Summary
Transformation efficiencies of Ras mutants at residue 61 range over three orders of magnitude, but the in vitro GTPase activity decreases 10-fold for all mutants. We show that Raf impairs the GTPase activity of RasQ61L, suggesting that the Ras/Raf complex differentially modulates transformation. Our crystal structures show that in transforming mutants, switch II takes part in a network of hydrophobic interactions burying the nucleotide and pre-catalytic water molecule. Our results suggest that Y32 and a water molecule bridging it to the γ-phosphate in the wild type structure play a role in GTP hydrolysis in lieu of the Arg finger in the absence of GAP. The bridging water molecule is absent in the transforming mutants, contributing to the burying of the nucleotide. We propose a mechanism for intrinsic hydrolysis in Raf-bound Ras and elucidate structural features in the Q61 mutants that correlate with their potency to transform cells.
Introduction
Ras is the canonical member of a large superfamily of small monomeric GTPase proteins that function as ‘molecular switches’ in a number of signaling pathways in the cell (Barbacid, 1987). Ras cycles between the inactive GDP and the active GTP bound forms through large conformational changes near the nucleotide binding site, localized to the switch I (30−38) and switch II (59−72) regions (Campbell et al., 1998). Both switch regions are generally involved in interactions with downstream effector proteins and with proteins that mediate the state of the switch. H-Ras has a low intrinsic rate of GTPase activity that is enhanced by at least 3 orders of magnitude in the presence of GTPase Activating Proteins (GAPs), resulting in the signal transduction switch being turned off (Scheffzek et al., 1997). The switch is turned on by Guanine Nucleotide Exchange Factors (GEFs) that accelerate the rate of nucleotide release to allow loading with GTP (Sprang, 1997).
Mutations in codons 12, 13, or 61 convert the ras gene into an active oncogene (Adari et al., 1988). These mutant proteins are constitutively active, resulting in unregulated cell proliferation and tumor formation. In particular, Q61L has one of the highest transformation efficiencies of any gain of function mutant. Not all substitutions for Q61, however, result in potent transforming efficiency of cells containing the Ras mutants, although they all decrease the in vitro GTPase activity of Ras about 10-fold (Der et al., 1986). The Q61L, Q61V and Q61K mutant Ras variants transform NIH-3T3 cells nearly 300-fold and 1000-fold more efficiently than the Q61G and Q61E mutants respectively. Interestingly, Q61I is an order of magnitude less efficient than the L, V and K mutants, and thus is only moderately transforming. Since the interaction of Ras-GTP with its effector Raf kinase mediates one of the major pathways through which Ras is involved in the control of cell proliferation (Hanahan and Weinberg, 2000), it is relevant to observe the influence of the mutations on switch II given the switch I conformation present in the Ras/Raf complex.
NMR spectroscopy experiments have shown that in solution the switch I and switch II regions in Ras bound to the GTP analogue, GppNHp, are dynamic and interconvert between two or more stable conformers in the millisecond time scale (Ito et al., 1997). Indeed, 31P NMR experiments, where the environment of the nucleotide phosphorus atoms can be monitored, show two distinct conformational states for the protein regions surrounding the triphosphate group of the nucleotide (Geyer et al., 1996). State 1 has been linked to a conformation where switch I residue Y32 is directed away from the nucleotide and state 2 to one in which Y32 is close enough to the nucleotide for the phosphate groups to experience a chemical shift due to the aromatic ring system (Geyer et al., 1996). These conformations are referred to throughout this article as the open and closed conformations respectively. Interestingly, 31P NMR experiments on Ras-GppNHp in complex with RasGAP shows Ras-GppNHp in state 1, consistent with the crystal structure of the complex, where Y32 is in an open conformation interacting intimately with RasGAP (Scheffzek et al., 1997). In the complex with Raf kinase-Ras Binding Domain (Raf-RBD) Ras-GppNHp is present entirely in state 2 (Geyer et al., 1996). Not surprisingly, the crystal structure of the Raps/Raf-RBD complex, where Raps is a mutant form of Rap containing the E30D, K31E double mutation that makes its effector region identical to that of Ras, shows Y32 closed over the nucleotide (Nassar et al., 1995).
A recently published series of crystal structures focus on the effects of switch II mutants on Ras-GppNHp, including two that crystallize with the symmetry of space group R32: A59G (Hall et al., 2002) and Q61G (Ford et al., 2006). In this crystal form switch I is in the closed conformation, with Y32 near the nucleotide as observed in the interaction with Raf kinase. Switch II is unhindered by crystal contacts. This is in contrast to the previously published structures of wild type Ras-GppNHp and its mutants from crystals having symmetry of space group P3221, in which both switch regions are modulated by crystal contacts and switch I has Y32 in an open conformation (Krengel et al., 1990). We have taken advantage of the new crystal form to explore the possibility that conformational properties of RasQ61 mutants might have a role in the potency of its oncogenic phenotype. The crystal structures of wild type Ras-GppNHp, of three highly transforming mutants and of a moderately transforming mutant from crystals having the symmetry of space group R32, together with a set of experiments showing a marked effect of Raf on the GTPase activity of RasQ61L, resolve the paradox associated with the wide range of transformation efficiencies of RasQ61 mutants.
Results
The construct of H-Ras used in the present studies contains the catalytic domain with 23 residues truncated from the C-terminus and is referred to simply as Ras throughout this article. The structures of wild type Ras-GppNHp, of the strongly transforming RasQ61L-GppNHp, RasQ61V-GppNHp, RasQ61K-GppNHp and of the moderately transforming RasQ61I-GppNHp were solved to 1.4 Å, 2.0 Å, 1.6 Å, 1.4 Å and 1.9 Å resolution respectively from crystals having symmetry of the space group R32. In addition, the structure of RasQ61I-GppNHp was solved to 1.5 Å resolution from crystals with P3221 symmetry. The mutants were chosen based on the published transforming efficiency of codon 61 ras mutants (Der et al., 1986), with L, V and K as the three most highly transforming mutants among 6 observed to produce foci at high efficiencies in NIH 3T3 cells. Of the moderately transforming mutants, Ile was chosen because its transforming efficiency is in the middle of this group's range. The published structure of the Q61G mutant (Ford et al., 2006) is used to represent the weakly transforming category. Diffraction data for all structures were collected at 100K at the SER-CAT synchrotron beamline 22-ID, APS (Argonne, IL). Table 1 shows the data collection and refinement statistics for the six crystallographic models presented in this study.
Table 1.
Data collection and refinement statistics. Rsym = ∑|Ii - 〈I〉| /∑I . Rwork = ∑||Fobs| - |Fcalc||/∑|Fobs|, calculated using 90% of the reflections against which the model was refined. Rfree = ∑||Fobs| - |Fcalc||/∑|Fobs|, calculated using a test set consisting of 10% of the total reflections, randomly selected from the original data set. Parentheses include information for the highest-resolution shell.
