Abstract
As a result of alternative splicing, the D2 gene of the dopamine receptor family exists in two isoforms. The D2 long is characterized by the insertion of 29 amino acids in the third cytoplasmic loop, which is absent in the short isoform. We have produced subtype-specific antibodies against both the D2 short and D2 long isoforms and found a unique compartmentalization between these two isoforms in the primate brain. The D2 short predominates in the cell bodies and projection axons of the dopaminergic cell groups of the mesencephalon and hypothalamus, whereas the D2 long is more strongly expressed by neurons in the striatum and nucleus accumbens, structures targeted by dopaminergic fibers. These results show that the splice variants of the dopamine D2 receptor are differentially distributed and possess distinct functions. The strategic localization of the D2 short isoform in dopaminergic cell bodies and axons strongly suggests that this isoform is the likely dopamine autoreceptor, whereas the D2 long isoform is primarily a postsynaptic receptor.
Among all the neurotransmitter receptors cloned to date, the D2 subtype of dopamine receptors has been the one most closely linked to the positive symptoms of schizophrenia (1, 2) and often implicated in extrapyramidal side effects (3, 4). Neuroanatomical (5, 6) and physiological (7, 8) studies indicate that this receptor has both autoreceptor and postsynaptic functions. Further, recent evidence of loss of autoreceptor function in D2-deficient mice (8) but sparing of this function in D3 mutant mice (9) strongly suggests that the D2 subtype is the only autoreceptor within the dopamine system. However, the D2 receptor exists in two isoforms (10, 11), and it is not known if both isoforms contribute to autoreceptor function. With the use of recently developed subtype-specific antibodies to dopamine D2 short (D2S) and D2 long (D2L) isoforms, we have found prevalent labeling of the D2S isoform in the cell bodies and axons of brain stem dopamine neurons, suggesting that this isoform is the autoreceptor of the dopamine system.
METHODS
Preparation of Antibodies.
The D2S peptide TPLKDAAR and D2L peptide SNGSFPVNRRRM corresponding to residues 238–245 and 259–270 (10, 11), respectively, were derived from the third cytoplasmic loop of the receptor. The D2S peptide was arranged by adopting four amino acids from each side of the insertion site to differentiate between D2S and D2L isoforms. A similar procedure has previously been used for the preparation of antibodies to γ-aminobutyric acid (GABA) receptors (12). The peptides were coupled to keyhole limpet hemocyanin (KLH) protein. Peptide–KLH conjugate (100 μg) emulsified in complete Freund’s adjuvant was injected into rabbits for antibody development. Affinity purification of the antisera was as described elsewhere (12). In brief, peptide (5 mg) was coupled to 1 g of activated thiopropyl-Sepharose 6B (Pharmacia LKB). Phosphate-buffered saline (PBS) diluted antiserum (1:5) was circulated through the column. After washing with PBS, the antibody was eluted with glycine⋅HCl, pH 2.3, and dialyzed against PBS.
Membrane Preparation.
Adult rhesus monkey (Macaca mulatta) brains were used for the preparation of tissue membranes as described earlier (13). Briefly, brain tissues were homogenized with 10% sucrose in Tris⋅HCl, pH 7.4, and centrifuged at 3,000 rpm in a Sorvall for 5 min. The supernatant was centrifuged at 35,000 rpm for 1 h in Beckman ultracentrifuge, and the resultant pellet was washed three times in 50 mM Tris⋅HCl, pH 7.4. The membranes were suspended, and their protein concentration was determined by the Lowry method.
Immunoblots.
Striatal and substantia nigra membrane proteins (17 μg per lane) and recombinant membranes of Sf9 cells (BioSignal) expressing various dopamine receptors (2 μg per lane) were separated by SDS/10% PAGE and transblotted. Nitrocellulose strips containing tissue membranes were incubated with 5 μg/ml antibodies to D2S and D2L, followed by incubation with anti-rabbit IgG (1:200, Sigma) and peroxidase–antiperoxidase (1:100, Sigma) and developed with 0.05% diaminobenzidine (Fig. 1). The nitrocellulose strips containing recombinant cell membranes were incubated with horseradish peroxidase-conjugated anti-rabbit IgG (1:2000, Amersham), and bands were visualized by using an ECL kit (Amersham; Fig. 1 a and b). Controls included preincubation with 25 μg/ml respective antigen peptide.
