Abstract
Axonal damage to adult peripheral neurons causes changes in neuronal gene expression. For example, axotomized sympathetic, sensory, and motor neurons begin to express galanin mRNA and protein, and recent evidence suggests that galanin plays a role in peripheral nerve regeneration. Previous studies in sympathetic and sensory neurons have established that galanin expression is triggered by two consequences of nerve transection: the induction of leukemia inhibitory factor (LIF) and the reduction in the availability of the target-derived factor, nerve growth factor. It is shown in the present study that no stimulation of galanin expression occurs following direct application of LIF to intact neurons in the superior cervical sympathetic ganglion. Injection of animals with an antiserum to nerve growth factor concomitant with the application of LIF, on the other hand, does stimulate galanin expression. The data suggest that the response of neurons to an injury factor, LIF, is affected by whether the neurons still receive trophic signals from their targets.
Axonal damage to adult peripheral neurons results in large morphological and biochemical changes and, eventually, in many cases, in regeneration of the axon and reinnervation of target tissues (1–4). Early investigations focused on histological changes, such as the involution of the rough endoplasmic reticulum, which is known as “chromatolysis.” More recent studies have examined alterations in gene expression including increases in the expression of α and β tubulin, major components of microtubules, and decreases in expression of proteins involved in the synthesis and synaptic actions of classical neurotransmitters (for review, see ref. 3). The adaptive significance of these morphological and biochemical changes is likely to involve a switch in the neuron’s status, produced by axotomy, from one specialized for chemical neurotransmission to one specialized for axonal regrowth (3, 5–7).
Dramatic changes in expression of certain neuropeptides are another common feature of peripheral neurons’ response to axotomy. Neuropeptides that are normally involved in synaptic transmission in these systems are downregulated; while at the same time, other peptides, not expressed by these neurons under normal conditions, are induced (3, 4). In the case of rat sympathetic ganglia, the majority of the neurons normally express neuropeptide Y, while very few express galanin, vasoactive intestinal peptide, or substance P. After axotomy, large decreases in neuropeptide Y mRNA occur together with large increases in mRNA and protein for galanin, vasoactive intestinal peptide, and substance P (8–12). Injured sensory and motor neurons also exhibit major changes in their neuropeptide phenotypes (3, 4). While the exact pattern of peptide changes is unique to each of these cell types, large inductions of galanin and vasoactive intestinal peptide occur in all three cases, and it has been speculated that these two peptides produce trophic effects on neurons at a time when they have been deprived of their normal target-derived trophic factors (3, 4). We have focused on the regulation of expression of galanin for two reasons: (i) this neuropeptide exhibits the largest increases in expression after nerve injury and (ii) recent data has demonstrated that regeneration of sensory neurons is delayed in mice in which the galanin gene has been knocked out by homologous recombination (13).
The signals involved in triggering these responses to axotomy have been studied in most detail with respect to the regulation of galanin expression in sympathetic neurons in the superior cervical ganglion (SCG). Postganglionic nerve transection causes the induction of the cytokine leukemia inhibitory factor (LIF) (8, 14–16) by nonneuronal cells within this ganglion. The role of LIF in the nervous system had originally been postulated to be as a “cholinergic differentiation factor,” involved in producing a cholinergic phenotype in a subpopulation of neonatal sympathetic neurons (17). Recently, however, a variety of studies have indicated that LIF probably does not play this developmental role (18–21). At the same time, the importance of LIF in the stimulation of galanin expression after axotomy in adult animals has been demonstrated in sympathetic neurons in organ culture using a LIF neutralizing antiserum (14) and in both sympathetic and sensory neurons in vivo using transgenic mice in which the gene for LIF had been knocked out (8, 18, 22, 23). The increase in galanin immunoreactivity in SCG from LIF −/− mice after axotomy was only about one-tenth the magnitude seen in wild-type animals (18).
A second known change in trophic/differentiation factor content of the SCG after axotomy is the drastic reduction in levels of nerve growth factor (NGF; 24, 25), a protein that normally reaches the ganglion primarily by retrograde transport from sympathetic target tissues. We have recently obtained evidence that decreased levels of NGF may also play a role in the regulation of galanin expression. Systemic administration of an antiserum to NGF (αNGF) causes an induction of galanin mRNA in the SCG in intact animals, while no response is seen after administration of normal serum (ref. 26; A.M.S., Y.S., and R.E.Z., unpublished manuscript).
