Abstract
The present study used functional magnetic resonance imaging to demonstrate that performance of visual spatial and visual nonspatial working memory tasks involve the same regions of the lateral prefrontal cortex when all factors unrelated to the type of stimulus material are appropriately controlled. These results provide evidence that spatial and nonspatial working memory may not be mediated, respectively, by mid-dorsolateral and mid-ventrolateral regions of the frontal lobe, as widely assumed, and support the alternative notion that specific regions of the lateral prefrontal cortex make identical executive functional contributions to both spatial and nonspatial working memory.
The frontal cortex plays a critical role in both spatial and nonspatial working memory. It has been suggested that there may be domain-specific subdivisions within dorsal and ventral regions of the lateral prefrontal cortex which subserve working memory for spatial and nonspatial information, respectively (1, 2). According to this view, working memory processing within the lateral prefrontal cortex is organized according to the type of information being processed, with the mid-dorsolateral prefrontal region being principally concerned with memory for spatial material and the ventrolateral prefrontal region with nonspatial visual material (1, 2). This hypothesis has formed the theoretical background against which the results of several recent functional neuroimaging studies of working memory in human subjects have been discussed (3–7). An alternative hypothesis, however, proposes that the difference between the mid-dorsolateral and ventrolateral prefrontal cortex does not lie in the type of information being processed but rather in the type of executive process subserved by those regions (8, 9). Specifically, the mid-dorsolateral frontal region is considered to subserve a level of executive processing involving monitoring and active manipulation of information within working memory, regardless of the nature of the stimulus material. According to this “process-specific” model, both spatial and nonspatial working memory may involve the mid-dorsolateral frontal cortex, if the particular tasks being performed demand the type of executive processing subserved by that area (8, 9).
The present study used functional MRI (fMRI) to investigate whether the same, or different, areas of the lateral prefrontal cortex are involved in visual spatial and visual nonspatial working memory when the executive processing is the same. Healthy volunteers were scanned while performing two experimental tasks and two control tasks (Fig. 1). The two experimental tasks had the same executive processing requirements, i.e., monitoring and manipulation of an ongoing series of visual stimuli within working memory, but differed in the type of visual stimuli to be remembered (locations versus abstract patterns). The first experimental task required that subjects continually monitor a sequence of “highlighted” locations on the screen, whereas, in the second experimental task, the same subjects were required to monitor a series of visual patterns presented in the same locations on the screen. The two experimental tasks were carefully matched in terms of the difficulty of their monitoring requirements, but differed in terms of the stimulus material presented (visual spatial versus visual abstract patterns). The requirements of these tasks are similar to those that have been shown to be critical in accounting for the impairment in spatial and nonspatial working memory observed after mid-dorsolateral prefrontal lesions in the monkey (10).
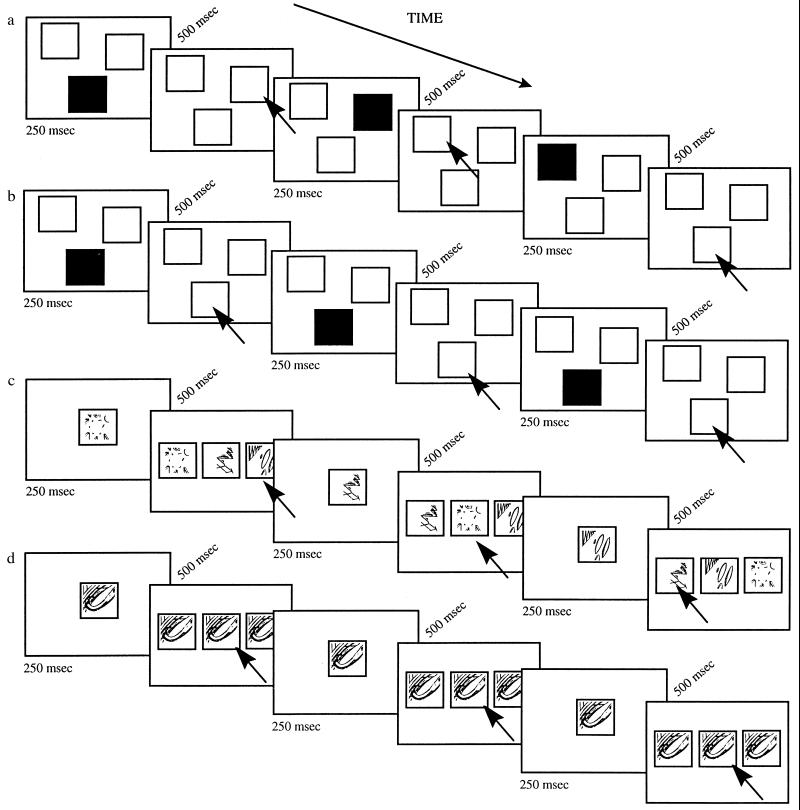
Figure 1.