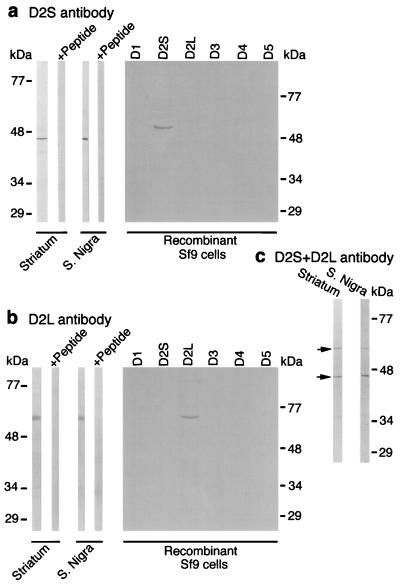
Figure 1.
Immonoblots of monkey brain tissues and crude membranes of recombinant Sf9 cell lines expressing dopaminergic human D1, D2S, D2L, D3rat, D4.2, and D5 receptors are shown with D2S (a) and D2L (b) antibodies. Both antibodies immunoreacted with single polypeptide band and do not show cross-reactivity. The relative abundance of the D2S and D2L receptor isoforms in the same strip (c) is shown.
Solubilization and Immunoprecipitation of Dopamine D2S and D2L Receptors.
The dopamine receptors were solubilized with 1% digitonin (14) (Sigma), and 500 μl of extracts containing 70 fmol of [3H]spiperone binding sites were incubated with 0–60 μl of antisera. The receptor–antibody complex was separated by incubation with 80 μl of protein A-agarose (Boehringer) followed by centrifugation and the supernatant of nonimmunoprecipitated receptors was used for binding assay. For the binding of [3H]spiperone and [3H]YM-09151 (both from DuPont/NEN), supernatant was incubated with 2 nM radioligand for 1 h at 24°C in total volume of 1 ml. The reaction was terminated by rapid filtration (15) through glass filters and counted for the radioactivity. The amount of immunoprecipitated receptors was calculated by subtracting the binding values of supernatant from the total (100%) binding of radioligands (Fig. 2). Incubation without addition of antisera represented 100% binding. The nonspecific binding was determined in the presence of 2 μM (+)-butaclamol HCl or fluphenazine and subtracted from the total.
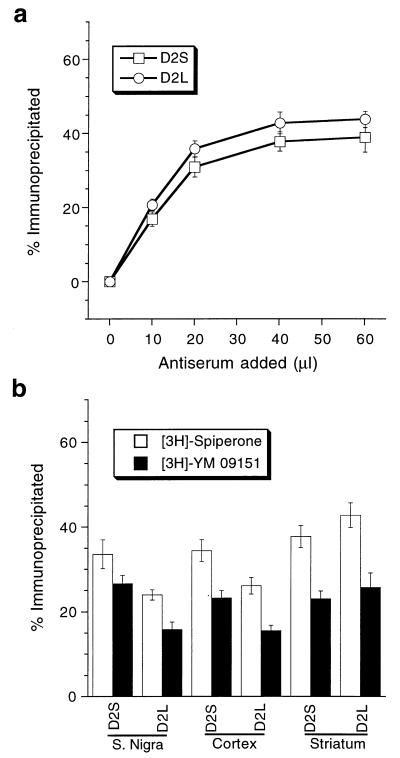
Figure 2.
Immunoprecipitation of dopamine receptor binding sites by D2S and D2L antibodies. (a) Antibody concentration-dependent immunoprecipitation demonstrated by [3H]spiperone binding in striatal tissues. Saturation point at 20 μl shows that both antibodies are equally sensitive. (b) Quantitative immunoprecipitation from substantia nigra, motor cortex, and striatum showing different amounts of D2S and D2L receptors. The results were confirmed with two different dopamine D2 receptor antagonists.
Immunocytochemistry.