EXPERIMENTAL PROCEDURES
Placement of LIF Pellet in Intact Animals.
Adult male Sprague–Dawley rats (≈200 g) were purchased from Zivic–Miller and kept on a 12 hr:12 hr light:dark cycle with ad libitum access to food and water. The rats were anesthetized with chloral hydrate (770 mg/kg), supplemented with ether as required. Their SCGs were exposed through a midline incision in the neck, the connective tissue sheath of the ganglion partially removed, and a 7-day slow-releasing pellet containing either 2.5 μg of LIF or a control pellet (containing the matrix material alone) was placed next to each ganglion. The pellets were obtained from Innovative Research of America. Two days after the pellets were implanted, animals were sacrificed using CO2 vapors, and their SCG were removed and quickly frozen on dry ice.
Administration of an NGF Antiserum.
Rats were injected i.p. with 0.5 ml of either NSS or αNGF daily for 3 days beginning 1 day before implantation of the LIF and control pellets. On day 4, animals were sacrificed, their SCG removed and frozen. The antiserum against NGF was generously supplied by Jack Diamond (McMaster University, Hamilton, Ontario) (27–29). A dose of 0.25 ml/100 g body weight has been shown to be 30–40% higher than that required to block NGF-dependent collateral sprouting of sensory nerves in rat skin (27). Previous studies have shown that, in addition to recognizing NGF, this antiserum can recognize neurotrophin 3, but not brain-derived neurotrophic factor, in an in vitro neurite outgrowth assay (29). Neurotrophin 3, however, unlike NGF, does not inhibit galanin expression (ref. 26; A.M.S., Y.S., and R.E.Z., unpublished manuscript).
Decentralization of the SCG.
In some animals, the SCGs and cervical sympathetic trunks (CSTs) were exposed through a midline incision in the neck. The nerve trunks were either left intact (sham-operated) or were transected bilaterally, 3 mm caudal to the ganglia. Two days after surgery, animals were sacrificed, their SCG removed and frozen quickly on dry ice. Unoperated animals were used as controls for all experiments.
Other animals were treated with either NSS or αNGF (as described above) 1 day before surgery, on the day of surgery, and 1 day later. On the second day of treatment, the animals were anesthetized, the SCG exposed and either the CST transected or exposed but left intact (sham). Two days after surgery, the animals were sacrificed, and their SCG removed.
Measurement of the Levels of Galanin and LIF mRNA.
RNA from 2 SCG per sample was extracted by using RNAzol B (Tel-Test, Friendswood, TX) as described (8). Samples were run on a 1.2% agarose gel and transferred to a nylon membrane. The membrane was hybridized with a 32P-labeled oligo probe to galanin (39-mer containing sequences 239–277) and a 32P-labeled cDNA probe for glyceraldehyde-3-phosphate dehydrogenase (GAPDH, from J. M. Blanchard, Université Montpellier II). Labeled membranes were exposed to a PhosphorImager screen overnight and radioactivity detected with a PhosphorImager (Molecular Dynamics) and quantified by using the imagequant program (Molecular Dynamics). Data are expressed as the intensity of the galanin mRNA band relative to that of the GAPDH band in the same sample. The same procedures were followed to measure LIF mRNA levels, using a 32P-labeled cDNA probe for LIF (from R. P. Hart of Rutgers University, Newark, NJ).
RESULTS
To determine whether LIF is a sufficient stimulus to induce galanin mRNA, rats were anesthetized and their SCG exposed and partially desheathed, taking care not to injure the ganglion’s pre- or postganglionic nerve trunks. A slow release pellet containing LIF or a control pellet was placed in contact with the SCG. Forty-eight hours later, a time after axotomy at which peak levels of galanin mRNA are found (8), no increase in galanin mRNA in the SCG from the LIF-treated animals was seen by Northern blot analysis (data not shown). This result suggests that intact sympathetic neurons are unresponsive to LIF, at least with respect to the regulation of galanin expression. Given that the induction and release of LIF plays an important role in the galanin response after axotomy, the data raise an intriguing hypothesis, namely, that axotomy not only induces LIF expression in the SCG but also increases the responsiveness of neurons to LIF.
