Abstract
Escherichia coli and Salmonella typhimurium strains grown in Luria–Bertani medium containing glucose secrete a small soluble heat labile organic molecule that is involved in intercellular communication. The factor is not produced when the strains are grown in Luria–Bertani medium in the absence of glucose. Maximal secretion of the substance occurs in midexponential phase, and the extracellular activity is degraded as the glucose is depleted from the medium or by the onset of stationary phase. Destruction of the signaling molecule in stationary phase indicates that, in contrast to other quorum-sensing systems, quorum sensing in E. coli and S. typhimurium is critical for regulating behavior in the prestationary phase of growth. Our results further suggest that the signaling factor produced by E. coli and S. typhimurium is used to communicate both the cell density and the metabolic potential of the environment. Several laboratory and clinical strains of E. coli and S. typhimurium were screened for production of the signaling molecule, and most strains make it under conditions similar to those shown here for E. coli AB1157 and S. typhimurium LT2. However, we also show that E. coli strain DH5α does not make the soluble factor, indicating that this highly domesticated strain has lost the gene(s) or biosynthetic machinery necessary to produce the signaling substance. Implications for the involvement of quorum sensing in pathogenesis are discussed.
Intercellular communication is used to regulate a wide variety of processes in bacteria, including quorum sensing in luminous Vibrio (1–3), competence and sporulation in Bacillus (4), and sporulation and motility in Myxococcus (5, 6). In each case, cell–cell communication is mediated by the synthesis, secretion, and detection of small extracellular signaling molecules. The signaling substances are distinct for the specific processes; acyl-homoserine lactone autoinducers regulate density sensing in Vibrio, peptides are used to induce competence and sporulation in Bacillus, and a complex mixture of amino acids and fragments of peptidoglycan control sporulation, social, and asocial motility in Myxococcus. In the case of the quorum- sensing bacterium Vibrio harveyi, two independent cell–cell communication systems are used to control luminescence (lux) expression in response to cell density (7). One of the V. harveyi systems (signaling system 1) is a high specificity, species-specific system, and the sensor responds to an acyl-homoserine lactone signal. The second system (signaling system 2) is a species-nonspecific system, and the signaling molecule(s) for this system has not yet been identified (8).
There have been preliminary indications that E. coli senses cell density (9–11). We took advantage of the species nonselectivity of the signaling system 2 sensor in V. harveyi to develop a sensitive assay for detection of extracellular signal molecules produced by E. coli and S. typhimurium. By using this assay we could determine the conditions under which many strains of E. coli and S. typhimurium synthesize, secrete, and degrade a signaling substance that will interact with the V. harveyi system 2 detector.
MATERIALS AND METHODS
Preparation of Cell-Free Culture Fluids.
E. coli strains AB1157 and DH5α and S. typhimurium strain LT2 were grown at 30°C overnight with aeration in Luria–Bertani (LB) broth containing glucose at the concentrations specified in the text. The next morning fresh LB medium containing the same concentration of glucose used for the overnight growth was inoculated at a 1:100 dilution with the overnight-grown cultures. The fresh cultures were grown for various times at 30°C with aeration. Cell-free culture fluids were prepared by removing the cells from the growth medium by centrifugation at 15,000 rpm for 5 min in a microcentrifuge. The cleared culture fluids were passed through 0.2-μm HT Tuffryn filters (Gelman) and stored at −20°C. Cell-free culture fluids containing V. harveyi autoinducer-2 were prepared from V. harveyi strain BB152 (autoinducer 1−, autoinducer 2+). V. harveyi BB120 (autoinducer 1+, autoinducer 2+) was used to prepare culture fluids containing autoinducer-1. In both cases, the V. harveyi strains were grown overnight at 30°C with aeration in AB (autoinducer bioassay) (7) medium. Cell-free culture fluids from V. harveyi were prepared from the overnight culture exactly as described above for E. coli and S. typhimurium.
Assay for Production of Signaling Molecules.