Four adult monkeys were deeply anesthetized (100 mg/ml nembutal) and perfused transcardially with a fixative containing 4% paraformaldehyde, 0.1–0.2% glutaraldehyde, and 15% picric acid. The coronal blocks of monkey brains were cut on a freezing microtome (30 μm) or a Vibratome (50 μm) and processed for immunohistochemistry at the light and electron microscopic levels. For light microscopic levels, sections were incubated with antibodies against D2S (15 μg diluted 1:100) and D2L (20 μg diluted 1:100) for 2 days (4°C) and further processed by the avidin–biotin method using biotinylated goat anti-rabbit antibody (1:200; Jackson ImmunoResearch) and ABC Elite kit (1:100, Vector). The bound antibodies were visualized by using either 0.05% diaminobenzidine and 0.01% hydrogen peroxide or diaminobenzidine–glucose oxidase reaction (Fig. 3 a–e and Fig. 5). For double-label immunofluorescence analysis, sections were incubated with either D2S or D2L antibodies and mouse monoclonal antibody to tyrosine hydroxylase (TH; 1:1000, Chemicon) followed by incubation with goat anti-rabbit IgG-FITC (1:100; Jackson ImmunoResearch) and goat anti-mouse IgG-Cy 3 (1:200; Jackson ImmunoResearch; Fig. 3 f–k).
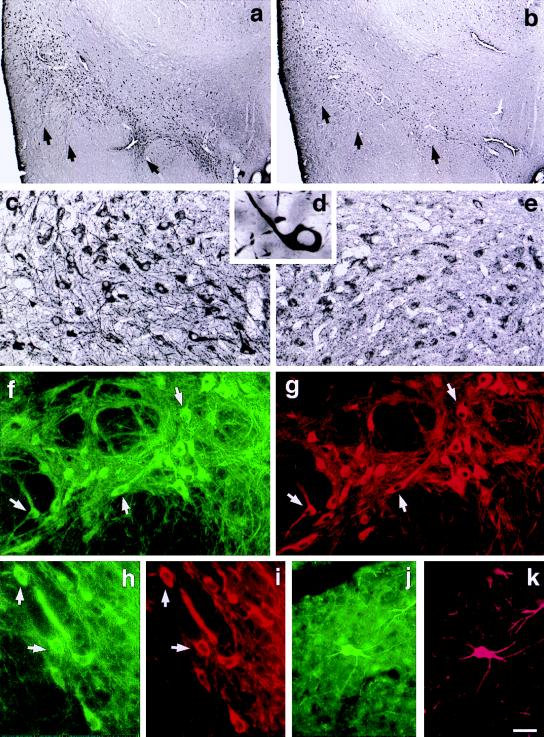
Figure 3.
Predominant expression of D2S protein in the substantia nigra (arrows) as shown with D2S (a, c, d) and D2L (b, e) antibodies. Double labeling immunofluorescence analysis shows colocalization of D2S (FITC, green) and TH immunoreactivity (Cy3, red) in dopaminergic neurons of substantia nigra (f, g), ventral tegmental area (h, i), and retrorubral field (j, k). (Scale bar: a and b, 400 μm; c, 50 μm; d, 11 μm; e, 50 μm; f–k, 50 μm.)
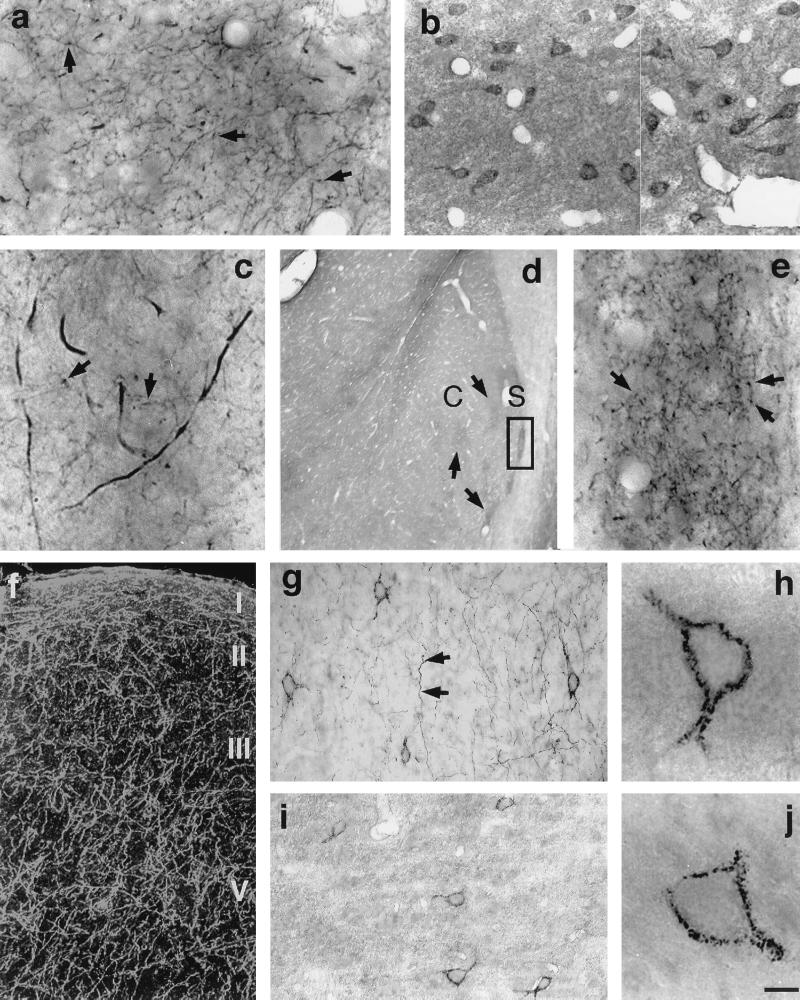
Figure 5.