| Wt Ras-GppNHp | Q61L Ras-GppNHp | Q61V Ras-GppNHp | Q61K Ras-GppNHp | Q61I Ras-GppNHp | Q61I Ras-GppNHp | |
|---|---|---|---|---|---|---|
| Space Group | R32 | R32 | R32 | R32 | R32 | P3(2)21 |
| Unit cell | a=89.63 b=89.63 c=134.51 a = b = 90° g = 120° |
a=88.95 b=88.95 c=134.02 a = b = 90° g = 120° |
a=88.43 b= 88.43 c = 132.69 Å a = b = 90° g = 120° |
a = 88.70 b= 88.70 c = 133.53 Å a = b = 90° g = 120° |
a = 88.34 b= 88.34 c = 133.42 Å a = b = 90° g = 120° |
a = 39.69 b= 39.69 c = 159.02 Å a = b = 90° g = 120° |
| Temperature | 100 K | 100 K | 100 K | 100 K | 100 K | 100 K |
| Resolution (Å) | 1.4 | 2.0 | 1.6 | 1.35 | 1.9 | 1.45 |
| # reflections | 39,942 | 13,810 | 26,113 | 41,677 | 15,426 | 25,870 |
| Completeness (%) | 97.2 | 98.3 | 98.4 | 93.6 | 96.1 | 98.4 |
| Redundancy | 8.3(7.2) | 10.7 (10.0) | 5(5) | 9.4 (5.2) | 10 (7) | 8.7 (6.7) |
| Rsym (%) | 0.09(0.4) | 0.08 (0.23) | 0.07 (0.5) | 0.06 (0.5) | 0.13 (0.6) | 0.10 (0.4) |
| Average I/σ | 20 (5) | 30 (14) | 39 (3.5) | 40 (2.4) | 22 (3) | 22 (6) |
| R-factor/R-free (%) | 20.4/22.0 | 18.0 / 21.4 | 19.0/21.1 | 19.8/21.7 | 17.7/20.8 | 21.1 / 22.8 |
| Bond length (Å) | 0.005 | 0.005 | 0.005 | 0.004 | 0.005 | 0.005 |
| Bond angle (°) | 1.2 | 1.1 | 1.2 | 1.1 | 1.1 | 1.2 |
| # protein atoms | 1,244 | 1,314 | 1,313 | 1,315 | 1,314 | 1,266 |
| # nucleotide atoms | 32 | 32 | 32 | 32 | 32 | 32 |
| # Magnesium molecules | 2 | 2 | 2 | 2 | 2 | 1 |
| # Calcium molecules | 1 | 1 | 1 | 1 | 1 | 0 |
| # water molecules | 180 | 158 | 150 | 195 | 142 | 147 |
The crystal structure of wild type Ras-GppNHp with switch I in the closed conformation
The new structure of Ras-GppNHp has switch I in the closed conformation, with Y32 stacked over the nucleotide. Its side chain hydroxyl group interacts with the γ-phosphate of GppNHp through a water molecule, precisely as observed in the Raps-GppNHp/Ras-RBD complex (Nassar et al., 1996). Superposition of the switch I regions based on alignment of the nucleotide in these two structures yields a Cα root mean square deviation (rmsd) of 0.34 Å. The crystal contacts in the R32 form result in a switch I conformation that mimics the Raps/Raf-RBD interface, including a Ca2+ ion provided by the crystallization mother liquor making similar interactions to those of Lys84 in Raps (Figure 1). This closed form of Ras-GppNHp precludes binding of Ras-GAP in the catalytically productive conformation observed in the Ras/RasGAP complex, consistent with the complete shift to the open form in the presence of GAP (Geyer et al., 1996). Interestingly, the previously published canonical structure (as exemplified by PDB code 1CTQ) (Scheidig et al., 1999) is very similar to the GAP-bound form of Ras (PDB code 1WQ1) (Scheffzek et al., 1997). Most importantly, switch I and in particular Y32 are in the same open conformation and superimpose well in the two structures.
Figure 1.
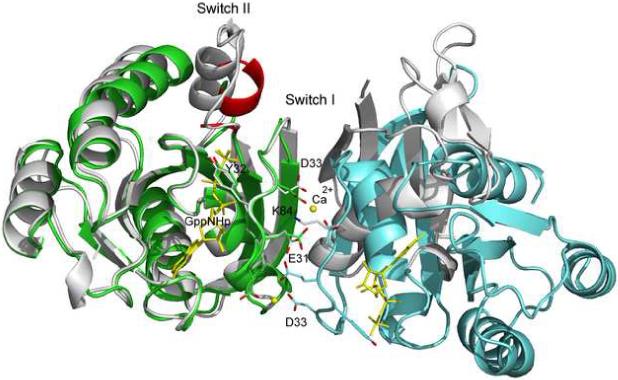
Ribbon diagram of Ras in the R32 crystal form. Two molecules are shown to illustrate crystal contacts along the 2-fold symmetry axes involving switch I. Switch II residues 61−68 are removed from the model. Residues 59−60 and 69−72 are shown in red. The GTP analog, GppNHp, as well as Ca2+ ions are yellow. The Raps/RafRBD complex (PDB code 1GUA) is shown in gray, superimposed on the Ras molecule in green based on the nucleotide. Ras (Raps) residues 31−33 and Raf Lys84 are in stick. Figures 1-5 were generated with PyMOL Molecular Graphics System (DeLano Scientific, San Carlos, CA).
While switch I has well defined electron density, switch II in the wild type Ras-GppNHp is completely disordered, with no electron density for residues 61−68 and with only main chain electron density for Q70 and Y71. At the beginning of the switch, G60 is well ordered and makes its usual amide interaction with the γ-phosphate of the nucleotide and beyond the switch R73 is anchored by crystal contacts. Residues 61−68 are not included in the model and residues 70 and 71 are modeled as alanine in our wild type structure.
Strongly transforming mutants of RasQ61: Q61L, Q61V and Q61K
Unlike the disordered switch II seen in wild type Ras-GppNHp, the strongly transforming Q61 mutants have an ordered switch II region (Figures 2A, 2B and 2C). Surprisingly, switch II in the L, V and K mutants is found in a very different conformation than previously observed for the L mutant (Krengel et al., 1990) and unique compared to all currently available structures of Ras. There is good electron density for nearly the entire switch II, with some weak areas along Ala66 and Met67 and no electron density for the side chain atoms of residues E62 and E63, which are modeled as alanine. In each of the mutant structures, switch I and switch II come together resulting in close interaction between Y32, P34, I36, L/V/K61 and Y64 to form a hydrophobic cluster over the nucleotide and the associated pre-catalytic water molecule (Figure 3). In the RasQ61K-GppNHp structure, the aliphatic portion of K61 participates in the hydrophobic cluster and its positively charged amino group is exposed to solvent. Surface accessibility calculations using a probe of radius equal to 1.4 Å (Lee and Richards, 1971) show that the hydrophilic complex of nucleotide and water molecule is completely buried in all three mutant structures (Figure 4A, 4B and 4C). The buried surface area of the nucleotide and water molecule, the B-factors for the mutant side chains, and distances between residue 61 and neighboring residues in the hydrophobic cluster are shown in Table 2 for all mutant structures presented in this article.
Figure 2.
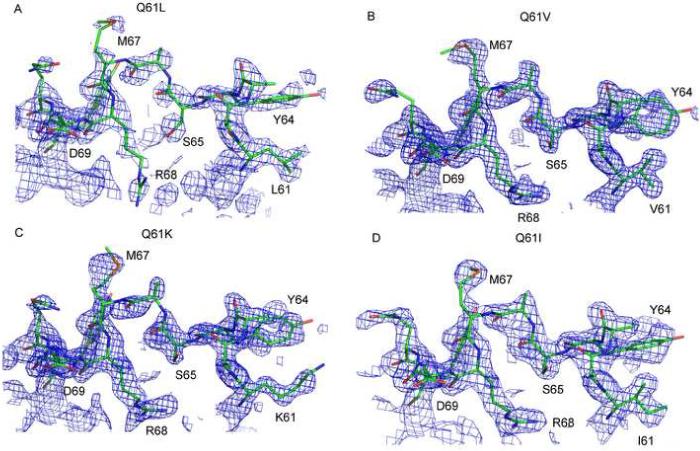
Electron density for switch II residues 61−70. (A) RasQ61L-GppNHp. (B) RasQ6V-GppNHp. (C) RasQ61K-GppNHp (D) RasQ61I-GppNHp (R32). All panels show final 2Fo-Fc electron density maps contoured at the 1σ level.
Figure 3.

Superposition of transforming Ras-GppNHp Q61 mutants (residues 32−35 and 61−64) and Ran-GppNHp/importinβ (residues 40−43 and 69−72). GppNHp is in yellow. The mutant structures are colored as follows: Q61L, cyan; Q61K, green; Q61I, magenta; Q61V, orange. Ran is in light gray. Wat175 corresponds to the pre-catalytic water molecule. Red dashed lines represent hydrogen bonds.