Cell-free culture fluids from E. coli, S. typhimurium, and V. harveyi strains were tested for the presence of signaling substances that could induce luminescence in the V. harveyi reporter strain BB170 or BB886. In the assays, 10 μl of cell-free culture fluids from E. coli AB1157, E. coli DH5α, and S. typhimurium LT2 strains grown and harvested as described above were added to 96-well microtiter dishes. The V. harveyi reporter strain BB170 or BB886 was grown for 16 hr at 30°C with aeration in AB medium and diluted 1:5,000 into fresh AB medium, and 90 μl of the diluted cells was added to the wells containing the E. coli and S. typhimurium cell-free culture fluids. Positive control wells contained 10 μl of cell-free culture fluid from strain V. harveyi BB152 (autoinducer-1−, autoinducer-2+) or V. harveyi BB120 (autoinducer-1+, autoinducer-2+). Negative control wells contained 10 μl of sterile growth medium. The microtiter dishes were shaken in a rotary shaker at 175 rpm at 30°C. Every hour, light production was measured by using a Wallac (Gaithersburg, MD) Model 1450 Microbeta Plus liquid scintillation counter in the chemiluminescence mode. The V. harveyi cell density was measured by diluting the same aliquots of cells used for measuring luminescence, spreading the dilutions onto solid Luria-marine medium (7), incubating the plates overnight at 30°C, and counting the resulting colonies the next day.
Preparation of E. coli and S. typhimurium Viable and UV-Killed Cells for the Activity Assay.
E. coli AB1157, E. coli DH5α, and S. typhimurium LT2 cultures were grown for 8 hr in LB medium containing 0.5% glucose at 30°C with aeration. The cultures were subjected to centrifugation for 5 min at 15,000 rpm in a microcentrifuge, and the growth medium was removed from the cell pellets by aspiration. The cell pellets were resuspended in AB medium and washed by vigorous mixing. The cells were again subjected to centrifugation for 5 min at 15,000 rpm. The AB wash medium was removed and discarded, and the cells were resuspended in fresh AB medium. Each cell suspension was diluted to give 1 × 106 cells/10 μl, and multiple 10-μl aliquots were added to wells of microtiter dishes. Half of the cell aliquots were treated with short- wavelength UV light for 15 min at a distance of 10 cm. This treatment was sufficient to kill all of the cells as judged by plating and incubating the UV-treated cells, and ensuring that no growth occurred by the next day. Ninety microliters of the diluted V. harveyi reporter strain BB170 was next added to the wells containing either the viable or dead E. coli and S. typhimurium cells, and the activity assay was carried out exactly as described in the previous section.
Analysis of Glucose in S. typhimurium LT2 Culture Fluids.
Glucose concentrations were determined in cell-free culture fluids prepared from S. typhimurium by using a Trinder assay (Diagnostic Chemicals, Oxford, CT) according to the recommendations of the manufacturer, except that the glucose standards were prepared in LB medium. The assay was sensitive to less than 0.002% glucose. No interfering substances were present in LB medium or spent LB culture fluids.
RESULTS AND DISCUSSION
E. coli AB1157 and S. typhimurium LT2 Produce a Signaling Substance That Induces One Specific Quorum-Sensing System of V. harveyi.
The V. harveyi reporter strain BB170 has the quorum-sensing phenotype sensor 1−, sensor 2+. It induces lux expression in response to extracellular signals that act exclusively through the signaling system 2 detector (7). Addition of 10% cell-free spent culture fluid prepared from V. harveyi strain BB152 (which contains the system 2 autoinducer) stimulates the reporter strain roughly 1,000-fold over the endogenous level of luminescence expression. In Fig. 1, the light production by V. harveyi BB170 induced by the addition of 10% cell-free spent culture fluids is normalized to 100% activity.
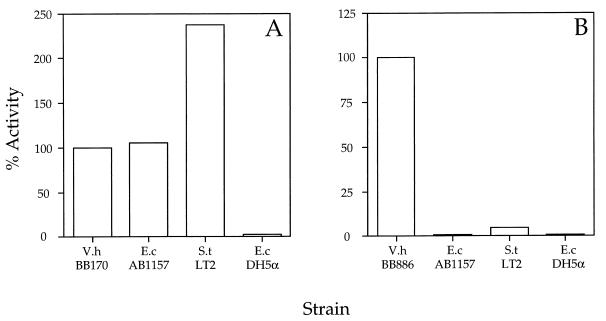
Figure 1.