Dense D2S immunopositive fiber network in the projection areas of dopaminergic neurons: striatum (a, c), nucleus accumbens (d, e), and cerebral cortex area 6 (f, darkfield micrograph; g). Immunopositive fibers bear numerous varicosities (arrows). (e) High-magnification micrograph depicted from square shown in d. D2L-positive neurons in striatum (b) and cerebral cortex area 6 (i, j) are shown. (g, h) Neurons labeled with D2S antibody in the area 6 of cerebral cortex. C, core; S, shell. (Scale bar: a and b, 25 μm; c, 8 μm; d, 400 μm; e, 25 μm; f, 80 μm; g and i, 67 μm; h and j, 10 μm.)
For double-labeling electron microscopic level analysis, Vibratome sections (40 μm) were incubated in a mixture containing either D2S or D2L antibody and antisera against dopaminergic markers such as TH or dopamine transporter (DAT; 1:500, Chemicon). The receptor isoforms were visualized using immunogold reaction and dopamine markers by the immunoperoxidase technique (Fig. 4). Briefly, the sections were incubated with goat anti-rabbit secondary antibody coupled to 1.4-nm gold particles (1:50, Nanoprobes) and mouse (TH) or rat (DAT) biotinylated secondary antibodies. This was followed by silver enhancement of gold particles (HQ Silver kit, Nanoprobes) and immunoperoxidase reaction using ABC Elite kit. Sections were osmicated, dehydrated, and flat embedded in Durcupan ACM (Fluka). The resin-embedded sections were cut into ultrathin sections on an ultramicrotome (Reichert) and examined in a JEOL transmission electron microscope.
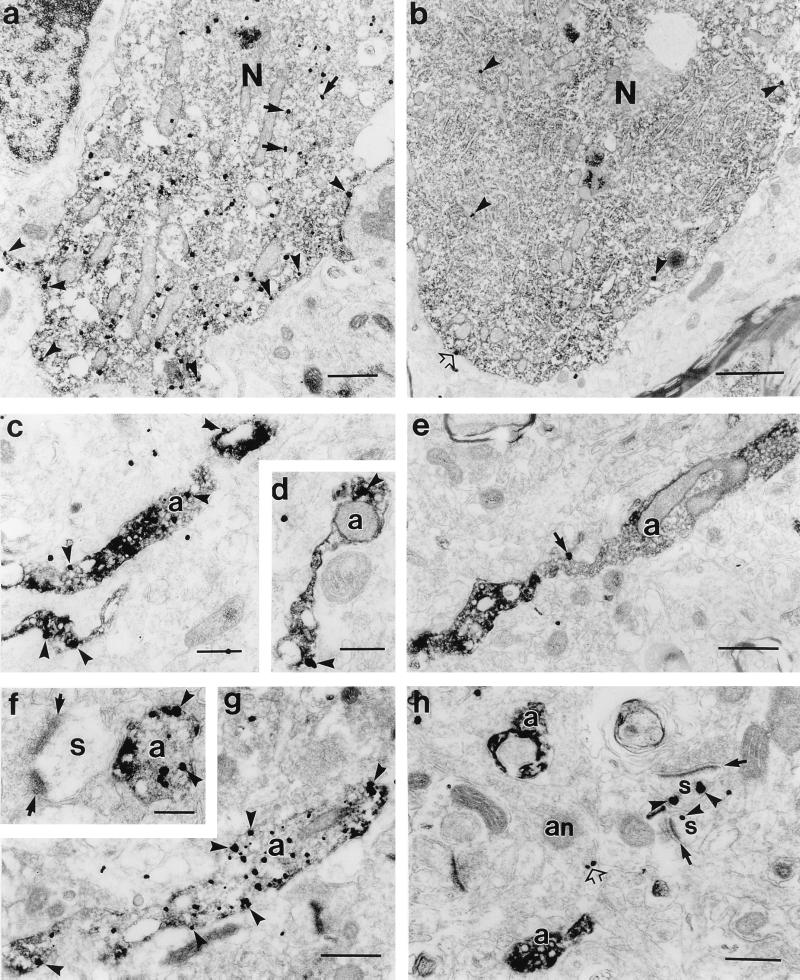
Figure 4.