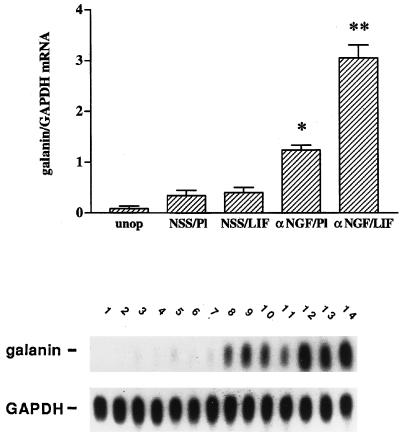
To determine whether the inhibition of LIF responsiveness in intact sympathetic neurons might be mediated by NGF, animals were injected with αNGF raised in sheep or with normal sheep serum (NSS), before and during LIF administration. As expected from the initial experiment described above, in the NSS-treated animals, no difference was found in the level of galanin mRNA between animals given LIF-containing or control pellets (Fig. 1). Administration of the NGF antiserum in animals given a control pellet showed a small but significant increase in galanin mRNA (P < 0.0001). Ganglia from animals treated with the NGF antiserum and a LIF-containing pellet exhibited a much larger increase in galanin mRNA (P < 0.0001 vs. αNGF/placebo). Thus, the combination of reducing NGF and increasing LIF had a clear synergistic effect over that of either treatment alone, resulting in an 8-fold increase in galanin mRNA over NSS/placebo.
Figure 1.
αNGF treatment alters responsiveness to LIF in intact sympathetic neurons in vivo. Messenger RNA levels were measured by northern blot analysis of SCG (20) that were either untreated (unop), or treated with NSS and a placebo pellet (NSS/Pl), NSS and a LIF-containing slow-release pellet (NSS/LIF), αNGF and a placebo pellet (αNGF/Pl), or αNGF and a LIF pellet (αNGF/LIF). Treatment with antiserum or control serum was for three days, while with LIF pellets or control pellets was for 2 days. In the NSS/Pl group, there was only a small increase in galanin mRNA levels over the unop group. This increase is most likely due to a small amount of neural damage occurring during the process of desheathing the SCG before pellet placement. Levels of galanin mRNA in the NSS/LIF group were not different from those in the NSS/Pl group. There was a 3.5-fold increase in galanin mRNA in the αNGF/Pl group as compared with NSS/Pl treatment. The αNGF/LIF group showed the highest levels of galanin mRNA in this experiment, being 2.3-fold higher than the αNGF/Pl group. Levels of galanin mRNA are expressed as a ratio of the intensity of the galanin band compared with that of the band corresponding to GAPDH. The bar graph represents the means ± SEM of data from five or six samples per group with 2 SCG per sample. The autoradiograph is from one representative experiment and contains multiple lanes of the groups described above. Lanes 1 and 2, unop; lanes 3–5, NSS/Pl; lanes 6–8, NSS/LIF; lanes 9–11, αNGF/Pl; and lanes 12–14, αNGF/LIF. After the blot was probed for galanin, it was stripped and reprobed for GAPDH. *, P < 0.0001 compared with NSS/Pl. **, P < 0.0001 compared with either NSS/LIF or αNGF/Pl.
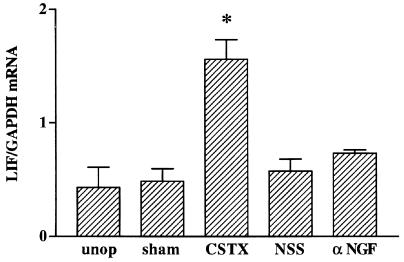
To determine whether αNGF might produce this effect by inducing LIF expression in the intact SCG and thereby increasing the content of LIF above a subthreshold level, LIF mRNA was measured after 3 days of antiserum treatment. Although, as we have found repeatedly, postganglionic axotomy produces a large increase in LIF mRNA in the SCG (8, 14), no increase in LIF mRNA was found after administration of the NGF antiserum or NSS (Fig. 2).
Figure 2.