E. coli AB1157 and S. typhimurium LT2 cell-free culture fluids contain a signaling substance that induces luminescence in V. harveyi. The responses of V. harveyi reporter strains BB170 (sensor 1−, sensor 2+) (A), and BB886 (sensor 1+, sensor 2−) (B) to signaling substances present in cell-free culture fluids from E. coli, S. typhimurium, and V. harveyi strains are shown. A bright culture of each reporter strain was diluted 1:5,000 into fresh medium, and the light production per cell then was measured during the growth of the diluted culture. Cell-free culture fluids or sterile growth medium were added at a final concentration of 10% (vol/vol) at the start of the experiment. The data for the 5-hr time point are shown and are presented as the percent of the activity obtained when V. harveyi cell-free spent culture fluids are added. V.h, V. harveyi; S.t, S. typhimurium, and E.c, E. coli.
E. coli strain AB1157 and S. typhimurium strain LT2 were grown for 8 hr in LB broth or LB broth containing 0.5% glucose. The E. coli and S. typhimurium cells were removed from the growth medium, and the cell-free culture fluids were prepared and assayed for an activity that could induce luminescence expression in V. harveyi. Addition of 10% cell-free culture fluid from S. typhimurium LT2 or E. coli AB1157 grown in LB medium containing glucose maximally induced luminescence in the reporter strain BB170, similar to culture fluids from V. harveyi BB152 (Fig. 1A). Specifically, E. coli AB1157 produced 106% and S. typhimurium produced 237% of the V. harveyi BB152 activity. When the E. coli and S. typhimurium were grown in LB medium without added glucose they did not produce the signaling factor. Substitution of 10% (vol/vol) of LB medium containing 0.5% glucose did not stimulate luminescence in the reporter strain, indicating that there is no substance in the LB-glucose growth medium that induces luminescence expression in V. harveyi. We tested obvious candidates for the signal, including glucose, amino acids, cAMP, acetate, homoserine lactone, α-ketoglutarate, and other keto acids that are known to be excreted (12). None of these compounds has activity. These results suggest that V. harveyi BB170 can respond to some substance secreted by E. coli AB1157 and S. typhimurium LT2 when they are grown on LB medium containing glucose.
Analogous experiments were performed with the V. harveyi reporter strain BB886 (sensor 1+, sensor 2−). V. harveyi BB886 is defective in its response to signaling molecules that act through the signaling system 2 detector, but it is an otherwise wild-type strain (13). Fig. 1B shows the normalized 100% activation of V. harveyi BB886 by cell-free spent culture fluids prepared from V. harveyi BB120. V. harveyi BB120 produces the system 1 autoinducer N-(3-hydroxybutanoyl)-l-homoserine lactone (7). Addition of S. typhimurium LT2 and E. coli AB1157 cell-free culture fluids to V. harveyi strain BB886 caused a 5% and a 1% increase, respectively, above the control level (Fig. 1B). Together the results of Fig. 1 A and B show that the signaling molecule produced by E. coli and S. typhimurium must act specifically through V. harveyi signaling system 2 and not some other, unidentified pathway.
Viable E. coli AB1157 and S. typhimurium LT2 Are Required for Secretion of the Signaling Molecule.
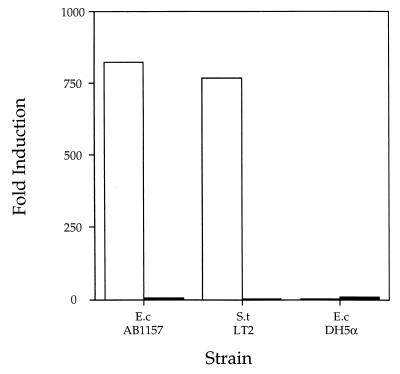
We considered the possibility that growth of E. coli AB1157 and S. typhimurium LT2 in LB medium containing glucose simply allowed them to use and therefore remove some pre-existing inhibitor of induction of luminescence. To show that the cells themselves produce the soluble signaling factor, we added washed E. coli and S. typhimurium cells directly to the luminescence assay. These results are presented in Fig. 2. In this experiment, E. coli AB1157 and S. typhimurium LT2 were grown for 8 hr in LB medium containing 0.5% glucose, the conditions for maximal production of the signaling factor. The cells were removed from the LB-glucose growth medium by centrifugation, and sterile V. harveyi luminescence assay medium was used to wash and resuspend the cell pellets. E. coli AB1157 or S. typhimurium LT2 cells (1 × 106) were added to the diluted V. harveyi BB170 culture at the start of the experiment. In Fig. 2, the empty bar in each series shows that the presence of washed E. coli AB1157 or S. typhimurium LT2 cells is sufficient to fully induce luminescence in V. harveyi BB170. E. coli AB1157 and S. typhimurium LT2 stimulated lux expression in V. harveyi BB170 821-fold and 766-fold, respectively. Identical aliquots of the washed E. coli or S. typhimurium cells were killed with short-wave UV light before addition to the assay. When dead cells were included in the assay, no stimulation of luminescence occurred. In Fig. 2, these results are shown in the filled bars for each strain. Taken together, the results show that the stimulatory factor is produced by the E. coli AB1157 and S. typhimurium LT2 cells themselves during the time course of the experiment; the factor could not have come from the medium in which the cells had been grown. This factor is actively released into the medium by E. coli and S. typhimurium because dead cells have no activity.