Electron micrographs of double labeled sections showing differential D2S and D2L receptor expression in dopaminergic neurons and axons. Dopaminergic markers, TH or dopamine transporter (DAT), are labeled by immunoperoxidase reaction and receptors by immunogold method. In dopaminergic neurons of substantia nigra, note the significantly higher number of immunogold particles (arrowheads) in the D2S (a) labeled cell body than D2L (b). D2S immunogold particles are frequently distributed at the cytoplasmic face of plasma membranes (arrowheads) and rough endoplasmic reticulum (arrows). D2L immunogold particles are only occasionally associated with plasma membranes (open arrow in b). TH immunolabeled axons (a) exhibit numerous D2S (c, d) immunogold particles (arrowheads), whereas D2L (e, h) immunoreactivity is not detected. In e, the immunogold particle is outside the TH-labeled axon (arrow). In h, D2L immunogold particles are localized along the cytoplasmic side of plasma membranes in dendritic spines (s; arrowheads), which receive asymmetric synapses (arrows). TH immunonegative axon (an) expresses D2L immunoreactivity (open arrow). (f–g) Dopaminergic axons in the area 6 labeled with DAT are strongly D2S positive. In f, D2S-labeled axon is apposed to the spine receiving an asymmetric input (arrows). N, nucleus. (Scale bars: a, 0.5 μm; b, 1 μm; c and d, 0.2 μm; e, 0.5 μm; f, 0.2 μm; g, 0.5 μm; h, 0.3 μm.)
RESULTS
Characterization of Antibodies.
To test the specificity and selectivity of the antibodies to the D2S and D2L, Western blots of tissue membranes and dopamine receptor cDNA-transfected recombinant Sf9 cell lines were carried out (Fig. 1). In striatal and substantia nigral membranes, the D2S and D2L antibodies immunoreacted to 47- and 59-kDa proteins, respectively (Fig. 1 a and b); however, preabsorption of the antibodies with their respective antigen peptide eliminated these immunoreactive bands. Antibodies to D2S and D2L were able to bind with the protein of the recombinant cell lines containing either D2S (Fig. 1a) or D2L (Fig. 1b) dopamine receptor. There was no cross-reactivity between D2S and D2L antibodies. Both antibodies also did not show any reactivity with other members of the dopamine receptors. In Fig. 1c, the strips resulting from the transfer of striatal and substantia nigra membranes were incubated with both the D2S and D2L antibodies together to demonstrate their approximate relative abundance.
The sensitivities of the D2S and D2L antisera were shown by immunoprecipitation of the dopamine D2 receptor binding sites (Fig. 2a) where a fixed amount of solubilized extract (70 fmol of [3H]spiperone binding sites) was incubated with various amounts (0–60 μl) of each antibody (Fig. 2a). As the quantity of antiserum was increased, higher levels of immunoprecipitation were observed, ultimately reaching saturation at 20 μl and maintained at a plateau until 60 μl, three times higher than the saturation point (Fig. 2a). Fig. 2b shows the amount of D2S and D2L receptors in the substantia nigra, cerebral cortex, and striatum as demonstrated by the immunoprecipitation. Both the dopamine D2 receptor antagonists ([3H]spiperone and 3[H]YM-09151) showed similar results. In substantia nigra and cerebral cortex, the D2S receptor is about 28% more abundant than D2L, whereas in striatum both are the same.