Figure 4.
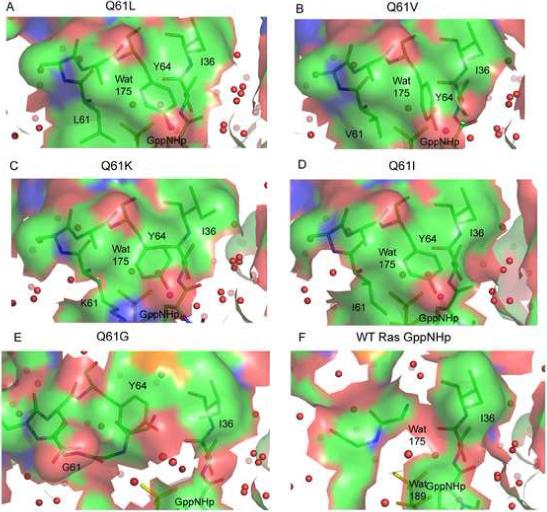
Active site surfaces in the RasQ61 mutants and in the wild type structure in complex with GppNHp (yellow). (A) Q61L; (B) Q61V; (C) Q61K; (D) Q61I (R32), (E) Q61G (PDB entry 1ZW6); (F) Wild Type (R32). Surfaces were constructed using PyMOL and colored based on atom type (N, blue; O, red; C, green). Only protein atoms were used to define the surface. Areas outlined in white define the protein/solvent interface. Wat175 represents the pre-catalytic water molecule.
Table 2.
Buried surface areas, average B-factors and interatomic distances for RasQ61 mutants in the non-catalytic conformation.
| Q61L Ras-GppNHp | Q61V Ras-GppNHp | Q61K Ras-GppNHp | Q61I Ras-GppNHp | |
|---|---|---|---|---|
| a Buried Surface Area | 934.87 Å2 | 926.71 Å2 | 934.35 Å2 | 936.03 Å2 |
| b Avg. B-factor (Residue 61) | 34.7 Å3 | 34.7 Å3 | 28.13 Å3 | 27.15 Å3 |
| 61 Cδ1... Tyr64 Cδ1 | 4.5 | n/a | 3.8 | 4.6 |
| 61 Cβ ... Tyr64 Cδ2 | 4.2 | 3.7 Å | 3.5 | 3.8 |
| 61 Cδ1 ... Pro34 Cβ | 4.0 | n/a | 3.6 | 3.8 |
| 61 C=O ... H-N Tyr64 | 2.9 | 2.9 | 2.8 | 2.7 |
| Tyr32 Cε2 ... Pro34 Cα | 3.8 | 3.8 | 3.8 | 3.7 |
| Tyr32 Cε2 ... Pro34 Cβ | 4.3 | 4.3 | 4.3 | 4.3 |
|
c Tyr64 Cε1 ... Pro34 Cβ Tyr64 Cε2 ... Pro34 Cβ |
3.3 | 3.3 | 4.0 | 4.0 |
| Tyr32 CZ ... Gly13 Cα | 3.9 | 3.9 | 3.9 | 3.9 |
|
d V61 Cγ1 ... H-N Glu63 I61 Cγ2 ... H-N Glu63 |
n/a | 3.9 | n/a | 3.9 |
| 61 N-H ... Wat 175 | 3.1 | 3.1 | 3.1 | 3.1 |
| Thr35 C=O...Wat 175 | 3.0 | 2.9 | 2.9 | 3.0 |
| O1G ... Wat 175 | 2.7 | 2.8 | 2.8 | 2.7 |
| Tyr32 OH ... O1G | 2.8 | 2.8 | 2.8 | 2.7 |
Buried surface Area was obtained using the Lee & Richards buried surface accessibility calculation in CNS.
The average B-factor was calculated using all atoms from each residue.
The closest distance was measured between Tyr64 and Pro34 and this was modulated by different orientations of the aromatic ring of Tyr64 relative to Q61.
Q61V and Q61I are the only mutants with carbon atoms that contact the upper portion of the switch. Distances are given in Å. A lack of interaction is indicated by ‘n/a’. Wat175 is the pre-catalytic water molecule.
Switch II in the canonical structures (P3221) and in the Ras/RasGAP complex forms an α-helix that spans residues 62 through 73 (PDB code 1CTQ). In our structures of the transforming mutants containing the hydrophobic cluster, the helix is observed only for the second part of the switch, spanning residues 68 through 73. The first part of switch II forms a type III β-turn with residues 61−64 representing the i, i+1, i+2 and i+3 positions respectively and a good H-bond between the C=O of residue 61 and the NH group of Y64 (Chou and Fasman, 1977) (Figure 3). The unwinding of the helix allows for extension of switch II toward switch I, and positioning of the residues 61 and 64 side chains to form the hydrophobic cluster. Residue 61 is central in this cluster, with its side chain making key van der Waals interactions to isolate the nucleotide from bulk solvent, with its amide N atom H-bonding to the pre-catalytic water molecule and its carbonyl group involved in the H-bond between residues i and i+3 in the type III β-turn (Table 2).
The moderately transforming mutant RasQ61I
The RasQ61I-GppNHp mutant crystallized in the presence of CaCl2 under conditions similar to those used to obtain crystals of the wild type and of the Q61L, Q61V and Q61K mutants with symmetry of space group R32. Interestingly, however, the vast majority of the RasQ61I-GppNHp crystals (about 80%) obtained under these conditions are of the canonical crystal form with symmetry of space group P3221. This was a surprise, since the crystallization conditions are different from those that normally yield the canonical form. In the more prominent form with P3221 symmetry, switch I has Y32 in the open conformation, switch II is disordered from residues 61−67 (removed from the model) and the end of the switch forms a helix as previously observed for the Q61L mutant in the canonical form (Krengel et al., 1990). Although the relative prominence of the two crystal forms in the crystallization drops cannot be correlated with the equilibrium constant between the open and closed forms of switch I in solution, it appears that the presence of I at position 61 is less favorable to the closed form than the L, V and K residues that result in strongly transforming mutations.
The structure of RasQ61I-GppNHp obtained from the R32 crystal form shows that switch II is highly ordered (Figure 2D) and that the I61 residue is involved in a hydrophobic cluster virtually identical to that seen in the strongly transforming mutants (Figure 3), with similar burying of the nucleotide and pre-catalytic water molecule (Figure 4 and Table 2). A superposition of this structure onto the RasQ61V, shows that the Cγ1 and Cγ2 atoms in the two mutants superimpose very well (Figure 3). Comparison with the RasQ61K structure shows good overlap of the Cβ, Cγ1 and Cδ atoms in I61 with the Cβ, Cγ and Cδ atoms of K61. Interestingly, in the Q61L and Q61K structures, where there is no branching from Cβ, the backbone of the Type III turn residues is in the order of 0.5 Å closer to switch I than in the Q61V and Q61I where the Cγ2 methyl group protrudes in the direction of the turn, forming a tight van der Waal's interaction within about 3.9 Å. On the other hand, the Cδ groups in K61 and I61 make tight van der Waal's contact with P34 within the hydrophobic cluster. In RasQ61V the close contact between the Cγ1 group and the Type III turn can be somewhat relieved due to the absence of a Cδ group, which provides more room toward switch I. In RasQ61L and RasQ61K, the presence of Cδ is compensated by a lack of strain toward the Type III turn. I61, however, encroaches both toward the turn and toward switch I. This may result in a more strained structure, which though it can exist, is less favored than in the strongly transforming mutants, thus resulting in a high prominence of the crystals with Y32 in the open conformation. Our results for RasQ61I suggest that the transforming power of the mutants at position 61 is correlated with the ability to form the hydrophobic cluster in the context of the switch I conformation seen in the Raps/Raf complex (Figure 5A).
Figure 5.