αNGF’s effects on neuropeptide expression are not mediated by LIF. Northern blot analysis was performed on samples from five experimental groups: unoperated animals (unop), animals that either underwent a sham operation (sham) or had their CST transected (CSTX) 2 days before sacrificing, or intact animals that had been injected daily for 3 days with either control serum (NSS) or αNGF. Transection of the CST produced a 3.2-fold increase in LIF mRNA compared with levels in ganglia from sham-operated animals. αNGF treatment had no effect on LIF mRNA levels when compared with NSS treatment. LIF mRNA levels are expressed as a ratio of the intensity of the LIF band to that of the GAPDH band. Bar = mean ± SEM of data from three or four samples per group, with 2 SCG per sample. *, P < 0.01 compared with all other groups.
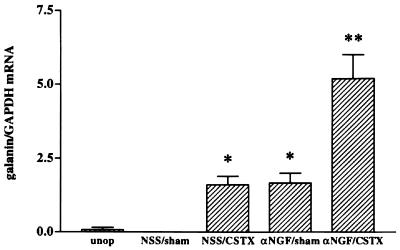
These data indicate that there is a synergistic interaction between the effects of increasing levels of LIF and reducing NGF levels. Having shown that the reduction in NGF potentiates the effect of exogenous LIF, we sought a paradigm where we could test whether a potentiating effect of the NGF antiserum could be seen when LIF was increased endogenously without altering the connection of the vast majority of the neurons to their target tissues. Such a paradigm is provided by transection of the predominantly preganglionic cervical sympathetic trunk, a procedure usually referred to as “decentralization” of the ganglion. Decentralization produced a significant increase in LIF mRNA (P < 0.005; Fig. 2), though not to the same extent as does postganglionic nerve transection (30). Previous studies indicated, however, that compared with axotomy, preganglionic nerve transection produced relatively small changes in galanin or vasoactive intestinal peptide mRNA (9, 10). To examine whether, under these conditions, the response of the decentralized but intact SCG neurons might be inhibited by endogenous NGF, animals whose CSTs had been transected were injected with αNGF or NSS. Some increase in galanin mRNA was found in animals whose SCG had been decentralized and who had been given NSS and also in animals receiving a sham operation and αNGF. The increase in galanin mRNA produced in animals whose SCG had been decentralized and who received NGF antiserum, however, was greater than the sum of the effects produced by either procedure alone (Fig. 3).
Figure 3.
Increased galanin mRNA after CST transection (CSTX) and αNGF treatment. Levels of galanin mRNA were nearly undetectable by Northern blot analysis in either the unoperated (unop) or sham-operated (sham), NSS-injected (NSS) animals. Galanin mRNA levels increased equally in both the NSS-injected/CSTX-operated (NSS/CSTX) and αNGF-injected/sham-operated (αNGF/sham) groups. The SCG from rats treated with αNGF that had their CST transected (αNGF/CSTX) had galanin mRNA levels approximately 3.1-fold higher than either NSS/CSTX or αNGF/sham. Levels of galanin mRNA are expressed as a ratio of the intensity of the galanin band as compared with that of GAPDH. The graph represents the means ± SEM of data from three or four samples per group, with 2 SCG per sample. *, P < 0.002 compared with NSS/sham. **, P < 0.01 compared with all other groups.
DISCUSSION
Together with our published results using LIF −/− mice (8, 18), the data presented here suggest that, while LIF plays an important role in triggering the increase in galanin mRNA after axotomy, it is not, by itself, a sufficient stimulus to produce such an increase. The data also raise the interesting hypothesis that the nature of the response of sympathetic neurons to LIF depends on whether the neurons are still connected to their targets and, thereby, receiving target-derived NGF. Such a hypothesis could also account for our previous findings that when only a portion of the neurons in a sympathetic ganglion are axotomized, the galanin response is highly localized to those neurons that have been axotomized (10, 11, 31), despite the fact that nearby intact neurons are probably also exposed to LIF released from nonneuronal cells (14). Finally, while LIF produces minimal changes in galanin expression in sympathetic neurons after decentralization in the absence of αNGF, it seems quite likely that it does stimulate a program of gene expression under these conditions, albeit one that differs from that seen after axotomy.
Even though treatment of intact SCG with both LIF and αNGF mimics the effects of axotomy on galanin mRNA, the effect of axotomy is quantitatively larger. The combined treatment leads to an 8-fold increase in galanin mRNA, while axotomy results in a 50–80-fold increase compared with levels seen in sham-operated controls (data not shown). These data suggest that stimulatory factors in addition to LIF or removal of inhibitory factors in addition to NGF are required to elicit the full axotomy response.