Predominance of D2S Receptor in the Midbrain.
The immunoreactivity of the D2S receptor was much more prevalent in the substantia nigra (Fig. 3 a and c) as compared with the D2L receptor (Fig. 3 b and e). Labeling with D2S antisera showed Golgi-like staining with intense immunoreactivity in the cell bodies and proximal and distal dendrites of neurons in the substantia nigra pars compacta (A9; Fig. 3 d and f), ventral tegmental area (A10; Fig. 3h), and retrorubral fields (A8, Fig. 3j), whereas D2L expression in these structures was much weaker and confined to the soma (Fig. 3 b and e). Fig. 3 f–k shows colocalization of D2S receptor (FITC, green) and tyrosine hydroxylase (a marker for the dopaminergic structures; Cy3, red) in substantia nigra pars compacta (Fig. 3 g and h), ventral tegmental area (Fig. 3 h and i), and retrorubral field (Fig. 3 j and k). We found that the D2S receptors were exclusively localized in the dopaminergic neurons.
The differences in expression of the D2S and D2L isoforms were confirmed at the electron microscopic level (Fig. 4 a and b). Dopamine neurons stained with the D2S antibody showed numerous immunogold particles (Fig. 4a) associated largely with the cytoplasmic surface of plasma membranes and rough endoplasmic reticulum, whereas D2L immunogold particles were sparse (Fig. 4b) and rarely associated with membranes.
D2S and D2L Receptors in the Projection Areas of the Dopaminergic Cell Groups.
In striatum (Fig. 5 a and c), nucleus accumbens (Fig. 5 d and e), and cerebral cortex (Fig. 5f), the D2S antibody stained a dense network of axons. These axons exhibited numerous varicosities. Indeed, double labeling experiments at the electron microscopic level showed that all of the D2S positive axons in the striatum (Fig. 4 c and d) and cerebral cortex (Fig. 4 f and g) were dopaminergic. In contrast, D2L immunoreactivity was virtually absent from dopaminergic axons (Fig. 4 e and h) and was observed in the non-dopaminergic structures.
The D2L immunoreactivity in the striatum was mainly in the medium-sized spiny neurons (Fig. 5b). Both the D2S and D2L isoforms were present in the cells of cerebral cortical layers III–V. (Fig. 5 g–j). These cells are GABAergic, as determined by parvalbumin immunoreactivity.
DISCUSSION
Western blot analysis of tissue membranes from monkey striatum and substantia nigra revealed that D2S and D2L antibodies react with single polypeptide bands of expected size (10, 11), 47 and 59 kDa, respectively (Fig. 1 a and b). These bands were not observed when antibodies were blocked with antigen peptides (Fig. 1 a and b), indicating that the observed protein bands are specific. We further demonstrated that these immunoreactive bands were indeed dopamine D2S and D2L receptors by immunoblots of membranes of known recombinant Sf9 cell lines (Fig. 1 a and b). The D2S and D2L antibodies reacted only with the cell lines expressing the D2S isoform (Fig. 1a) and the D2L isoform (Fig, 1b), respectively. Furthermore, the precipitated D2S and D2L immunoreactive proteins from solubilized extract of monkey tissues showed specific binding to antagonists (spiperone and YM-09151) of the dopamine D2 receptor (Fig. 2b). The immunoblot results from recombinant Sf9 cells also show that D2S and D2L antibodies do not cross-react and are subtype-specific (Fig. 1 a and b), which was further established by immunoprecipitation of both D2S and D2L isoforms together. The immunoprecipitation value (77.9 ± 3.5%) was similar to the value obtained after addition of individual receptors (D2S, 37.8 ± 2.6% + D2L, 42.9 ± 2.9%), indicating that there is no cross-reactivity between D2S and D2L antibodies.