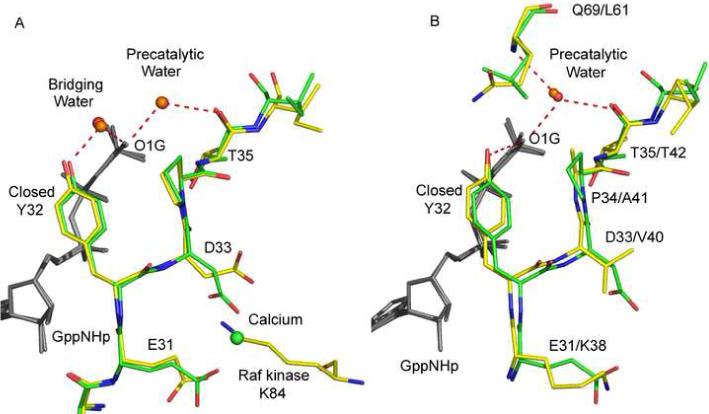
Switch I in the wild type and mutant Ras structures: comparisons with biologically relevant complexes. (A) Wild type Ras-GppNHp (green with water molecules in red) and Raps-GppNHp/Raf-RBD (yellow with water molecules in orange). (B) RasQ61L-GppNHp (green with water molecules in red) and Ran-GppNHp-Importinβ (yellow with water molecules in orange). The nucleotide is in gray. Red dashed lines represent hydrogen bonds.
The hydrophobic cluster is observed in a non-catalytic form of Ran-GTPase
A search through all of the GTPase structures in the Protein Data Bank revealed a striking similarity between the switch II conformation in transforming Ras61 mutants and that found in the Ran-GppNHp/importin-β complex (PDB code 1IBR) (Vetter et al., 1999) (Figure 3). Superposition of the nucleotide in the RasQ61L and Ran structures yields an average Cα rmsd of 0.48 Å for the switch II residues 60−65 and the arrangement of the active site is identical in the two structures (Figure 5B). Inhibition of the intrinsic Ran-GTP hydrolysis reaction by importin-β is an important aspect in the spatial control of nuclear import of proteins (Gorlich et al., 1997; Vetter et al., 1999). Importin-β that has released a cargo protein is transported by Ran-GTP from the nucleus to the cytoplasm, where RanBP1 and other factors aid in the release of importin-β from RanGTP and GAP catalyzed GTP hydrolysis converts Ran to the GDP bound form (Floer et al., 1997). It is critical to this transport mechanism that Ran remain in the GTP bound form until it reaches the cytoplasm, consistent with the experimental finding that Ran-GTP bound to importin-β is catalytically inactive (Gorlich et al., 1997). We call the associated structure the non-catalytic conformation of switch II. This non-catalytic conformation in the wild type Ran is stabilized by interactions with importin-β, but in Ras it is attained by the capacity of residue 61 to stabilize the hydrophobic cluster with switch I in the Ras/Raf complex.
Raf impairs intrinsic hydrolysis in RasQ61L, but not in wild type Ras
As a test to the idea that it is in the context of the Ras/Raf complex that the RasQ61 mutants exhibit their oncogenic phenotypes, a set of experiments were performed to qualitatively assess the effect of Raf on the hydrolysis of GTP by RasQ61L relative to its effect on the wild type protein. There are two domains in Raf known to interact with Ras. The first is the RBD, mentioned in the introduction, which interacts preferentially with Ras-GTP (or GTP analogue). The second is the Cystein Rich Domain (CRD), known to mediate activation of the Raf kinase and to bind Ras independent of the state of bound nucleotide (Thapar et al., 2004). In our experiments we used a construct of C-Raf containing residues 52−196, including both the RBD and CRD domains fused at the N-terminus to a 54-residue GB1 domain (referred to as Raf from here on).
Samples of freshly prepared RasQ61L-GTP were allowed to hydrolyze at room temperature either in the presence or absence of stoichiometric amounts of Raf. The reaction times ranged from 6 to 7 hours, beyond the time expected for completion of hydrolysis in the wild type, which has a half-life of 25 minutes at 37 °C (Herrmann et al., 1995), and in free RasQ61L even with its 10-fold decrease in GTPase activity (Der et al., 1986). Raf was added to the free RasQ61L sample before analysis by gel filtration chromatography. This step is important because Raf binds with nanomolar affinity to Ras-GTP and with only micromolar affinity to Ras-GDP, providing excellent separation between the two forms in gel filtration chromatography (Herrmann et al., 1995). The result for the RasQ61L/Raf hydrolysis is shown in Figure 6A. The first peak corresponds to elution of the RasQ61L/Raf complex, and the second peak represents free Ras (in the GDP-bound form). This is shown by SDS gel in the insert. The elution profile for the RasQ61L/Raf mixture soon after the GDP/GTP exchange reaction (not shown) is the same as at the end of the incubation period, indicating that the RasQ61L-GDP was initially there due to incomplete exchange, rather than resulting from GTP hydrolysis by RasQ61L/Raf. In the presence of Raf the RasQ61L mutant is essentially unable to hydrolyze GTP within the time frame of the experiment. Figures 6B, 6C and 6D represent the RasQ61L, wild type Ras/Raf and wild type Ras reactions respectively and show corresponding peaks in similar elution volumes, although the relative intensities of the peaks are reversed compared to those in the RasQ61L/Raf situation. In the absence of Raf, RasQ61L is able to hydrolyze GTP and at the end of 7 hours the results are indistinguishable from those for wild type either with or without Raf. In all three cases there is a small peak representing the complex due to the fact the some binding of Raf to Ras-GDP is expected in the micromolar concentrations in which the experiments were performed (Herrmann et al., 1995).
Figure 6.
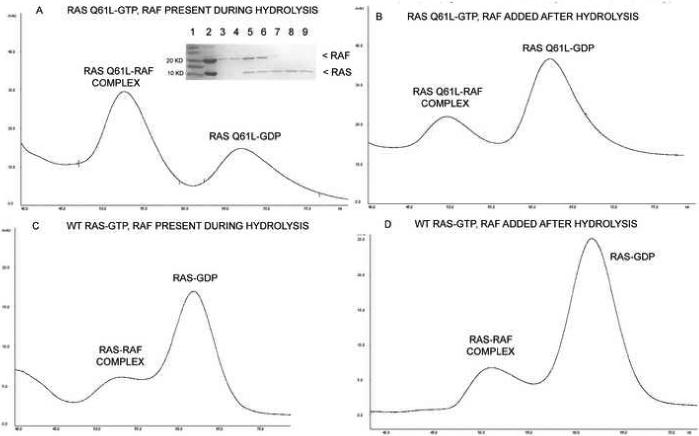
Gel filtration chromatography results from the hydrolysis experiments. A) RasQ61L incubated with Raf. The contents of the elution peaks are shown in the inserted SDS gel. Lane 1: MW markers; Lane 2: sample injected into the column; Lanes 3 and 4: Raf, which tends to aggregate at 4°C and elute near the void volume (not shown in elution profile); Lanes 5 and 6: fractions taken from the first elution peak showing a 1:1 ratio of Raf and Ras; Lanes 7, 8 and 9: second elution peak containing the Ras protein. B) RasQ61L incubated without Raf. C) Wild type Ras incubated with Raf. D) Wild type Ras incubated without Raf. For results shown in B and D Raf was added before gel filtration.
