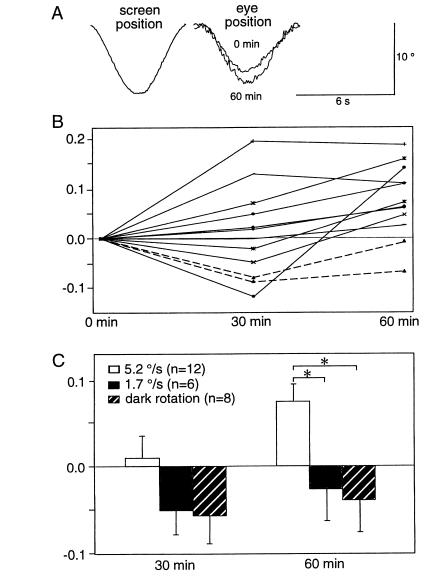
The HOKR was generally stable and was affected very little by the general conditions of the mice. On the other hand, the HVOR was rather sensitive to the states of alertness and/or stress of the mice. For example, external somatic stimuli, e.g., a gentle tail touch, affected the gain of the HOKR very little, but increased the gain of HVOR by 0.2 on average in C57BL/6 mice in preliminary experiments (data not shown). Thus in the present experiments, the adaptation of the HOKR was investigated by a 1-h sustained sinusoidal screen oscillation in the light in C57BL/6 mice. Sustained oscillation of the screen by 10° at 0.17 Hz (5.2°/s) for 1 h, whereby the gain of the HOKR was relatively low (Fig. 2A), so that a sufficient amount of retinal slips occurred, adaptively increased the gain of the HOKR by 0.03–0.19 in 10 of 12 mice (Fig. 3 A and B). The mean increase in the HOKR gain for these 12 mice was 0.08 (Fig. 3C). Meanwhile, sustained oscillation of the screen by 5° at 0.11 Hz (1.7°/s), whereby relatively small retinal slips occurred, affected the gain of the HOKR very little (P < 0.05 by Student’s t test) (Fig. 3C). Similarly, sustained turntable oscillation in darkness by 10° at 0.17 Hz, whereby no retinal slips occurred, did not increase the gain of the HOKR (P < 0.05 by Student’s t test) (Fig. 3C).
Figure 3.
C57BL/6 mice showed adaptation in the HOKR. (A) Examples of the averaged eye position traces before and after 60 min of sustained screen oscillations. (B) Changes in HOKR gains during continuous screen oscillations in individual mice. The screen was oscillated at a maximum velocity of 5.2°/s (10° at 0.17 Hz). Ten of 12 mice, indicated by solid lines, exhibited increased gains at 1 h. Only two of these 12 mice showed a decrease in the HOKR gain at 1 h (broken lines). (C) Stimulation parameter-dependence of the adaptation of the HOKR. The screen oscillation at low velocity (5° at 0.11 Hz, 1.7°/s) induces relatively small retinal slips (see Fig. 2A). The sustained oscillation of the turntable by 10° at 0.17 Hz in darkness induced no retinal slips. These results suggest that exposure to a sufficient amount of retinal slips is required for the adaptation of the HOKR. ∗, P < 0.05, Student’s t test.