The sensitivity of the antibodies was shown by immunoprecipitation studies (Fig. 2a). Both the D2S and D2L antibodies showed similar sensitivity because their saturation point was the same (20 μl). In parallel experiments, we found no immunoprecipitation when an unrelated antibody, anti-β of the GABAA receptor (bd17, Boehringer), preimmune serum, or D2S and D2L antibodies blocked with corresponding antigen peptide were used (not shown). In addition, the sensitivity of the D2S and D2L antibodies was also evident from the relative abundance of immunoreactive proteins of both isoforms in substantia nigra and striatal tissue immunocytochemistry (Figs. 3, c and e, and 5, a and b), Western blot (Fig. 1c), and immunoprecipitation (Fig. 2b). The D2S isoform showed stronger immunoreactivity in the substantia nigra than the D2L isoform, whereas in the striatum, both were expressed to a similar extent. In brain areas such as the thalamus and globus pallidus, the D2L isoform showed higher immunoreactivity than D2S (not shown).
Both the D2S and D2L receptors were observed in the dopaminergic cell groups of the midbrain and hypothalamus, but with strikingly different levels of expression. The D2S antibody showed strong immunoreactivity in the cell bodies and proximal and distal dendrites of neurons in the midbrain, whereas D2L expression was much weaker and associated with soma. A similar pattern of D2S and D2L staining was observed in hypothalamic neurons of the arcuate nucleus and zona incerta (A11–14; not shown). Furthermore, quantitative immunoprecipitation studies (Fig. 2b) showed higher levels of D2S protein in the substantia nigra, which was also evident from the relative blot analysis (Fig. 1c). The areas targeted by midbrain and hypothalamic dopaminergic neurons include the cerebral cortex, striatum, nucleus accumbens, lateral septum, and median eminence. In all of these structures, the D2S antibody stained a dense network of axons identical to those described for dopaminergic fibers (16). A large proportion of the D2S immunoreactivity was associated with the cytoplasmic surface of plasma membranes in the cell body and dendrites of dopaminergic neurons as was anticipated by the intracellular location of the epitope (Fig. 4a). In contrast, the D2L receptors were absent from dopaminergic axons (Fig. 4 e and h), indicating that the long isoform protein is not shuttled from the site of synthesis to potential functional membrane sites.
In contrast to reports in the literature of a predominance of the D2L mRNA in numerous brain regions (17, 18), protein levels of this isoform in the substantia nigra and cerebral cortex are lower than the D2S isoform (Fig. 2b). In striatum, the D2L splice variant is expressed more strongly in medium-sized GABAergic (Fig. 5b) and large cholinergic neurons, whereas the D2S receptor is found mainly in axons. The GABAergic D2 neurons of the striatum are the origin of the “indirect” pathway extending from the striatum to the external segment of globus pallidus (6, 19). Transmission through this pathway is increased in Parkinson’s disease, resulting in hypokinesia (6, 20). The D2L isoform may therefore be implicated in Parkinson symptomology. This isoform is also the receptor involved in the well known dopaminergic modulation of acetylcholine release from striatal interneurons (21). D2L immunoreactivity also predominates in the GABAergic neurons of the nucleus accumbens, lateral septum and globus pallidus as well as in thalamic neurons, which utilize excitatory amino acid neurotransmitters such as glutamate (22). The abundance of D2L in these non-dopaminergic neurons suggests that this receptor is involved in postsynaptic modulation. Like other D2-like dopamine receptors (ref. 23; Z.U.K., A.G., R. Martin, A. Peñafiel, A. Rivera, and A.D.C., unpublished results), both isoforms were also observed in nonpyramidal neurons (Fig. 3 g–j), which are parvalbumin immunoreactive (not shown), indicating that the D2S and D2L isoforms may be equally involved in the modulation of GABAergic transmission.
The localization of the D2S isoform in the membranes of the somatodendritic and axonal domains of dopamine neurons represents strong evidence that the D2S isoform is the autoreceptor in dopaminergic pathways and the morphological substrate for controlling dopamine release (5–7) and also possibly for up-regulation of the D2 receptor in target structures after treatment with antipsychotic drugs (24). The D2S isoform is also the presumed autoreceptor in the hypothalamic dopamine pathway, which regulates prolactin secretion (25). Our findings thus open the possibility that some of the effects of antipsychotics may in large part be mediated through the short isoform of the dopamine D2 receptor. As dopamine modulates motivational (26) and cognitive (27) functions through mesocortical dopamine pathways (28, 29), the D2S isoform may be a useful target of future antipsychotic drug design.