Water molecules near the γ-phosphate in wild type Ras-GppNHp
A thorough analysis of water molecules in the active site of Ras with Y32 in an open conformation has previously been published based on the Ras-GppNHp (PDB code 1CTQ) and Ras-GTP (PDB code 1QRA) structures, both solved from the canonical crystal form with P3221 symmetry (Scheidig et al., 1999). Except for the water molecules that coordinate the Mg2+ ion, the arrangement of active site water in our wild type Ras-GppNHp structure, where Y32 is closed over the nucleotide, is significantly different from that seen in the open form and exactly as observed for the Raps-GppNHp/Raf-RBD structure (Figure 5A) (PDB code 1GUA) (Nassar et al., 1995). In our wild type structure, two water molecules H-bond to the least buried oxygen atom of the γ-phosphate (O1G). The first is the so-called pre-catalytic water molecule proposed to be the nucleophile in the hydrolysis reaction. It is 2.7 Å from the O1G atom of the γ-phosphate and 2.9 Å from the carbonyl oxygen atom of T35. The pre-catalytic water molecule is analogous to Wat175 in the Ras-GTP structure (1QRA), not far from the position found in the GAP catalyzed transition state mimic (Scheffzek et al., 1997). The second water molecule in the active site is 2.6 Å from the O1G atom and 2.5 Å from the hydroxyl group of Y32, bridging between the two groups (Figure 5A). In the previously published weakly transforming mutant RasQ61G-GppNHp (PDB code 1ZW6) (Figure 4E) as well as in our wild type Ras-GppNHp structure (Figure 4F) a water channel links the γ-phosphate to the bulk solvent. It has been suggested that a water channel plays an important role in both facilitating a shift of the pre-catalytic water molecule for inline nucleophilic attack on the γ-phosphate and in providing a path for product release (Pasqualato and Cherfils, 2005). The water channel is completely absent in the strongly transforming Q61L, Q61V and Q61K mutants (Figures 4A, 4B and 4C respectively) as well as in the closed conformation of the moderately transforming mutant Q61I (Figure 4D).
Water molecules near the γ-phosphate in the Ras mutants
The structures the Q61L, Q61V, Q61K and Q61I mutants of Ras-GppNHp reveal a somewhat different water arrangement. The pre-catalytic water molecule is found in the same location as in the wild type, with nearly ideal H-bonds to the O1G atom of the γ-phosphate and to the carbonyl oxygen atom of T35 (Table 2). In addition, this water molecule makes a good H-bond to the backbone amide of residue 61 in the respective structures and is isolated from bulk solvent by the hydrophobic cluster that closes over the γ-phosphate group, obstructing the water channel that exists both in the wild type and in the weakly transforming Q61G mutant. The bridging water molecule is completely absent in our four transforming mutant structures and instead, there is a direct H-bond between the hydroxyl group of Y32 and the O1G atom of the γ-phosphate. This is exactly the situation observed in the Ran/importin-β structure, where the hydroxyl group of Y39 makes a 2.9 Å H-bond to the γ-phosphate oxygen atom of GppNHp (Figure 5B).
Discussion
Since the transformation experiments with RasQ61 mutants (Der et al., 1986), the crystal structures of several oncogenic Ras mutants have been solved, including those of RasQ61L (highly transforming) and RasQ61H (moderately transforming) (Krengel et al., 1990). However, these structures were derived from crystals having symmetry of space group P3221, which most closely mimics the conformation of the GAP-bound Ras structure. While these structures offer important insights into the overall reduced activity of these mutants, they do not explain the large variation in transformation efficiency between different mutations at residue 61. In thinking about transformation it is particularly important to understand how the structural features of switch II might affect intrinsic hydrolysis in the Ras/Raf complex, where switch I is locked in the closed conformation with Y32 interacting with the γ-phosphate through the bridging water molecule (Nassar et al., 1996) and with RasGAP unable to bind Ras (Moodie et al., 1995). There is strong experimental evidence that switch II is not involved in the Ras/Raf interaction (Thapar et al., 2004) and it is therefore a reasonable assumption that it may be disordered in the complex. This situation is very closely mimicked in the context of the crystalline environment having symmetry of space group R32, with switch I in the conformation found in the Raps/Raf complex and switch II disordered in the wild type protein.
Proposed contribution of Y32 and bridging water molecule to intrinsic catalysis in Ras
Two critical residues in GAP catalyzed GTP hydrolysis are Ras Q61 and RasGAP R789 (Arg finger) (Scheffzek et al., 1997). A role for Q61 as a general base for activation of the catalytic water is unlikely, but it is thought to be a critical residue in positioning the water molecule during the reaction (Maegley et al., 1996). The role of the Arg finger, inserted into the active site, is to stabilize the negative charge that develops on the bridging oxygen between the β and γ phosphate atoms of the nucleotide during catalysis (Kosloff and Selinger, 2001; Li and Zhang, 2004). In the RasGAP catalyzed reaction, Y32 is in an open conformation, interacting intimately at the Ras/RasGAP interface where it is not involved directly in catalysis. In the presence of Raf, catalysis must occur with Y32 in the pocket that is occupied by R789 in the Ras/RasGAP complex. In this situation, it is much more likely that Y32 is involved in the catalytic mechanism.
There is evidence in the literature that the base that activates the water molecule for nucleophilic attack in the hydrolysis reaction is the γ-phosphate of GTP itself, in a substrate assisted catalytic mechanism where the abstracted proton ends up being shared by the Pi and the β-phosphate of the GDP leaving group in the product (Kosloff and Selinger, 2001; Pasqualato and Cherfils, 2005). One objection to this mechanism is that electron density would be stabilized at the γ-phosphorous atom, which is inconsistent with a reaction mechanism where the outcome is an increase in negative charge at the oxygen bridging the β and γ-phosphorus atoms (Maegley et al., 1996). In the GAP catalyzed reaction, where the transition state and product are highly stabilized by the Arg finger in the Ras/RasGAP complex, this may be a negligible effect, but in intrinsic catalysis it could be more of a problem. This problem would be alleviated, however, if the abstracted proton were to become part of a hydrogen-bonding network where it could be donated to an electronegative acceptor. This is exactly the situation observed in our wild type Ras-GppNHp structure and in the Raps-GppNHp/Raf-RBD complex, where the O1G oxygen atom of the γ-phosphate is 2.7 Å from the pre-catalytic water molecule and 2.6 Å from the water molecule bridging to the hydroxyl group of the Y32 side chain (Figure 5A). During the course of the reaction, there is inversion of configuration at the γ-phosphate. The arrangement of the bridging water molecule and Y32 allows flexibility for this water to accompany the reaction coordinate, following the dynamic charge shifts that must occur during the reaction, and facilitating transfer of the proton to the β-phosphate as negative charge accumulates there in the transition state and as GDP is formed. This situation would be one in which the interaction between R789 and both the β and γ-phosphate oxygen atoms seen in GAP catalyzed hydrolysis would be mimicked, albeit in a much weaker form, by the bridging water molecule and Y32 together during intrinsic catalysis. In this context the importance of Y32 is two-fold: the lone pair of electrons on its hydroxyl group can accept an H-bond from the bridging water molecule, which increases the tendency of this molecule to accept the H-bond from the O1G γ-phosphate oxygen atom; and the Y side chain has the ability to precisely position the bridging water molecule within the active site.
In the ground state the pre-catalytic water molecule donates an H-bond to the carbonyl oxygen atom of T35 (2.9Å). It is not inline with the γ-phosphorous atom, but is shifted toward the O1G oxygen of the phosphate group (Pasqualato and Cherfils, 2005). Q61 is disordered in the ground state. During the reaction, as a hydrogen is abstracted by the γ-phosphate O1G atom and donated in an H-bonding interaction to the bridging water molecule, the catalytic water is activated and the H-bond to the carbonyl group of T35 is no longer favored. This perhaps facilitates the shift in position necessary for inline nucleophilic attack on the γ-phosphate and as the reaction proceeds Q61 may interact with the newly formed transition state, as it does in the Ras/RasGAP transition state mimic (Scheffzek et al., 1997).