Illustrated are: (a) the spatial working memory task, (b) the spatial control task, (c) the nonspatial working memory task, and (d) the nonspatial control task. A number of trials are shown in each case. Trials were presented sequentially with a constant 500-ms interval in between. Black arrows, subjects’ responses.
Each experimental task was compared with its own control task which, in each case, involved similar stimuli as the working memory task and required the same type and number of responses. If the type of stimulus material is the critical variable determining which areas of the prefrontal cortex are involved, then the comparison between the spatial working memory task and its control would be expected to yield greater activity in the dorsolateral prefrontal cortex, whereas the comparison between the nonspatial visual pattern working memory task and its control would be expected to involve the ventrolateral prefrontal cortex. In contrast, if the type of executive processing is the critical variable, involvement of the dorsolateral prefrontal cortex would be expected in both tasks given that they were matched in terms of the executive processes assumed to be subserved by that area.
METHODS
Scanning Methods and Data Analysis.
High-speed echo-planar imaging techniques (11, 12), evaluated fMRI signal changes throughout the brain during the four tasks used in the present study. MRI was performed using a high-speed 1.5 Tesla scanner (General Electric Signa scanner, Milwaukee, WI, modified by Advanced NMR Systems, Wilmington, MA). Twenty 7-mm-thick contiguous slices were positioned with 3 × 3 mm in-plane resolution coronally from the frontal pole to the occipital lobe. A series of high-resolution, T1-weighted images was taken for anatomically defining the high-speed functional images. A receive-only radio frequency quadrature head volume coil, an automatic shimming technique, and an asymmetric spin-echo imaging sequence were used (TR = 2500, TE = 50) (13–15). The data for each subject were manually concatenated to produce one continuous data set for the spatial working memory and spatial control tasks (comprising three separate runs) and a second continuous data set for the nonspatial working memory and nonspatial control tasks (also comprising three separate runs).
Task-induced changes in fMRI signal intensity were assessed using a nonparametric statistical analysis procedure that tests whether data acquired during an experimental task are likely to come from the same distribution as data acquired during control tasks (16). This analysis was performed using the following procedure: All slices and time points were reconstructed using unfiltered Fourier transforms from complete k-space data to form a volumetric time series magnitude image dataset. Each successive time point in the volumetric time series was registered to the first time point to compensate for slow motion of the subject’s head that occurred during a scan (17). Every magnitude image in the time series was spatially filtered using a two-dimensional Hamming window, resulting in a voxel size of 6.25 mm × 6.25 mm × 7.0 mm (FWHM). Each voxel location was treated independently to estimate the empirical cumulative distribution functions during the control and experimental states. The point(s) of maximal difference between the two estimated distribution functions, i.e., the Kolmogorov–Smirnov statistic, was computed for each voxel and the probability that this maximal difference could have occurred due to chance for each voxel was assembled into a volumetric probability map. The probability map was converted to a logarithmic color scale and was merged with anatomical images of the same location. Each scan was converted into Talairach space (18) and the coordinates of peak changes were computed.
Testing Procedure.