Figure 2.
Viable E. coli and S. typhimurium actively secrete the signaling molecule. The response of the V. harveyi reporter strain BB170 (sensor 1−, sensor 2+) to a signaling substance produced and secreted by E. coli AB1157 and S. typhimurium LT2 but not E. coli DH5α is shown. V. harveyi reporter strain BB170 was diluted 1:5,000 in AB medium and light output per cell was monitored during growth. At the start of the experiment, either 1 × 106 E. coli AB1157, S. typhimurium LT2, or E. coli DH5α washed and resuspended viable cells (empty bars) or UV-killed cells (filled bars) was added. The data are presented as the fold-activation above the endogenous level of luminescence expressed by V. harveyi BB170 at the 5-hr time point. S.t, S. typhimurium; E.c, E. coli. Results for replicates were within 10%.
E. coli DH5α Does Not Produce the Signaling Activity.
Clinical isolates of E. coli and Salmonella also produce the signaling compound. Ten clinical isolates of Salmonella and five pathogenic isolates of E. coli O157 were assayed, and all produced the activity (not shown). It was conceivable that the signal was some normal byproduct of glucose metabolism that simply diffuses out of the cells. This postulation is not the case, however, because we show that E. coli DH5α, which is equally capable of using glucose as E. coli AB1157 and S. typhimurium LT2 (14), does not produce the signaling activity. Fig. 1A demonstrates that unlike E. coli AB1157 and S. typhimurium LT2, the addition of 10% cell-free culture fluid prepared from E. coli DH5α grown 8 hr in LB medium containing 0.5% glucose does not stimulate light production in V. harveyi BB170. Similarly, inclusion of washed viable or killed E. coli DH5α cells in the luminescence assay does not stimulate V. harveyi BB170 to produce light (Fig. 2). The inability of E. coli DH5α to produce the activity indicates that this highly domesticated strain lacks the gene or genes necessary for either the production or the export of the signaling activity. We assayed other laboratory strains of E. coli for the signaling activity (Table 1). Only E. coli DH5α was completely defective in producing the extracellular signal.
Table 1.
The induction of luminescence in V. harveyi reporter strain BB170 by cell-free culture fluids from V. harveyi, S. typhimurium, and E. coli
| Species and strain | Induction of luminescence, % |
|---|---|
| V. harveyi | |
| V. harveyi BB152 | 100 |
| Salmonella | |
| S. typhirmurium LT2 | 237 |
| E. coli | |
| E. coli AB1157 | 106 |
| E. coli DH5α | 5 |
| E. coli JM109 | 76 |
| E. coli MG1655 | 100 |
| E. coli MC4100 | 93 |
Cell-free culture fluids were prepared from various strains of V. harveyi, S. typhimurium, and E. coli as described and tested for production of a signaling substance that could stimulate light production in the reporter strain. V. harveyi BB170. The level of V. harveyi stimulation was normalized to 100%. The data for the 5-hr time point are shown.
Glucose Regulates the Production and Degradation of the Signaling Factor by S. typhimurium LT2.
Cell-free culture fluids from S. typhimurium LT2 and E. coli AB1157 cells grown in LB medium without added glucose did not stimulate the expression of luminescence in the reporter strain, indicating that metabolism of glucose is necessary for the production of the signal. We tested other carbohydrates, including mannose, mannitol, fructose, glucosamine, sucrose, and maltose. In general, growth in the presence of phosphotransferase system (PTS) sugars (15) enabled E. coli AB1157 and S. typhimurium LT2 to produce the signal. Of the sugars tested, growth on glucose induced the synthesis of the highest level of activity. Growth on other carbon sources, for example tricarboxylic acid cycle intermediates and glycerol, did not induce significant production of the signaling activity.