The structure of switch II in transforming mutants is consistent with a non-catalytic Ras
The crystal structures presented here show that in the context of a closed conformation of switch I, with Y32 over the nucleotide, transformation is highly correlated with the ability of the mutated residue to form the hydrophobic cluster. There are two important features of this conformation consistent with a non-catalytic version of the GTPases. One is that the hydrophobic cluster completely shields the γ-phosphate and pre-catalytic water molecule from the bulk solvent (Figure 4), so that even if the reaction were to occur, the release of the Pi would be severely hindered. In contrast, the wild type and the weakly transforming mutant RasQ61G (Ford et al., 2006) have an open water channel into the active site (Figure 4). The second feature of the conformation found in the transforming mutants is that the hydrogen-bonding network in the active site is changed by Y32 making a direct H-bond to the γ-phosphate O1G atom (excluding the bridging water molecule) and by the formation of a good H-bond between the backbone amide group of residue 61 and the pre-catalytic water molecule (Figures 3 and 5; Table 2). In this situation the pre-catalytic water molecule could still donate a hydrogen bond to the O1G atom, which could in turn be donated to the hydroxyl group of Y32. However, because of the resonance involving the aromatic ring, the hydroxyl oxygen atom is sp2 hybridized, with its H-atom and lone pair tending to stay in the plane of the ring (Thanki et al., 1988). The H-bonding orientation is therefore highly controlled by the overall rotational freedom of the Y32 side chain. This side chain in turn is sandwiched between P34, G13 and the sugar moiety of the nucleotide in a restricted conformation that would not favor transfer of the abstracted hydrogen atom to the β-γ bridging oxygen during the reaction. Interestingly, the hydroxyl oxygen atom is positioned to interact ideally with the O1G atom of the γ-phosphate, which is in the plane of the ring in the ground state.
In the non-catalytic conformation, the buried pre-catalytic water molecule still donates an H-bond to the backbone carbonyl group of T35 and to the O1G atom of the γ-phosphate, but in addition it accepts an H-bond from the backbone amide of residue 61. This new H-bond is likely to diminish the nucleophilicity of the water molecule, helping to stabilize the entire system in a non-reactive ground state. Taken together, the features resulting from formation of the hydrophobic cluster create a strongly anti-catalytic situation, where the H-bonding network is ideal in the ground state, the pre-catalytic water is more difficult to activate and the entire complex is trapped in the active site.
Intrinsic catalysis in vitro versus transformation efficiency in cells
Over 20 years ago it was proposed that residue 61 represents a strong conformation-determining region in Ras that modulates differences in affinity for effectors or regulatory molecules in accordance with the observed transforming potency (Der et al., 1986). However, in spite of a large number of biochemical and structural studies on Ras, its mutants and related GTPases, there has been no explanation for the fact that all mutations at residue 61 show approximately 10-fold decrease in Ras intrinsic GTPase activity measured in vitro while there is a 1000-fold range in in vivo transformation efficiency. The structures presented here shed light on this problem, supporting the idea that the conformation of residue 61 is indeed associated with transformation efficiency in the cell.
We propose that the key to understanding the differences in the in vitro versus the in vivo results is to consider that intrinsic hydrolysis was measured in vitro in the absence of Raf, with switch I in equilibrium between two or more conformations as shown by NMR (Geyer et al., 1996). In this situation, the hydrophobic cluster, which involves Y32 and other switch I residues, is unstable and does not result in a non-catalytic Ras. The nature of the mutation may then be negligible and the uniform reduction in GTPase activity is due to detrimental changes that occur when the Q61 side chain is removed. In the in vivo experiments the situation is much more complex and we propose that it is in the Raf-bound state that the identity of the mutation becomes important. This idea is supported by our hydrolysis experiments showing a strong damping effect of Raf on the RasQ61L GTPase reaction, which was not observed for wild type Ras (Figure 6). When bound to Raf, Ras is entirely in state 2, with Y32 in the closed conformation (Geyer et al., 1996). This provides a stable docking surface composed of Y32 and P34 with which the aliphatic portions of a variety of side chains at position 61 can interact to form the non-catalytic conformation. This is completely consistent with the finding that V, L, K, A, C and R are strongly transforming mutations (Der et al., 1986). All of these, except for K and R, have side chains with hydrophobic character (Radzicka and Wolfenden, 1988) and based on the common non-catalytic conformation, are expected to fit well into the hydrophobic cluster. The R side chain has an aliphatic portion that is one carbon shorter than that of K, but it is probably positioned similarly, with its charged head group toward the bulk solvent. On the other hand, Y, W and F are too bulky to significantly sample the non-catalytic conformation and accordingly have much weaker transformation efficiencies (Der et al., 1986). We propose that the moderately transforming mutants N, H, I, M and T have side chains that frequently sample the non-catalytic conformation but are either a little too bulky or too polar for optimal stabilization of the hydrophobic cluster. Gly is the most weakly transforming of all the mutations, while Pro and Glu are equivalent to the wild type and essentially show no transformation unless highly overexpressed (Der et al., 1986). Gly, with no side chain, cannot contribute to the hydrophobic cluster. In this structure, there is a direct H-bond between Y32 and the O1G atom of the γ-phosphate, a feature that could perhaps explain the measurable, although weakly transforming phenotype of the mutant. Glu would be expected to be similar to Q in size, shape and the ability participate in intrinsic catalysis (Frech et al., 1994). Interestingly, P would be somewhat strained with a ϕ dihedral angle of about −85° in the non-catalytic conformation of switch II, given that its ideal ϕ angle is −60°. However, even if it were able to attain this conformation, its backbone N atom could not donate an H-bond to the pre-catalytic water molecule, disrupting a component of the anti-catalytic arrangement observed for the transforming mutants. These two features together could be sufficient to destabilize the non-catalytic conformation in the G61P mutant, opening the water channel to the active site.
Our results are consistent with a scenario where the transformation efficiency of the mutant is directly correlated with its ability to stabilize the non-catalytic conformation in the context of the Ras/Raf complex, rather than one in which each mutant stabilizes a different conformation which is more or less transforming. They offer a rational for the previously obtained transformation efficiencies observed for RasQ61 mutants and provide a mechanism to explain how the conformation first observed and proven to be non-catalytic in the Ran/importin-β complex can result in a severely impaired enzyme in the context of the Ras/Raf complex.
Experimental Procedures
All of the experiments were done using a truncated version of H-Ras, containing residues 1−166. Dr. Sharon Campbell (UNC - Chapel Hill) provided the expression systems for wild type Ras, the RasQ61L mutant and the C-Raf construct containing the RBD and CRD domains (residues 52−196). The QuikChange II Site-Directed Mutagenesis Kit from Stratagene was used, following the manufacturers instructions, to obtain RasQ61V, RasQ61K and RasQ61I. The DNA coding for each protein was cloned into the pET21A(+) vector (Novagen) and transformed into E. coli BL21 cells (Novagen) for expression and purification.
Wild type and mutant Ras: expression, purification and crystallization
Plasmids containing the wild type and the Q61 mutant Ras genes were expressed in BL21 E. coli cells and purified as previously described (Buhrman et al., 2003). The GDP was exchanged for the GTP analogue GppNHp using published procedures (Stumber et al., 2002). Protein in a buffer solution containing 20 mM Tris pH 8.0, 50 mM NaCl, 5 mM MgCl2 , 1 mM DTT, 5% glycerol and 20μM GppNHp was concentrated and used immediately for crystallization or stored in 50 μL aliquots at −80 °C. Crystals were grown by the hanging drop vapor diffusion method at 18 °C. The initial crystallization drops contained 4 μL of the protein solution and 4 μL of the reservoir solution. For crystals of wild type Ras-GppNHp the purified protein solution was concentrated to 15−20 mg/mL and the reservoir solution consisted of 200 mM CaCl2, 20% PEG 3350 (PEG Ion Screen condition #7 from Hampton Research). For crystals of the Q61L mutant the purified protein was concentrated to 10−15 mg/mL and the reservoir solution consisted of 200 mM CaCl2, 25 % PEG3350 and 1mM DTT. Crystals grew in 5−10 days to an average size of 0.5 mm3 and were flash-frozen in liquid nitrogen directly from the crystallization drop without additional cryoprotectant.
The final protein concentration for RasQ61K was 12mg/mL in a buffer solution containing 20mM Hepes pH 7.5, 20mM MgCl2, 50mM NaCl and 1mM DTT (stabilization buffer). Crystals were obtained using the hanging drop vapor diffusion method at 18 °C, in drops containing 2 μL of protein solution and 2 μL of reservoir solution (500μL PEG Ion Screen #7 and 200 uL of stabilization buffer). Crystals of Q61K took 3−5 days to form. Crystals were exchanged into a cryoprotectant solution consisting of 800 uL of PEG Ion Screen #7 and 200 uL PEG 400 immediately prior to flash freezing in liquid nitrogen.