The visual stimuli were projected, via a computer and a back-projection television system, onto a screen viewed by the subjects lying in the MRI scanner through an overhead mirror. In all tasks, the subjects were requested to fixate on a central marker which, by changing from a − to a + sign, indicated to the subjects when they should respond. The subjects responded by pressing one of three buttons which corresponded to the left, middle, and right locations. There were two experimental tasks (a spatial and a nonspatial working memory task) and two control tasks, one for each experimental task. In the experimental working memory tasks, the subjects were required to monitor continually a sequence of stimuli (locations or abstract patterns) presented on the screen and to respond after each stimulus by selecting one that had been presented earlier in the sequence. In the present study, it was essential that the spatial and nonspatial working memory tasks be matched in terms of the difficulty of their monitoring requirements to be certain that any difference in activation within the frontal cortex could be ascribed to the type of the information being processed. We carried out pilot testing with other normal subjects to find out how to equate the spatial and nonspatial working memory tasks in terms of the difficulty of their monitoring demands. The pilot testing indicated that requiring the subjects to respond after each stimulus by selecting the one that was presented two steps earlier (N-2) in the sequence in the spatial working memory task and one step earlier (N-1) in the nonspatial pattern task ensured high but equal levels of performance in the two tasks. Thus, the spatial and nonspatial working memory tasks were matched in terms of the difficulty of their monitoring requirements but differed in terms of the type of stimulus to be processed (visual spatial versus visual nonspatial).
Before entering the MRI scanner, all subjects received extensive training in the testing procedure, which was as follows for the various tasks. In the spatial working memory task, different locations were highlighted in a continual sequence on the screen. On each trial of the ongoing sequence, one of the three white locations (which were present on the screen throughout the scan) was randomly selected by the computer program and momentarily (250 ms) changed color to black and then back again to white, indicating that it was the next in the series to be remembered (Fig. 1a). Following a 500-ms delay, the subjects responded by pressing the button corresponding not to the box just indicated, but to the one that had appeared two steps earlier in the sequence (i.e., N-2). In doing so, they were required to monitor an ongoing sequence of locations in memory, continually responding to one that had been presented earlier in the series. During the spatial control task, the same three white boxes were present on the screen. Following a 500-ms delay, one box changed color to black and returned to white only once the subject had responded by pressing the corresponding button (Fig. 1b). Thus, in the control condition, the stimuli and response rate were the same as those used during the spatial working memory condition, but without the monitoring requirements of the working memory task. In the nonspatial working memory task, abstract patterns that had been made familiar to the subject prior to scanning were presented in the center of the screen in a continual sequence. On each trial of the ongoing sequence, one of three possible patterns was selected randomly by the computer and appeared in the center of the screen for 250 ms. Following a 500-ms delay, the three patterns were presented simultaneously on the screen, each pattern being randomly positioned within one of the three central boxes (Fig. 1c). The subjects responded by pressing the button corresponding, not to the pattern that they had just seen, but to the one before that (i.e., N-1).
In the nonspatial control task, only one familiar pattern was presented in the central box for 250 ms (Fig. 1d). Following a 500-ms delay, the same pattern was presented simultaneously in the three boxes on the screen and the subject responded by pressing the middle button. Thus, in the control task, the type of stimuli used and the responses required were equivalent to those in the nonspatial working memory task, but without the monitoring requirements of the latter.
Scanning occurred over successive 4-min blocks during which fMRI images were acquired throughout the brain every 2.5 s. The 4-min blocks comprised either (i) 1 min of the spatial working memory task, 1 min of the spatial control task, and two 1-min blocks of tasks not related to the study presented here or (ii) 1 min of the nonspatial visual working memory task, 1 min of the nonspatial control task, and two 1-min blocks of unrelated tasks. Each scanning sequence was repeated three times in a counterbalanced order to produce three “spatial working memory versus spatial control” data sets and three “nonspatial working memory versus nonspatial control” data sets for each of the six subjects. We chose to study runs which alternated the spatial experimental task with the spatial control task and other runs which alternated the nonspatial experimental task with the nonspatial control task to answer the critical question whether the same prefrontal areas would be activated by these two experimental tasks which involve different stimulus material but the same executive process.