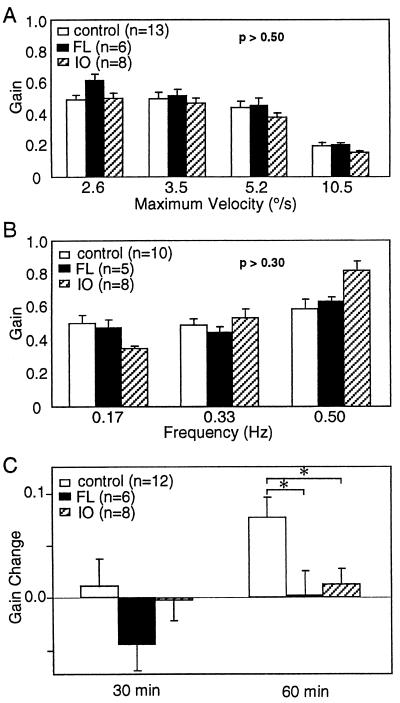
To locate the neural areas involved in the adaptation of the HOKR, bilateral lesions were made either in the cerebellar flocculi (n = 6) or inferior olivary nuclei (n = 8) in C57BL/6 mice. Floccular lesions caused by local application of ibotenic acid little affected either the gain (Fig. 4 A and B) or the phase (data not shown) in the HOKR or HVOR, and abolished the adaptation of the HOKR (Fig. 4C). On the contrary, lesions in the paraflocculi, sparing the flocculi, did not impair either the dynamics (HOKR gains: 0.54 ± 0.04 at 2.6°/s, 0.46 ± 0.05 at 3.5°/s, 0.38 ± 0.02 at 5.2°/s, 0.14 ± 0.01 at 10.5°/s, mean ± SE, n = 6) or the adaptation of the HOKR (gain increase: 0.08 ± 0.02, mean ± SE, n = 6). Inferior olivary nuclei lesions caused by i.p. injection of 3-AP did not affect either the gain (Fig. 4A) or phase (data not shown) of the HOKR, but markedly depressed the adaptation of the HOKR (Fig. 4C). The gains of the HVOR fluctuated stimulus frequency dependently, which was not attached with statistical significance (P > 0.30 by two-way ANOVA) by inferior olivary nuclei lesions (Fig. 4B). Stimulus frequency-dependent changes were seen in the phase of the HVOR. The phases were delayed by about 30° at low frequencies (0.17–0.33 Hz), and about 10° at the highest frequency (0.50 Hz) (P < 0.001 by two-way ANOVA, data not shown).
Figure 4.
Eye movement dynamics and adaptation in intact, flocculus (FL)- and olivary (IO)-lesioned mice. Lesions in the flocculi and the inferior olivary nuclei did not significantly affect the gains of the HOKR (A) and HVOR (B). The P-values were obtained by the two-way ANOVA. (C) Lesions of the flocculi and the inferior olivary nuclei abolished the adaptation of the HOKR. The screen was oscillated at a maximum velocity of 5.2°/s (10° at 0.17 Hz). ∗, P < 0.05, Student’s t test.
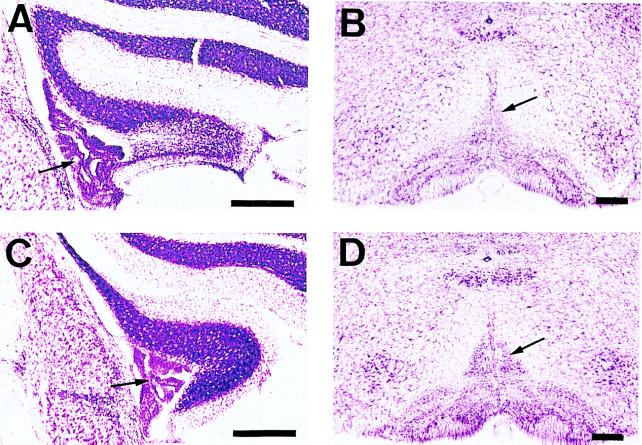
Microscopic inspections revealed that substantial numbers of the granule cells were destroyed and Purkinje cells disappeared after microinjection of ibotenic acid into the flocculi (Fig. 5A). The injection preferentially injured middle and caudal parts of the flocculus, where 20–60% of the granule cells were lost. As a consequence, the flocculus slightly shrank. Large fractions of the inferior olivary nuclei involving medial accessory nuclei cell groups were deformed because of the degeneration of neurons after the application of 3-AP (Fig. 5B). To quantify the lesions, the cell numbers within the dorsal cap of Kooy were numerated from three representative sections per mouse. The relative cell numbers in the dorsal cap of Kooy in the treated mice were decreased by 35.1 ± 0.05% (n = 8) compared with those of C57BL/6 untreated mice (n = 2). We did not detect abnormalities in any other regions in the brains of these mice.
Figure 5.