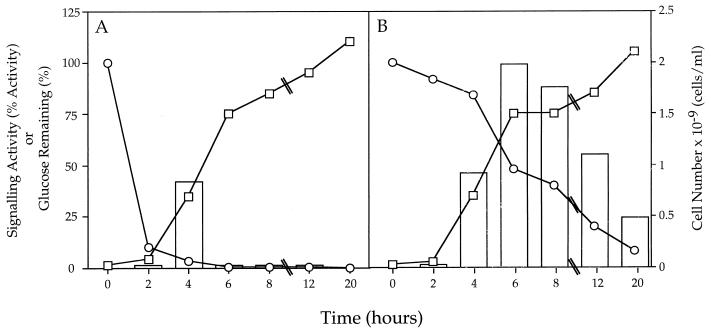
We tested whether the presence of glucose was required for the cells to continue to produce the signal. Fig. 3 shows results with S. typhimurium LT2 grown in LB medium containing limiting (0.1%) and nonlimiting (0.5%) glucose concentrations. Fig. 3A shows that when glucose is limiting, S. typhimurium LT2 produces the signal in midexponential phase (after 4 hr of growth), but stops producing the signaling activity once glucose is depleted from the medium. Fig. 3B shows that when glucose does not become limiting, S. typhimurium LT2 produces greater total activity and continues to produce the signaling activity throughout exponential phase, with maximal activity at 6 hr of growth. Furthermore, Fig. 3 also shows that the signaling activity synthesized by midexponential phase cells is degraded by the time the cells reach stationary phase. In conditions of limiting glucose, no activity remained at stationary phase, and when glucose was plentiful, only 24% of the activity remained. Increasing the concentration of glucose in the growth medium did not change these results, i.e., the activity was secreted during midexponential growth and severely reduced activity remained in the spent culture fluids by stationary phase.
Figure 3.
Effect of glucose depletion on the production and degradation of the signaling activity by S. typhimurium LT2. S. typhimurium LT2 was grown in LB medium containing either 0.1% glucose (A) or 0.5% glucose (B). At the specified times cell-free culture fluids were prepared and assayed for signaling activity in the luminescence stimulation assay (bars), and the concentration of glucose remaining (○). The cell number was determined at each time by diluting and plating the S. typhimurium LT2 on LB medium and counting colonies the next day (□). The signaling activity is presented as the percent of the activity obtained when V. harveyi cell-free spent culture fluids are added. These data correspond to the 5-hr time point in the luminescence stimulation assay. The glucose concentration is shown as % glucose remaining. Cell number is cells/ml × 10−9. ⑊ indicates that the time axis is not drawn to scale after 8 hr. Replicate samples agreed within 10%.
A Possible Role for Quorum Sensing in E. coli and S. typhimurium.
Our results show that E. coli and S. typhimurium produce a signaling substance that stimulates one specific quorum-sensing system in V. harveyi. Many other bacteria previously have been assayed for such an activity, and only rarely were species identified that are positive for production of this factor (8). Furthermore, as shown here, the E. coli and S. typhimurium signal is potent; these bacteria make activity equal to that of V. harveyi. The degradation of the E. coli and S. typhimurium signal before stationary phase indicates that quorum sensing in these bacteria is tuned to a lower cell density community than described in other quorum-sensing bacteria. This result suggests that quorum sensing in E. coli and S. typhimurium is modulated so that the response to the signal does not persist into stationary phase. Additionally, quorum sensing in E. coli and S. typhimurium is influenced by several environmental factors. The production and the degradation of the signal are sensitive not only to growth phase but also to the metabolic activity of the cells. These results indicate that the quorum-sensing signal in E. coli and S. typhimurium has two functions; it allows the cells to communicate their growth phase and also the metabolic potential of the environment to one another.
Understanding the regulation of quorum sensing in E. coli and S. typhimurium could be important for understanding community structure and cell–cell interactions in pathogenesis. In the wild, pathogenic E. coli and S. typhimurium may never reach stationary phase because dispersion is critical. It is therefore appropriate that quorum sensing in E. coli and S. typhimurium should be functioning before stationary phase. This situation is in contrast to that of V. fischeri, the luminescent marine symbiont, where the quorum-sensing system is only operational at high cell densities, cell densities indicative of existence inside the specialized light organ of the host. The specific quorum-sensing systems of V. fischeri as well as E. coli and S. typhimurium appear appropriately regulated for the niche in which each organism exists. In both cases, quorum sensing could be useful for communicating that the bacteria reside in the host and are not free-living in the environment. Additional complexity exists in the E. coli and S. typhimurium systems because these bacteria channel both cell density information and metabolic cues into the quorum-sensing circuit. Again, signals relaying information regarding the abundance of glucose or other metabolites could communicate to the bacteria that they should undergo the transition from a free-living mode to the mode of existence inside the host.