RESULTS
The spatial and nonspatial working memory tasks were well matched for the difficulty of monitoring required with subjects scoring 80 and 83% correct, respectively (t(5) = 0.32, P = 0.76). The regions of the frontal cortex showing statistically significant differences between the experimental working memory conditions and the appropriate control conditions were identified in each individual subject by using the sulcal landmarks seen on the functional and high-resolution anatomical images. In particular, the mid-dorsolateral frontal region (areas 46 and 9/46), which was of a major interest in the present study, lies on the middle frontal gyrus above the inferior frontal sulcus at mid-levels of the frontal lobe (19).
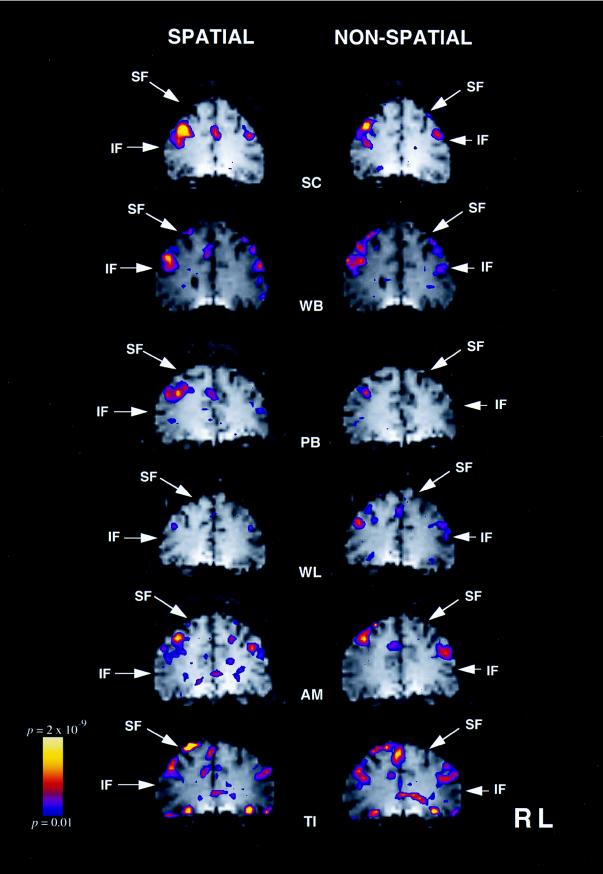
When activity in the spatial working memory condition was compared with that in the spatial control condition, significant increases in signal intensity were observed, bilaterally, in the mid-dorsolateral prefrontal cortex, in five of the six subjects (Fig. 2 and Table 1). In the sixth subject, this change only reached significance in the right hemisphere. The pattern of frontal signal intensity changes observed when the nonspatial working memory task was compared with the nonspatial control task was almost identical. Thus, significantly increased signal intensity was observed bilaterally in the mid-dorsolateral prefrontal cortex in five of the six subjects studied and only in the right mid-dorsolateral prefrontal region in the sixth subject (Fig. 2 and Table 1).
Figure 2.
fMRI signal increases in the mid-dorsolateral frontal cortex for each of the six subjects during the spatial working memory task (left column) and during the nonspatial working memory task (right column). The color bar on the left gives the range of significance values, with blue representing P < 0.01 and yellow a level of P < 2 × 10−9. The left hemisphere appears on the right of each image. SF, superior frontal sulcus; IF, inferior frontal sulcus.
Table 1.