Extents of floccular and inferior olivary lesions. (A) Coronal section in the middle part of the rostrocaudal extent of the lesioned flocculus, ipsilateral to the observed eye, 7 days after local injection of 0.2 μl of 0.1% ibotenic acid. Note marked cell loss about 60% in granular and Purkinje cell layers. (B) Coronal section of the lesioned inferior olivary nuclei 21 days after i.p. injection of 500 mg/kg of 3-AP followed by 500 mg/kg of nicotinamide. The loss and degeneration of the neurons in the inferior olivary nuclei involving the medial accessory nuclei cell groups are clearly seen. (C) and (D) The intact flocculus and inferior olivary nuclei, respectively. Arrows in A and C indicate choroidal plexus of the IVth ventricle. Arrows in B and D indicate dorsal cap of Kooy. (Bars, 250 μm.)
DISCUSSION
In this study, we quantitatively evaluated the dynamic characteristics of mouse HOKR and HVOR. The HVOR gain in C57BL/6 mice (around 0.5) was the same as those of pigmented rabbits (0.4–0.6, ref. 13), chickens (0.4–0.6, ref. 15), and rats (0.4–0.7, ref. 16), whereas the gains of the HOKR at low-velocity screen oscillations were low (0.5) in C57BL/6 mice compared with other afoveate animals. The gain of the HOKR at low-velocity screen oscillation is known to be almost unity (0.8–1.0) in pigmented rabbits (13), cats (17), and goldfish (18). The dynamic characteristics of the HOKR were not reported in other animals. Sustained optokinetic stimulation in the presence of sufficient retinal slips induced adaptation of the HOKR in C57BL/6 mice. The adaptation of the HOKR by sustained screen oscillations has been reported in pigmented rabbits (13, 19, 20) and goldfish (21).
The 129/Sv mice showed a very low gain in the HVOR but not in the HOKR. In contrast, 129/Sv mice also showed a large delay in the phase of the HOKR but not of the HVOR. The neural circuitry for the HVOR and HOKR is supposed to be common both in the brainstem and cerebellar flocculus reflex path, because some groups of vestibular nuclear neurons (cat, ref. 22; monkey, ref. 23) and flocculus Purkinje cells (rabbit, ref. 24) exhibit strong responses both to vestibular and optokinetic stimulations. Thus, the deficits in the HVOR gain in 129/Sv mice seem more likely to be caused by dysfunctions in the vestibular side rather than to those in the motor side of the vestibulo-ocular system. With the same reasons, we assume that dysfunctions in the visual systems involving the retina or accessory optic pathways, which send visual inputs to the vestibular nuclear or floccular neurons, cause the anomaly in the HOKR phase. Previous studies implied that the phase of eye movements is controlled independently of the gain of eye movements, because lesions in area I of the hindbrain selectively impaired the phase of the HVOR in goldfish (25). Similar results were reported in prepositus hypoglossi nucleus-lesioned cats (26). The present data suggest that the gain and phase of the HOKR are also under the control of independent neural mechanisms.
The 129/Sv mouse is known to exhibit several genetically based disorders in cognitive behaviors, i.e., in the water navigation task (9). Embryonic stem cells derived from the 129 strains are widely used to generate gene-knockout mice. Thus, for the analysis of behavior including sensory-motor tasks in knockout mice derived from the 129 strains, sufficient backcrossing to the C57BL/6 strain seems to be necessary.