The final protein concentration for the Q61V mutant was 5 mg/mL in stabilization buffer. Crystals were obtained from wells containing 400 uL of PEG Ion Screen #7, 100 uL of stabilization buffer and 150 uL PEG 400. They grew in 3−5 days to an average size of 0.1 mm3 and were cryoprotected as with Q61K.
The final protein concentration for the Q61I mutant was 12 mg/mL in stabilization buffer. Crystals of Q61I were obtained from wells containing 500 uL of PEG Ion Screen #7 and 150 uL of 50% PEG 6000 or 8000. Crystals of Q61I grew in 7 days. The cryoprotectant for the RasQ61I crystals was a solution of 500μL of PEG Ion Screen #7, 200μL of stabilization buffer, 100μL of 50% PEG 6000 and 200μL of glycerol.
For the hydrolysis experiments Ras-GDP was exchanged for Ras-GTP following a published procedure (Cheng et al., 2001). The protein was kept at 4 °C for immediate use in the experiments.
Data Collection and structure refinement
High resolution data for the wild type Ras and the Q61 mutants were collected at 100K on the Ser-CAT ID-22 beamline at APS (Argonne, IL), using a Mar CCD detector. The X-rays were tuned to a wavelength of 1.0 Å. Exposure was from 1 to 3 seconds with an oscillation angle of 1° and a crystal to detector distance of 120mm. The data were processed with HKL2000 (Otwinowski and Minor, 1997).
The structure of H-Ras 166 (pdb code 1CTQ) with all non protein atoms and residues 61−71 deleted from the model and with atomic B-factors set at 30 Å3, was used as an initial search model for molecular replacement using the program Crystallography and NMR System (CNS) (Brunger et al., 1998). CNS was also used for all reciprocal space refinement with 10% of the unique reflections set aside for the calculation of Rfree (Kleywegt and Brunger, 1996). The best molecular replacement solution was applied to generate a model used for rigid body refinement at 2.5 Å, followed by rigid body refinement at 2.0 Å, simulated annealing, energy minimization and group B-factor refinement in CNS prior to generation of 2Fo-Fc and Fo-Fc electron density maps. Manual rebuilding was done in O (Jones et al., 1991) and COOT (Emsley and Cowtan, 2004). CNS was used in successive rounds of energy minimization and individual B-factor refinement. The GppNHp molecule was added to the model early in the refinement. Water molecules and ions were added in successive rounds of manual rebuilding. The final model for the wild type protein was used to phase the RasQ61L mutant. The final model for the RasQ61L mutant structure was used to phase RasQ61K, RasQ61V and RasQ61I. The initial search model (pdb code 1CTQ) was used to phase the RasQ61I mutant structure from crystals with the symmetry of the P3221 space group.
Coordinates and structure factors have been deposited in the PDB with the following accession codes: WT Ras ; Q61L ; Q61V ; Q61K ; Q61I (R32) ; Q61I (P3221).
C-Raf(RBD-CRD): expression and purification
The C-Raf(52−196) construct was expressed from a Protein G expression vector (GEV2) with a N-terminal GB1 tag for increased solubility and a C-terminal His tag for affinity purification. After standard overexpression in E. coli, Raf was purified by affinity chromatography, using a 5mL Nickel-NTA column (Amersham Pharmacia). Protein was solubilized in Buffer A (20mM Hepes pH 7.5, 500 mM NaCl, 30mM imidazole, 5mM β- mercaptoethanol) and eluted from a gradient (80% in 80 mLs) of increasing Buffer B (20mM Hepes pH 7.5, 50mM NaCl, 500mM Imidazole, 5 mM β-mercaptoethanol). Raf elutes from 50−70% Buffer B. Raf protein was pooled, dialyzed into Buffer A minus the imidazole, concentrated to 3 mg/mL by placing the dialysis bag on a bed of polyethylene glycol (M.W. 20,000) and stored at −80 °C.
Hydrolysis experiments
The hydrolysis reactions were performed at room temperature in a total volume of 2.5 mL of Hydrolysis Buffer (50mM Tris pH 7.5, 10mM DTT, 50mM NaCl, 10mM MgCl2) and allowed to proceed for 6−7 hours. The experiments were repeated independently to insure reproducibility. Either RasQ61L or wild type Ras was present initially at a concentration of 8−10 μM after exchange to GTP and allowed to incubate in two sets of parallel experiments: one in which a stoichiometric amount of Raf was added at the onset of the reaction and one in which it was added 30 minutes before being transferred to 4 °C, where the reaction in all cases was slowed down for analysis. Samples were run on S-100 gel filtration columns pre-equilibrated in Hydrolysis Buffer + 5% glycerol at 4 °C before and after the incubation period. Gel filtration with Ras-GDP/Raf showed a small amount of complex.
Acknowledgments
Diffraction data were collected at the Southeast Regional Collaborative Access Team (SER-CAT) 22-ID beamline at the Advance Photon Source, Argonne National Laboratory. Use of the Advanced Photon Source was supported by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences, under Contract No. W-31-109-Eng-38. Winnell Newman made the RasQ61V mutant and Dr. Zhongmin Jin collected and processed the data set for this mutant. Susan Fetics helped with details of the Raf purification. This research is supported by a grant from the NIH (1 R01 CA096867-01A1).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adari H, Lowy DR, Willumsen BM, Der CJ, McCormick F. Guanosine triphosphatase activating protein (GAP) interacts with the p21 ras effector binding domain. Science. 1988;240:518–521. doi: 10.1126/science.2833817. [DOI] [PubMed] [Google Scholar]
- Barbacid M. ras genes. Annual review of biochemistry. 1987;56:779–827. doi: 10.1146/annurev.bi.56.070187.004023. [DOI] [PubMed] [Google Scholar]
- Brunger AT, Adams PD, Clore GM, DeLano WL, Gros P, Grosse-Kunstleve RW, Jiang JS, Kuszewski J, Nilges M, Pannu NS, et al. Crystallography & NMR system: A new software suite for macromolecular structure determination. Acta crystallographica. 1998;54:905–921. doi: 10.1107/s0907444998003254. [DOI] [PubMed] [Google Scholar]
- Buhrman G, de Serrano V, Mattos C. Organic solvents order the dynamic switch II in Ras crystals. Structure. 2003;11:747–751. doi: 10.1016/s0969-2126(03)00128-x. [DOI] [PubMed] [Google Scholar]
- Campbell SL, Khosravi-Far R, Rossman KL, Clark GJ, Der CJ. Increasing complexity of Ras signaling. Oncogene. 1998;17:1395–1413. doi: 10.1038/sj.onc.1202174. [DOI] [PubMed] [Google Scholar]
- Cheng H, Sukal S, Deng H, Leyh TS, Callender R. Vibrational structure of GDP and GTP bound to RAS: an isotope-edited FTIR study. Biochemistry. 2001;40:4035–4043. doi: 10.1021/bi0021131. [DOI] [PubMed] [Google Scholar]
- Chou PY, Fasman GD. Beta-turns in proteins. Journal of molecular biology. 1977;115:135–175. doi: 10.1016/0022-2836(77)90094-8. [DOI] [PubMed] [Google Scholar]
- Der CJ, Finkel T, Cooper GM. Biological and biochemical properties of human rasH genes mutated at codon 61. Cell. 1986;44:167–176. doi: 10.1016/0092-8674(86)90495-2. [DOI] [PubMed] [Google Scholar]