Stereotaxic coordinates of maximal fMRI signal increases in the mid-dorsolateral frontal cortex during spatial and nonspatial working memory
| Spatial working memory
|
Nonspatial working memory
|
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Left hemisphere
|
Right hemisphere
|
Left hemisphere
|
Right hemisphere
|
|||||||||||||
| Subject | X | Y | Z | P | X | Y | Z | P | X | Y | Z | P | X | Y | Z | P |
| SC | −43 | 39 | 31 | 6.6 × 10−8 | 21 | 42 | 37 | 2.8 × 10−14 | −43 | 36 | 31 | 1.2 × 10−7 | 18 | 42 | 31 | 3.5 × 10−9 |
| WB | −34 | 36 | 34 | 2.7 × 10−6 | 37 | 39 | 25 | 3.7 × 10−8 | −31 | 48 | 40 | 2.7 × 10−6 | 31 | 39 | 25 | 3.9 × 10−11 |
| PB | NS | 37 | 45 | 29 | 9.8 × 10−12 | NS | 35 | 45 | 29 | 1.8 × 10−9 | ||||||
| WL | −37 | 42 | 31 | 5.9 × 10−7 | 40 | 48 | 34 | 2.5 × 10−5 | −37 | 42 | 31 | 1.9 × 10−9 | 40 | 48 | 34 | 2.0 × 10−7 |
| AM | −34 | 54 | 28 | 2.4 × 10−12 | 34 | 48 | 37 | 1.3 × 10−13 | −37 | 57 | 28 | 1.7 × 10−6 | 34 | 48 | 37 | 2.8 × 10−10 |
| TI | −43 | 39 | 28 | 3.7 × 10−8 | 43 | 39 | 34 | 3.5 × 10−9 | −46 | 36 | 31 | 1.9 × 10−9 | 43 | 45 | 34 | 1.2 × 10−7 |
| Mean (SD) | −38(5), 42(7), 30(3) | 35(8), 44(4), 33(5) | −39(6), 44(9), 32(5) | 34(9), 45(4), 32(4) | ||||||||||||
The stereotaxic coordinates are expressed in mm and are based on the system used in the brain atlas of Talairach and Tournoux (18). X = medial-to-lateral distance relative to the midline (positive = right hemisphere); Y = anterior-to-posterior distance relative to the anterior commissure (positive = anterior); Z = superior-to-inferior distance relative to the anterior commissure-posterior commissure line (positive = superior). Significance level (P) is given as uncorrected probability. Subjects are identified according to their initials which appear on the left. NS, not significant.
The average stereotaxic coordinates of the areas showing increased signal intensity changes outside of the prefrontal cortex are listed in Table 2. In the posterior neocortex, there were several significant peaks within the posterior parietal cortex bilaterally when the spatial working memory task was compared with its control. In contrast, when the nonspatial working memory task was compared with its control, there were several significant peaks within the anterior and inferior temporal cortex.
Table 2.
Stereotaxic coordinates of (group) mean maximal fMRI signal increases outside the prefrontal cortex during spatial and nonspatial working memory
| X | Y | Z | P value | |
|---|---|---|---|---|
| Spatial 2-Back Task compared with the Spatial Control Task | ||||
| Right hemisphere | ||||
| 46 | −36 | 43 | 3.2 × 10−4 | Area 40, posterior parietal cortex |
| 31 | −42 | 43 | 6.3 × 10−4 | Area 40, posterior parietal cortex |
| 21 | −57 | 34 | 1.4 × 10−3 | Area 40, posterior parietal cortex |
| 12 | −63 | 46 | 2.9 × 10−4 | Area 7, posterior parietal cortex |
| 21 | −3 | 43 | 1.0 × 10−6 | Area 6, premotor cortex |
| 40 | 9 | 43 | 2.6 × 10−5 | Area 6/8, premotor cortex |
| 6 | 31 | 31 | 1.3 × 10−4 | Area 32/8, medial frontal cortex |
| Left hemisphere | ||||
| −37 | −42 | 43 | 7.8 × 10−5 | Area 40, posterior parietal cortex |
| −59 | −42 | −3 | 1.7 × 10−4 | Area 37, temporo-occipital cortex |
| −37 | 6 | 37 | 2.0 × 10−3 | Area 6, premotor cortex |
| −18 | 3 | 65 | 3.8 × 10−6 | Area 6, premotor cortex |
| Bilateral | ||||
| 0 | 9 | 56 | 2.3 × 10−6 | Area 6, supplementary motor area |
| Nonspatial 1-Back Task compared with the Nonspatial Control Task | ||||
| Right hemisphere | ||||
| 31 | 15 | −40 | 1.0 × 10−3 | Area 38, anterior temporal cortex |
| 56 | −33 | −6 | 4.6 × 10−3 | Area 21, mid-temporal cortex |
| 46 | −39 | 18 | 3.5 × 10−3 | Area 22, mid-temporal cortex |
| 21 | −3 | 43 | 1.5 × 10−5 | Area 6, premotor cortex |
| 40 | 12 | 46 | 4.2 × 10−5 | Area 6/8, premotor cortex |
| Left hemisphere | ||||
| −31 | 18 | −40 | 4.2 × 10−5 | Area 38, anterior temporal cortex |
| −34 | −48 | −21 | 1.9 × 10−3 | Area 37, ventral temporo-occipital cortex |
| −56 | −48 | −15 | 1.8 × 10−3 | Area 37, ventral temporo-occipital cortex |
| −40 | 6 | 37 | 7.1 × 10−5 | Area 6, premotor cortex |
| −6 | 6 | 53 | 6.0 × 10−5 | Area 6, supplementary motor cortex |
DISCUSSION