The HOKR and HVOR are known to be adaptively modified by 1–3 h of simple visual-vestibular or visual stimulations in the presence of a sufficient amount of retinal slips. Adaptations of the HVOR gain are known in rabbits (13), monkeys (27, 28), rats (16), and goldfish (29), and adaptations of the HOKR are known in rabbits (13, 19, 20) and goldfish (21). Lesion studies consistently suggest that the cerebellar flocculus is essential for the adaptation of the HVOR and HOKR in rabbits (13) and for the adaptation of the HVOR in monkeys (30). Unit recording studies revealed neuronal responses well correlated with the adaptation of the HVOR (rabbit, ref. 31; monkey, ref. 28) and HOKR (rabbit, ref. 20) in a group of flocculus Purkinje cells. Lesions of the inferior olivary nuclei or the severance of visual pathways to the inferior olivary nuclei are known to abolish adaptation of the HVOR in rabbits (32) and rats (16). The lack of adaptation of the HOKR in flocculus-lesioned mice suggests that the flocculus plays an essential role in the adaptation of the HOKR in mice as well as in other animals. The lesions preferentially located in the middle and caudal parts of the flocculus. The results seem to be consistent with the previous findings that the neuronal activities responding to the optokinetic stimulation were most prominent in the middle sections of the rostrocaudal extent of the flocculus in rat (33). It should be noted that the paraflocculus-lesioned mice showed the adaptation of the HOKR. Furthermore, the mice i.p. injected with 3-AP failed to show the adaptation of the HOKR. Because 3-AP selectively destroyed neurons in the inferior olivary nuclei, the inferior olivary nuclei seem to also play a crucial role in the adaptation of the HOKR. Thus, we suggest that the main neuronal circuitry for the reflex eye movements is phylogenetically conserved. However, subtle differences may exist among species. For instance, flocculus lesions reduce the gains of the HVOR and HOKR in rabbits (13), but not in mice, varied the gain of the HVOR inconsistently in monkeys (30). The species difference in the effects of lesions on the HVOR are supposed to be induced by the relative strength of the out-of-phase and in-phase modulating flocculus Purkinje cells, which, respectively, act to enhance and depress the gain of the HVOR, in these animal species. Actually, the population of the out-of-phase modulating Purkinje cells are dominant in rabbits (20), but equivalent to that of the in-phase modulating Purkinje cells in monkeys (34) within the flocculus. Furthermore, we found that the HVOR, but not HOKR, in mice, was highly sensitive to external somatic stimuli. This is not the case in rabbits. The result may imply that neural circuitry of the HVOR is much more susceptible to the effects of the other neural circuitry than that of the HOKR. For instance, the extracerebellar neural circuitry may play a role in the eyeblink conditioning at a certain condition (35).
Several lines of experimental evidence, thus far obtained for various animal species, seem to be consistent with the suggestion that the adaptations of the HVOR and HOKR are caused by plastic changes within the flocculus (36). Chemical agents (hemoglobin or l-NG-monomethyl-N-arginine), which have been shown to interfere with the LTD of the parallel fiber-Purkinje cell synapses by the climbing fiber inputs in slice preparations, actually blocked the adaptation of the HVOR in the rabbit and monkey (37) and the goldfish (38). A different view is proposed regarding the role of the monkey flocculus (39) in the adaptation of the HVOR. Lisberger et al. suggested that a group of brain stem cells play a larger role than the flocculus during the adaptation of HVOR. However, some of the experimental observations on which this view is based seem to relate to the paraflocculus rather than the flocculus (34, 40).
Gene-knockout techniques have produced mice with defects in the type 1 metabotropic glutamate receptor (4), the δ2 subunit of the glutamate receptor (5), the γ isoform of C kinase (7), and glial fibrillary acidic proteins (8). Some of these mice show ataxic movement disorders and/or acquisition of the conditioned eyeblink response, and also deficiencies in the synaptic plasticity in cerebellar neurons (LTD) and/or an anomaly in the cerebellar circuit genesis. Whether these mice also show impairment of the adaptation of the reflex eye movements, which is a most primitive model of motor learning, should be clarified in future experiments.
Acknowledgments
We thank Drs. J. Yamada and T. Kitamura (Tokyo Medical College), M. Yamakado, K. Takahashi, and K. Kitamura (Jichi Medical School) for their technical advice and discussions regarding histology. We thank all members of our laboratories for their encouragement and discussions. This work was supported by grants from the Shionogi Institute for Medical Science and the ministry of Health to S.I., and a Grant-in-aid from the ministry of Science, Education and Culture (09680799) and a grant from Toyota Riken to S.N.