Purification of the E. coli, S. typhimurium, and V. harveyi signal currently is underway. Under all the conditions we have tested, the activity does not extract quantitatively into organic solvents and it does not bind to either a cation or anion exchange column. Our preliminary characterization indicates that the signal is a small (less than 1,000 Mr) polar, but apparently uncharged, organic compound. The activity is acid stabile and base labile; it is heat resistant to 80°C but not 100°C. Identification of the signaling molecule, the sensory transduction pathway, and downstream targets will enable us to address the different roles that quorum sensing plays in community interactions and intercellular signaling in V. harveyi, E. coli, and Salmonella.
Acknowledgments
We thank Dr. T.J. Silhavy for many informative discussions. This work was supported by a National Science Foundation grant (B.B.) and grants from the Alberta Heritage Foundation for Medical Research and The Medical Research Council of Canada (M.S.).
ABBREVIATIONS
- LB
Luria–Bertani
- AB
autoinducer bioassay
Footnotes
A commentary on this article begins on page 6571.
References
- 1.Engebrecht J, Nealson K, Silverman M. Cell. 1983;32:773–781. doi: 10.1016/0092-8674(83)90063-6. [DOI] [PubMed] [Google Scholar]
- 2.Fuqua W C, Winans S C, Greenberg E P. J Bacteriol. 1994;176:269–275. doi: 10.1128/jb.176.2.269-275.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bassler B L, Silverman M R. In: Two-Component Signal Transduction. Hoch J A, Silhavy T J, editors. Washington, DC: Am. Soc. Microbiol.; 1995. pp. 431–445. [Google Scholar]
- 4.Perego M, Hoch J A. Proc Natl Acad Sci USA. 1996;93:1549–1553. doi: 10.1073/pnas.93.4.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaplan H B, Plamam L. FEMS Microbiol Lett. 1996;139:89–95. doi: 10.1111/j.1574-6968.1996.tb08185.x. [DOI] [PubMed] [Google Scholar]
- 6.Kaiser D, Losick R. Cell. 1993;73:873–885. doi: 10.1016/0092-8674(93)90268-u. [DOI] [PubMed] [Google Scholar]
- 7.Bassler B L, Wright M, Showalter R E, Silverman M R. Mol Microbiol. 1993;9:773–786. doi: 10.1111/j.1365-2958.1993.tb01737.x. [DOI] [PubMed] [Google Scholar]
- 8.Bassler B L, Greenberg E P, Stevens A M. J Bacteriol. 1997;179:4043–4045. doi: 10.1128/jb.179.12.4043-4045.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huisman G W, Kolter R. Science. 1994;265:537–539. doi: 10.1126/science.7545940. [DOI] [PubMed] [Google Scholar]
- 10.Sitnikov D M, Schineller J B, Baldwin T O. Proc Natl Acad Sci USA. 1996;93:336–341. doi: 10.1073/pnas.93.1.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garcia-Lara J, Shang L H, Rothfield L I. J Bacteriol. 1996;178:2742–2748. doi: 10.1128/jb.178.10.2742-2748.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reissbrodt R, Kingsley R, Rabsch W, Beer W, Roberts M, Williams P H. J Bacteriol. 1997;179:4538–4544. doi: 10.1128/jb.179.14.4538-4544.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bassler B L, Wright M, Silverman M R. Mol Microbiol. 1994;13:273–286. doi: 10.1111/j.1365-2958.1994.tb00422.x. [DOI] [PubMed] [Google Scholar]
- 14.Miller J H. A Short Course in Bacterial Genetics: A Laboratory Manual and Handbook for Escherichia coli and Related Bacteria. Plainview, NY: Cold Spring Harbor Lab. Press; 1992. p. 6.18. [Google Scholar]
- 15.Postma P W, Lengeler J W, Jacobson G R. In: Escherichia coli and Salmonella Cellular and Molecular Biology. Neidhardt F C, editor. Washington, DC: Am. Soc. Microbiol.; 1996. pp. 1149–1174. [Google Scholar]