The present investigation examined activation patterns within the frontal cortex in two working memory tasks that required monitoring of information within working memory. The monitoring requirements of the two tasks were carefully matched, but in the spatial working memory task performance depended on remembering the locations of the stimuli, whereas in the nonspatial working memory task, location was irrelevant, performance requiring memory for visual patterns. The results demonstrated that spatial and nonspatial working memory tasks activate similar regions within the human lateral prefrontal cortex when factors other than those directly related to the type of stimulus material and, which are likely to affect patterns of frontal lobe activation, are carefully controlled. As can be seen in Fig. 2, performance of both the spatial and the nonspatial working memory tasks resulted in increased activity in the mid-dorsolateral frontal region, namely, the cortex that lies on the middle frontal gyrus above the inferior frontal sulcus. Furthermore, inspection of Fig. 2 shows that performance of the visual nonspatial working memory task did not lead to increased activity in the ventrolateral frontal region. The above pattern of results is consistent with the hypothesis that the mid-dorsolateral frontal cortex is critically involved in the monitoring of information within working memory, regardless of the nature of the stimulus material (8, 9). It does not, however, support the theoretical position that views the functional distinction between the dorsolateral and the ventrolateral prefrontal cortex in terms of the type of stimulus material being held in working memory: spatial in the case of the dorsolateral and nonspatial in the case of the ventrolateral prefrontal cortex (1, 2).
Previous functional neuroimaging studies of spatial or nonspatial working memory did not directly address this issue because the tasks that the subjects performed differed both in terms of the nature of the stimulus material to be remembered and in terms of the executive demands of the tasks. For example, McCarthy et al. (7) reported increased activity in the mid-dorsolateral frontal cortex (area 46) during performance of a spatial working memory task, whereas Jonides et al. (6) reported increased activation in the ventrolateral frontal cortex (area 47) during performance of another spatial working memory task. Owen et al. (20) demonstrated that either, or both, of these two lateral frontal areas can be activated in spatial working memory tasks, depending on the precise executive processes that are called on by the task that is being performed. In other work, Courtney et al. (5) reported increased activity in a frontal region which included dorsolateral areas 9 and 46 during the performance of a delayed face-matching task, whereas a delayed location-matching task did not result in increased activity in this region. Petrides et al. (21, 22) have consistently reported increased activity in the mid-dorsolateral frontal cortex (areas 46 and 9/46) during the performance of working memory tasks that taxed monitoring requirements, regardless of whether the stimulus material to be remembered was visual abstract patterns or auditory verbal.
The present results, indicating that two working memory tasks that have the same monitoring demands yield increased activity in the mid-dorsolateral frontal cortical region, regardless of whether the stimulus material is visual spatial or not, are consistent with findings from behavioral-lesion studies in nonhuman primates (10) that demonstrated the critical nature of the mid-dorsolateral frontal cortex for the monitoring of information within working memory. The present findings are also consistent with a more recent examination of delay-related activity at the single-neuron level in the lateral prefrontal cortex (23). In the latter study, approximately half of the neurons with delay-related activity were tuned both to visual pattern and location. Furthermore, neurons that were tuned only to location or only to visual pattern were equally distributed between the dorsolateral and the ventrolateral prefrontal cortex, neurons tuned to location not being predominant in the dorsolateral prefrontal cortex.