ABBREVIATIONS
- HVOR
horizontal vestibulo-ocular reflex
- HOKR
horizontal optokinetic response eye movement
- LTD
long-term depression
- 3-AP
3-acetylpyridine
References
- 1.Grant S G N, O’Dell T J, Karl K A, Stein P L, Soriano P, Kandel E R. Science. 1992;258:1903–1910. doi: 10.1126/science.1361685. [DOI] [PubMed] [Google Scholar]
- 2.Takahashi J S, Pinto L H, Vitaterna M H. Science. 1994;264:1724–1733. doi: 10.1126/science.8209253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen C, Tonegawa S. Annu Rev Neurosci. 1997;20:157–184. doi: 10.1146/annurev.neuro.20.1.157. [DOI] [PubMed] [Google Scholar]
- 4.Aiba A, Kano M, Chen C, Stanton M E, Fox G D, Herrup K, Zwingmann T A, Tonegawa S. Cell. 1994;79:377–388. [PubMed] [Google Scholar]
- 5.Kashiwabuchi N, Ikeda K, Araki K, Hirano T, Shibuki K, Takayama C, Inoue Y, Kutsuwada T, Yagi T, Kang Y, et al. Cell. 1995;81:245–252. doi: 10.1016/0092-8674(95)90334-8. [DOI] [PubMed] [Google Scholar]
- 6.Kano M, Hashimoto K, Chen C, Abeliovich A, Aiba A, Kurihara H, Watanabe M, Inoue Y, Tonegawa S. Cell. 1995;83:1223–1231. doi: 10.1016/0092-8674(95)90147-7. [DOI] [PubMed] [Google Scholar]
- 7.Chen C, Kano M, Abeliovich A, Chen L, Bao S, Kim J J, Hashimoto K, Thompson R F, Tonegawa S. Cell. 1995;83:1233–1242. doi: 10.1016/0092-8674(95)90148-5. [DOI] [PubMed] [Google Scholar]
- 8.Shibuki K, Gomi H, Chen L, Bao S, Kim J J, Wakatsuki H, Fujisaki T, Fujimoto K, Katoh A, Ikeda T, et al. Neuron. 1996;16:587–599. doi: 10.1016/s0896-6273(00)80078-1. [DOI] [PubMed] [Google Scholar]
- 9.Gerlai R. Trends Neurosci. 1996;19:177–181. doi: 10.1016/s0166-2236(96)20020-7. [DOI] [PubMed] [Google Scholar]
- 10.Ito M, Nagao S. Comp Biochem Physiol. 1991;98:221–228. [PubMed] [Google Scholar]
- 11.Nagao S. Neurosci Res. 1990;8:210–213. doi: 10.1016/0168-0102(90)90022-7. [DOI] [PubMed] [Google Scholar]
- 12.Jastreboff P J. Biol Cybern. 1979;33:113–120. doi: 10.1007/BF00355259. [DOI] [PubMed] [Google Scholar]
- 13.Nagao S. Exp Brain Res. 1983;53:36–46. doi: 10.1007/BF00239396. [DOI] [PubMed] [Google Scholar]
- 14.Jones N, Marec N L, Stelz T, Caston J. Brain Res. 1994;656:257–262. doi: 10.1016/0006-8993(94)91468-0. [DOI] [PubMed] [Google Scholar]
- 15.Wallman J, Velez J, Weinstein B, Green A E. J Neurophysiol. 1982;48:952–967. doi: 10.1152/jn.1982.48.4.952. [DOI] [PubMed] [Google Scholar]
- 16.Tempia F, Dieringer N, Strata P. Exp Brain Res. 1991;86:568–578. doi: 10.1007/BF00230530. [DOI] [PubMed] [Google Scholar]
- 17.Godaux E, Gobert C, Halleux J. Exp Neurol. 1983;80:42–54. doi: 10.1016/0014-4886(83)90005-5. [DOI] [PubMed] [Google Scholar]
- 18.Marsh E, Baker R. J Neurophysiol. 1997;77:1099–1118. doi: 10.1152/jn.1997.77.3.1099. [DOI] [PubMed] [Google Scholar]