The mechanism by which reduction of NGF levels alters the responsiveness of adult SCG neurons to LIF remains to be determined. Among other possibilities, it could represent a change in LIF receptors, in the generation of intracellular signals (e.g., via the JAK-STAT pathway), or in the ability of these signals to induce gene expression. The antagonistic interaction between LIF and NGF seen in the adult SCG differs from previous reports of synergistic or additive interactions between cytokines and neurotrophins. For example, LIF and NGF have synergistic actions on development of embryonic dorsal root ganglion neurons in culture (32). In addition, ciliary neurotrophic factor, a cytokine belonging to the same family as LIF, acts synergistically with NGF to induce neuronal development in MAH cells (immortalized sympathoadrenal progenitor cells) that have been transfected to express Trk receptors (33). Also, ciliary neurotrophic factor has an additive effect with either neurotrophin 3 or brain-derived neurotrophic factor, on the induction of choline acetyltransferase activity in embryonic spinal cord neurons (34). The inhibition by NGF of the galanin-inducing activity of LIF further broadens the types of interactions exhibited by neurotrophins and cytokines. It will be interesting to determine whether other actions of LIF on sympathetic neurons are inhibited by endogenous NGF, and whether similar interactions occur between other injury cytokines (e.g., interleukin 6) and other target-derived trophic factors.
Acknowledgments
This research was supported by National Institutes of Health Grant NS 17512. A.M.S. was supported by National Institutes of Health Training Grant NS 07118.
ABBREVIATIONS
- LIF
leukemia inhibitory factor
- NGF
nerve growth factor
- αNGF
antiserum to nerve growth factor
- NSS
normal sheep serum
- SCG
superior cervical ganglion
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- CST
cervical sympathetic trunk
- CSTX
CST transection
References
- 1.Lieberman A R. In: International Review of Neurobiology. Pfeiffer C C, Smythies J R, editors. Vol. 14. New York: Academic; 1971. pp. 49–124. [DOI] [PubMed] [Google Scholar]
- 2.Watson W E. Br Med Bull. 1974;30:112–115. doi: 10.1093/oxfordjournals.bmb.a071179. [DOI] [PubMed] [Google Scholar]
- 3.Zigmond R E, Hyatt-Sachs H, Mohney R P, Schreiber R, Shadiack A M, Sun Y, Vaccariello S A. Perspect Dev Neurobiol. 1996;4:75–90. [PubMed] [Google Scholar]
- 4.Zigmond R E. Neuroscientist. 1997;3:176–185. [Google Scholar]
- 5.Hårkönen, M. (1964) Acta Physiol. Scand. 63 (Suppl. 237), 1–94.
- 6.Cheah T B, Geffen L B. J Neurobiol. 1973;4:443–452. doi: 10.1002/neu.480040505. [DOI] [PubMed] [Google Scholar]
- 7.Matthews M R, Raisman G. Proc R Soc London B. 1972;181:43–79. doi: 10.1098/rspb.1972.0040. [DOI] [PubMed] [Google Scholar]
- 8.Sun Y, Zigmond R E. J Neurochem. 1996;67:1751–1760. doi: 10.1046/j.1471-4159.1996.67041751.x. [DOI] [PubMed] [Google Scholar]
- 9.Hyatt-Sachs H, Schreiber R C, Bennett T A, Zigmond R E. J Neurosci. 1993;13:1642–1653. doi: 10.1523/JNEUROSCI.13-04-01642.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schreiber R C, Hyatt-Sachs H, Bennett T A, Zigmond R E. Neuroscience. 1994;60:17–27. doi: 10.1016/0306-4522(94)90200-3. [DOI] [PubMed] [Google Scholar]