By providing direct evidence for similar patterns of frontal activation in the same subjects during visual spatial and visual nonspatial working memory tasks that have been matched in terms of the difficulty of their monitoring requirements, the present study demonstrated that the nature of stimulus material being processed may not be the decisive factor determining activity differences between the mid-dorsolateral and the mid-ventrolateral frontal cortex, as previously thought (1, 2). These results are consistent with the alternative view that executive processing rather than stimulus material is the important factor determining where activity will be increased within the prefrontal cortex (8, 9).
It could be argued that subtle differences may still exist in the location of the activation foci that are below the resolution of current neuroimaging techniques and the particular methods used here. It must be remembered, however, that the hypothesis being tested is that spatial working memory processing engages selectively mid-dorsolateral prefrontal cortex (i.e., cortex lying on the middle frontal gyrus), whereas nonspatial pattern working memory processing engages selectively ventrolateral prefrontal cortex (i.e., cortex on the inferior frontal gyrus). The functional neuroimaging methods used here are certainly adequate for detecting activation differences at this level and clearly show that activation of mid-dorsolateral prefrontal cortex was not uniquely related to the spatial task.
It is clear from Table 2 that the spatial working memory task also produced a considerable number of activity peaks in the posterior parietal cortex, whereas the nonspatial task yielded several peaks in the anterior and mid-temporal cortex. This differential pattern of activation in posterior neocortex is consistent with the existing literature (e.g., ref. 5) and, along with the fact that these tasks yielded the same activity changes within the prefrontal cortex, provides further support for the processing-specific hypothesis of prefrontal organization.
The present study does not rule out the possibility that some functional differentiation based on stimulus material might still exist within a particular frontal cortical region. Nevertheless, even if one were to detect such subtle differences in activation (e.g., activation related to the spatial task being separated by a few millimeters from activation related to the nonspatial task) that would still not be evidence for functional separation between mid-dorsolateral and mid-ventrolateral prefrontal cortex in terms of type of information; it would only be evidence that the mid-dorsolateral prefrontal cortex is involved in both spatial and nonspatial working memory, although within this region processing may be separated slightly according to stimulus material. As pointed out above, such a subtle distinction was recently tested at the level of single-neuron recording in nonhuman primates and was not confirmed (23).
Acknowledgments
We thank K. K. Kwong, R. M. Weisskoff, T. L. Davis, J. R. Baker, T. Reese, D. Kennedy, G. Bush, and A. Jiang for the development of the fMRI imaging protocols (K.K., R.M.W., J.R.B.), statistical software (R.M.W., T.L.D.), motion correction software (A.J., D.K.), Talairach transformation software (A.J., D.K., G.B.), and automatic shimming protocols (T.R.). P. M. Collins and A. C. Roberts are also thanked for suggestions relating to the task design. The work reported here was supported by the McDonnell–Pew Program in Cognitive Neuroscience.