- 19.Collewijn H, Grootendorst A F. Prog Brain Res. 1979;50:771–781. doi: 10.1016/S0079-6123(08)60874-2. [DOI] [PubMed] [Google Scholar]
- 20.Nagao S. Exp Brain Res. 1988;73:489–497. doi: 10.1007/BF00406606. [DOI] [PubMed] [Google Scholar]
- 21.Schairer J O, Bennett V L. Brain Res. 1986;373:177–181. doi: 10.1016/0006-8993(86)90328-8. [DOI] [PubMed] [Google Scholar]
- 22.Keller E L, Precht W. Neuroscience. 1979;4:1599–1613. doi: 10.1016/0306-4522(79)90023-x. [DOI] [PubMed] [Google Scholar]
- 23.Waespe W, Henn V. Exp Brain Res. 1977;27:523–538. doi: 10.1007/BF00239041. [DOI] [PubMed] [Google Scholar]
- 24.Nagao S. Exp Brain Res. 1990;80:221–224. doi: 10.1007/BF00228867. [DOI] [PubMed] [Google Scholar]
- 25.Pastor A M, De La Cruz R R, Baker R. Proc Natl Acad Sci USA. 1994;91:807–811. doi: 10.1073/pnas.91.2.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheron G, Godaux E, Laune J M, Vanderkelen B. J Physiol. 1986;372:75–94. doi: 10.1113/jphysiol.1986.sp015998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miles F A, Eighmy B B. J Neurophysiol. 1980;43:1406–1425. doi: 10.1152/jn.1980.43.5.1406. [DOI] [PubMed] [Google Scholar]
- 28.Watanabe E. Neurosci Res. 1985;3:20–38. doi: 10.1016/0168-0102(85)90036-7. [DOI] [PubMed] [Google Scholar]
- 29.Schairer J O, Bennett V L. Brain Res. 1986;373:164–176. doi: 10.1016/0006-8993(86)90327-6. [DOI] [PubMed] [Google Scholar]
- 30.Lisberger S G, Miles F A, Zee D S. J Neurophysiol. 1984;52:1140–1153. doi: 10.1152/jn.1984.52.6.1140. [DOI] [PubMed] [Google Scholar]
- 31.Nagao S. Exp Brain Res. 1989;77:531–540. doi: 10.1007/BF00249606. [DOI] [PubMed] [Google Scholar]
- 32.Ito M, Miyashita Y. Proc Japan Acad. 1975;512:716–720. [Google Scholar]
- 33.Mori K, Miyashita Y. Neurosci Res. 1988;5:258–264. doi: 10.1016/0168-0102(88)90054-5. [DOI] [PubMed] [Google Scholar]
- 34.Nagao S. NeuroReport. 1992;3:13–16. doi: 10.1097/00001756-199201000-00003. [DOI] [PubMed] [Google Scholar]
- 35.Thompson R F, Kim J J. Proc Natl Acad Sci USA. 1996;93:13438–13444. doi: 10.1073/pnas.93.24.13438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ito M. The Cerebellum and Neural Control. New York: Raven; 1984. [Google Scholar]
- 37.Nagao S, Ito M. NeuroReport. 1991;2:193–196. doi: 10.1097/00001756-199104000-00008. [DOI] [PubMed] [Google Scholar]
- 38.Li J, Smith S S, McElligott J G. J Neurophysiol. 1995;74:489–494. doi: 10.1152/jn.1995.74.1.489. [DOI] [PubMed] [Google Scholar]
- 39.Lisberger S G, Pavelko T A, Broussard D M. J Neurophysiol. 1994;72:909–927. doi: 10.1152/jn.1994.72.2.909. [DOI] [PubMed] [Google Scholar]
- 40.Nagao S, Kitamura T, Nakamura N, Hiramatsu T, Yamada J. J Comp Neurol. 1997;382:480–498. doi: 10.1002/(sici)1096-9861(19970616)382:4<480::aid-cne5>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]