- Emsley P, Cowtan K. Coot: model-building tools for molecular graphics. Acta crystallographica. 2004;60:2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- Floer M, Blobel G, Rexach M. Disassembly of RanGTP-karyopherin beta complex, an intermediate in nuclear protein import. The Journal of biological chemistry. 1997;272:19538–19546. doi: 10.1074/jbc.272.31.19538. [DOI] [PubMed] [Google Scholar]
- Ford B, Hornak V, Kleinman H, Nassar N. Structure of a transient intermediate for GTP hydrolysis by ras. Structure. 2006;14:427–436. doi: 10.1016/j.str.2005.12.010. [DOI] [PubMed] [Google Scholar]
- Frech M, Darden TA, Pedersen LG, Foley CK, Charifson PS, Anderson MW, Wittinghofer A. Role of glutamine-61 in the hydrolysis of GTP by p21H-ras: an experimental and theoretical study. Biochemistry. 1994;33:3237–3244. doi: 10.1021/bi00177a014. [DOI] [PubMed] [Google Scholar]
- Geyer M, Schweins T, Herrmann C, Prisner T, Wittinghofer A, Kalbitzer HR. Conformational transitions in p21ras and in its complexes with the effector protein Raf-RBD and the GTPase activating protein GAP. Biochemistry. 1996;35:10308–10320. doi: 10.1021/bi952858k. [DOI] [PubMed] [Google Scholar]
- Gorlich D, Dabrowski M, Bischoff FR, Kutay U, Bork P, Hartmann E, Prehn S, Izaurralde E. A novel class of RanGTP binding proteins. The Journal of cell biology. 1997;138:65–80. doi: 10.1083/jcb.138.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall BE, Bar-Sagi D, Nassar N. The structural basis for the transition from Ras-GTP to Ras-GDP. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:12138–12142. doi: 10.1073/pnas.192453199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- Herrmann C, Martin GA, Wittinghofer A. Quantitative analysis of the complex between p21ras and the Ras-binding domain of the human Raf-1 protein kinase. The Journal of biological chemistry. 1995;270:2901–2905. doi: 10.1074/jbc.270.7.2901. [DOI] [PubMed] [Google Scholar]
- Ito Y, Yamasaki K, Iwahara J, Terada T, Kamiya A, Shirouzu M, Muto Y, Kawai G, Yokoyama S, Laue ED, et al. Regional polysterism in the GTP-bound form of the human c-Ha-Ras protein. Biochemistry. 1997;36:9109–9119. doi: 10.1021/bi970296u. [DOI] [PubMed] [Google Scholar]
- Jones TA, Zou JY, Cowan SW, Kjeldgaard M. Improved methods for building protein models in electron density maps and the location of errors in these models. Acta Crystallogr A. 1991;47(Pt 2):110–119. doi: 10.1107/s0108767390010224. [DOI] [PubMed] [Google Scholar]
- Kleywegt GJ, Brunger AT. Checking your imagination: applications of the free R value. Structure. 1996;4:897–904. doi: 10.1016/s0969-2126(96)00097-4. [DOI] [PubMed] [Google Scholar]
- Kosloff M, Selinger Z. Substrate assisted catalysis -- application to G proteins. Trends in biochemical sciences. 2001;26:161–166. doi: 10.1016/s0968-0004(00)01748-5. [DOI] [PubMed] [Google Scholar]
- Krengel U, Schlichting L, Scherer A, Schumann R, Frech M, John J, Kabsch W, Pai EF, Wittinghofer A. Three-dimensional structures of H-ras p21 mutants: molecular basis for their inability to function as signal switch molecules. Cell. 1990;62:539–548. doi: 10.1016/0092-8674(90)90018-a. [DOI] [PubMed] [Google Scholar]
- Lee B, Richards FM. The interpretation of protein structures: estimation of static accessibility. Journal of molecular biology. 1971;55:379–400. doi: 10.1016/0022-2836(71)90324-x. [DOI] [PubMed] [Google Scholar]
- Li G, Zhang XC. GTP hydrolysis mechanism of Ras-like GTPases. Journal of molecular biology. 2004;340:921–932. doi: 10.1016/j.jmb.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Maegley KA, Admiraal SJ, Herschlag D. Ras-catalyzed hydrolysis of GTP: a new perspective from model studies. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:8160–8166. doi: 10.1073/pnas.93.16.8160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moodie SA, Paris M, Villafranca E, Kirshmeier P, Willumsen BM, Wolfman A. Different structural requirements within the switch II region of the Ras protein for interactions with specific downstream targets. Oncogene. 1995;11:447–454. [PubMed] [Google Scholar]
- Nassar N, Horn G, Herrmann C, Block C, Janknecht R, Wittinghofer A. Ras/Rap effector specificity determined by charge reversal. Nature structural biology. 1996;3:723–729. doi: 10.1038/nsb0896-723. [DOI] [PubMed] [Google Scholar]
- Nassar N, Horn G, Herrmann C, Scherer A, McCormick F, Wittinghofer A. The 2.2 A crystal structure of the Ras-binding domain of the serine/threonine kinase c-Raf1 in complex with Rap1A and a GTP analogue. Nature. 1995;375:554–560. doi: 10.1038/375554a0. [DOI] [PubMed] [Google Scholar]
- Otwinowski Z, Minor W. Processing of X-ray diffraction data collected in oscillation mode. Methods in Enzymology. 1997;276:307–326. doi: 10.1016/S0076-6879(97)76066-X. [DOI] [PubMed] [Google Scholar]
- Pasqualato S, Cherfils J. Crystallographic evidence for substrate-assisted GTP hydrolysis by a small GTP binding protein. Structure. 2005;13:533–540. doi: 10.1016/j.str.2005.01.014. [DOI] [PubMed] [Google Scholar]
- Radzicka A, Wolfenden R. Comparing the Polarities of the Amino Acids: Side-Chain Distribution Coefficients between the Vapor Phase, Cyclohexane, 1-Octanol, and Neutral Aqueous Solution. Biochemistry. 1988;27:1664–1670. [Google Scholar]
- Scheffzek K, Ahmadian MR, Kabsch W, Wiesmuller L, Lautwein A, Schmitz F, Wittinghofer A. The Ras-RasGAP complex: structural basis for GTPase activation and its loss in oncogenic Ras mutants. Science. 1997;277:333–338. doi: 10.1126/science.277.5324.333. [DOI] [PubMed] [Google Scholar]
- Scheidig AJ, Burmester C, Goody RS. The pre-hydrolysis state of p21(ras) in complex with GTP: new insights into the role of water molecules in the GTP hydrolysis reaction of ras-like proteins. Structure. 1999;7:1311–1324. doi: 10.1016/s0969-2126(00)80021-0. [DOI] [PubMed] [Google Scholar]
- Sprang SR. G protein mechanisms: insights from structural analysis. Annual review of biochemistry. 1997;66:639–678. doi: 10.1146/annurev.biochem.66.1.639. [DOI] [PubMed] [Google Scholar]
- Stumber M, Geyer M, Graf R, Kalbitzer HR, Scheffzek K, Haeberlen U. Observation of slow dynamic exchange processes in Ras protein crystals by 31P solid state NMR spectroscopy. Journal of molecular biology. 2002;323:899–907. doi: 10.1016/s0022-2836(02)01010-0. [DOI] [PubMed] [Google Scholar]
- Thanki N, Thornton JM, Goodfellow JM. Distributions of water around amino acid residues in proteins. Journal of molecular biology. 1988;202:637–657. doi: 10.1016/0022-2836(88)90292-6. [DOI] [PubMed] [Google Scholar]
- Thapar R, Williams JG, Campbell SL. NMR characterization of full-length farnesylated and non-farnesylated H-Ras and its implications for Raf activation. Journal of molecular biology. 2004;343:1391–1408. doi: 10.1016/j.jmb.2004.08.106. [DOI] [PubMed] [Google Scholar]
- Vetter IR, Arndt A, Kutay U, Gorlich D, Wittinghofer A. Structural view of the Ran-Importin beta interaction at 2.3 A resolution. Cell. 1999;97:635–646. doi: 10.1016/s0092-8674(00)80774-6. [DOI] [PubMed] [Google Scholar]