- 11.Mohney R P, Siegel R E, Zigmond R E. J Neurobiol. 1994;25:108–118. doi: 10.1002/neu.480250203. [DOI] [PubMed] [Google Scholar]
- 12.Rao M S, Sun Y, Vaidyanathan U, Landis S C, Zigmond R E. J Neurobiol. 1993;24:571–580. doi: 10.1002/neu.480240504. [DOI] [PubMed] [Google Scholar]
- 13.Holmes F E, McMahon S B, Murphy D, Wynick D. Soc Neurosci Abstr. 1997;23:1955. [Google Scholar]
- 14.Sun Y, M S, Rao M S, Zigmond R E, Landis S C. J Neurobiol. 1994;25:415–430. doi: 10.1002/neu.480250407. [DOI] [PubMed] [Google Scholar]
- 15.Banner L R, Patterson P H. Proc Natl Acad Sci USA. 1994;91:7109–7113. doi: 10.1073/pnas.91.15.7109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun Y, Landis S C, Zigmond R E. Mol Cell Neurosci. 1996;7:152–163. doi: 10.1006/mcne.1996.0012. [DOI] [PubMed] [Google Scholar]
- 17.Yamamori T, Fukada K, Aebersold R, Korsching S, Fann M J, Patterson P H. Science. 1989;246:1412–1416. doi: 10.1126/science.2512641. [DOI] [PubMed] [Google Scholar]
- 18.Rao M S, Sun Y, Escary J L, Perreau J, Tresser S, Patterson P H, Zigmond R E, Brulet P, Landis S C. Neuron. 1993;11:1175–1185. doi: 10.1016/0896-6273(93)90229-k. [DOI] [PubMed] [Google Scholar]
- 19.Rao M S, Landis S C, Patterson P H. Dev Biol. 1990;139:65–74. doi: 10.1016/0012-1606(90)90279-r. [DOI] [PubMed] [Google Scholar]
- 20.Rao M S, Patterson P H, Landis S C. Development (Cambridge, UK) 1992;116:731–744. doi: 10.1242/dev.116.3.731. [DOI] [PubMed] [Google Scholar]
- 21.Francis N J, Asmus S E, Landis S C. Dev Biol. 1997;182:76–87. doi: 10.1006/dbio.1996.8464. [DOI] [PubMed] [Google Scholar]
- 22.Sun Y, Zigmond R E. Eur J Neurosci. 1996;8:2213–2220. doi: 10.1111/j.1460-9568.1996.tb00744.x. [DOI] [PubMed] [Google Scholar]
- 23.Corness J, Shi T J, Xu Z Q, Brulet P, Hökfelt T. Exp Brain Res. 1996;112:79–88. doi: 10.1007/BF00227180. [DOI] [PubMed] [Google Scholar]
- 24.Korsching S, Thoenen H. J Neurosci. 1985;5:1058–1061. doi: 10.1523/JNEUROSCI.05-04-01058.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou X F, Zettler C, Rush R A. J Neurosci Methods. 1994;54:95–102. doi: 10.1016/0165-0270(94)90163-5. [DOI] [PubMed] [Google Scholar]
- 26.Zigmond R E, Shadiack A M, Sun Y. Soc Neurosci Abstr. 1995;21:1052. [Google Scholar]
- 27.Diamond J, Holmes M, Coughlin M. J Neurosci. 1992;12:1454–1466. doi: 10.1523/JNEUROSCI.12-04-01454.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gloster A, Diamond J. J Comp Neurol. 1995;359:586–94. doi: 10.1002/cne.903590406. [DOI] [PubMed] [Google Scholar]
- 29.Van der Zee C E, Rashid K, Le K, Moore K A, Stanisz J, Diamond J, Racine R J, Fahnestock M. J Neurosci. 1995;15:5316–5323. doi: 10.1523/JNEUROSCI.15-07-05316.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun Y. Ph.D. thesis. Cleveland, OH: Case Western Reserve University; 1996. [Google Scholar]
- 31.Shadiack A M, Vaccarriello S A, Zigmond R E. Neuroscience. 1995;65:1119–1127. doi: 10.1016/0306-4522(95)00022-b. [DOI] [PubMed] [Google Scholar]
- 32.Murphy M, Reid K, Brown M A, Bartlett P F. Development (Cambridge, UK) 1993;117:1173–1182. doi: 10.1242/dev.117.3.1173. [DOI] [PubMed] [Google Scholar]
- 33.Ip N Y, Yancopoulos G D. Annu Rev Neurosci. 1996;19:491–515. doi: 10.1146/annurev.ne.19.030196.002423. [DOI] [PubMed] [Google Scholar]
- 34.Kato A C, Lindsay R M. Exp Neurol. 1994;130:196–201. doi: 10.1006/exnr.1994.1198. [DOI] [PubMed] [Google Scholar]