ABBREVIATION
- fMRI
functional MRI
References
- 1.Wilson F A W,, Scalaidhe S P O, Goldman-Rakic P S. Science. 1993;260:1955–1958. doi: 10.1126/science.8316836. [DOI] [PubMed] [Google Scholar]
- 2.Goldman-Rakic P S. Ann N Y Acad Sci. 1995;769:71–83. doi: 10.1111/j.1749-6632.1995.tb38132.x. [DOI] [PubMed] [Google Scholar]
- 3.Cohen J D, Forman S D, Braver T S, Casey B J, Servan-Schreiber D, Noll D C. Hum Brain Mapp. 1994;1:293–304. doi: 10.1002/hbm.460010407. [DOI] [PubMed] [Google Scholar]
- 4.Smith E E, Jonides J J, Koeppe R A, Awh E, Schumacher E H, Minoshima S. J Cognit Neurosci. 1995;7:337–356. doi: 10.1162/jocn.1995.7.3.337. [DOI] [PubMed] [Google Scholar]
- 5.Courtney S M, Ungerlieder L G, Keil K, Haxby J V. Cereb Cortex. 1996;6:39–49. doi: 10.1093/cercor/6.1.39. [DOI] [PubMed] [Google Scholar]
- 6.Jonides J, Smith E E, Koeppe R A, Awh E, Minoshima S, Mintun M A. Nature (London) 1993;363:623–625. doi: 10.1038/363623a0. [DOI] [PubMed] [Google Scholar]
- 7.McCarthy G, Blamire A M, Puce A, Nobre A C, Bloch G, Hyder F, Goldman-Rakic P, Shulman R G. Proc Natl Acad Sci USA. 1994;91:8690–8694. doi: 10.1073/pnas.91.18.8690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Petrides M. In: Handbook of Neuropsychology. Boller F, Grafman J, editors. Vol. 9. Amsterdam: Elsevier; 1994. pp. 59–82. [Google Scholar]
- 9.Petrides M. Philos Trans R Soc London B. 1996;351:1455–1462. doi: 10.1098/rstb.1996.0130. [DOI] [PubMed] [Google Scholar]
- 10.Petrides M. J Neurosci. 1995;15:359–375. doi: 10.1523/JNEUROSCI.15-01-00359.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kwong K K, Belliveau J W, Chesler D A, Goldberg I E, Weisskoff R M, Poncelet B P, Kennedy D N, Hoppel B E, Cohen M S, Turner R, et al. Proc Natl Acad Sci USA. 1992;89:5675–5679. doi: 10.1073/pnas.89.12.5675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ogawa S, Tank D W, Menon R, Ellermann J M, Kim S, Merkle H, Ugurbil K. Proc Natl Acad Sci USA. 1992;89:5951–5955. doi: 10.1073/pnas.89.13.5951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wismer G L, Buxton R B, Rosen B R, Fisel C R, Oot R F, Brady T J, Davis K R. J Comput Assist Tomogr. 1988;12:259–265. doi: 10.1097/00004728-198803000-00014. [DOI] [PubMed] [Google Scholar]
- 14.Dixon W T. Radiology. 1984;153:189–194. doi: 10.1148/radiology.153.1.6089263. [DOI] [PubMed] [Google Scholar]
- 15.Sepponen R E, Sepponen J T, Tanttu J I. J Comput Assist Tomogr. 1984;8:585–587. doi: 10.1097/00004728-198408000-00001. [DOI] [PubMed] [Google Scholar]
- 16.Stuart A, Ord J K. Kendall’s Advanced Theory of Statistics. New York: Oxford Univ. Press; 1991. [Google Scholar]
- 17.Jiang A, Kennedy D N, Baker J R, Weisskoff R M, Tootell R B H, Woods R P, Benson R R, Kwong K K, Brady T J, Rosen B R, Belliveau J W. Hum Brain Mapp. 1995;3:224–235. [Google Scholar]
- 18.Talairach J, Tournoux P. Co-planar Stereotaxic Atlas of the Human Brain. New York: Thieme; 1988. [Google Scholar]
- 19.Petrides M, Pandya D N. In: Handbook of Neuropsychology. Boller F, Grafman J, editors. Vol. 9. Amsterdam: Elsevier; 1994. pp. 17–58. [Google Scholar]
- 20.Owen A M, Evans A C, Petrides M. Cereb Cortex. 1996;6:31–38. doi: 10.1093/cercor/6.1.31. [DOI] [PubMed] [Google Scholar]
- 21.Petrides M, Alivisatos B, Evans A C, Meyer E. Proc Natl Acad Sci USA. 1993;90:873–877. doi: 10.1073/pnas.90.3.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Petrides M, Alivisatos B, Meyer E, Evans A C. Proc Natl Acad Sci USA. 1993;90:878–882. doi: 10.1073/pnas.90.3.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rao S C, Rainer G, Miller E K. Science. 1997;276:821–824. doi: 10.1126/science.276.5313.821. [DOI] [PubMed] [Google Scholar]