Acknowledgments
We thank M. Pappy, K. Szigeti, and Joe Musco for expert technical assistance. This research was supported by grants from the National Institute on Drug Abuse, the National Institute of Mental Health (to P.G.R.), and the European Commission (to A.D.C.); a fellowship from Junta de Andalucia (to Z.K.); and a National Alliance for Research on Schizophrenia and Depression Young Investigator Award (to L.M.).
ABBREVIATIONS
- D2S
dopamine D2 short isoform
- D2L
dopamine D2 long isoform
- DAT
dopamine transporter
- GABA
γ-aminobutyric acid
- TH
tyrosine hydroxylase
- FITC
fluorescein isothiocyanate
References
- 1.Joyce J N, Meador-Woodruff J H. Neuropsychopharmacol. 1997;16:375–384. doi: 10.1016/S0893-133X(96)00276-X. [DOI] [PubMed] [Google Scholar]
- 2.Reynolds J P. Biochem Soc Trans. 1996;24:202–205. [PubMed] [Google Scholar]
- 3.Nordstrom A L, Farde L, Wiesel F A, Forslund K, Pauli S, Halldin C, Uppfeldt G. Biol Psychiatry. 1993;33:227–235. doi: 10.1016/0006-3223(93)90288-o. [DOI] [PubMed] [Google Scholar]
- 4.Calabresi P, Saiardi A, Pisani A, Baik J, Centonze D, Mercuri N B, Bernardi G, Borrelli E. J Neurosci. 1997;17:4536–4544. doi: 10.1523/JNEUROSCI.17-12-04536.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sesack S R, Aoki C, Pickel V. J Neurosci. 1994;14:88–106. doi: 10.1523/JNEUROSCI.14-01-00088.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hersch S M, Ciliax B J, Gutekunst C A, Rees H D, Heilm C J, Yung K K L, Bolam J P, Ince E, Yi H, Levey A. J Neurosci. 1995;15:5222–5237. doi: 10.1523/JNEUROSCI.15-07-05222.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cragg S J, Greenfield S A. J Neurosci. 1997;17:5738–5746. doi: 10.1523/JNEUROSCI.17-15-05738.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mercury N B, Saiardi A, Bonci A, Picetti R, Calabresi P, Bernardi G, Borrelli E. Neuroscience. 1997;79:323–327. doi: 10.1016/s0306-4522(97)00135-8. [DOI] [PubMed] [Google Scholar]
- 9.Koeltzow T E, Xu M, Cooper D C, Hu X, Tonegawa S, Wolf M E, White F J. J Neurosci. 1998;18:2231–2238. doi: 10.1523/JNEUROSCI.18-06-02231.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giros B, Sokoloff P, Martres M P, Riou J F, Emorine L J, Schwartz J C. Nature (London) 1989;342:923–926. doi: 10.1038/342923a0. [DOI] [PubMed] [Google Scholar]
- 11.Monsma F J, McVittie L D, Gerfen C R, Mahan L C, Sibley D R. Nature (London) 1989;342:926–929. doi: 10.1038/342926a0. [DOI] [PubMed] [Google Scholar]
- 12.Khan Z U, Gutierrez A, De Blas A L. J Neurochem. 1994;63:1466–1476. doi: 10.1046/j.1471-4159.1994.63041466.x. [DOI] [PubMed] [Google Scholar]
- 13.Khan Z U, Fernando L P, Escriba P, Busquets X, Mallet J, Miralles C P, Filla M, De Blas A L. J Neurochem. 1993;60:961–971. doi: 10.1111/j.1471-4159.1993.tb03243.x. [DOI] [PubMed] [Google Scholar]
- 14.Gorrisen H, Laduron P. Nature (London) 1979;279:72–74. doi: 10.1038/279072a0. [DOI] [PubMed] [Google Scholar]
- 15.Bruns R F, Lawson-Wendling K, Pugsley T A. Anal Biochem. 1983;132:74–81. doi: 10.1016/0003-2697(83)90427-x. [DOI] [PubMed] [Google Scholar]
- 16.Williams S M, Goldman-Rakic P S. Cereb Cortex. 1993;3:199–222. doi: 10.1093/cercor/3.3.199. [DOI] [PubMed] [Google Scholar]
- 17.Snyder L A, Roberts J L, Sealfon S C. Neurosci Lett. 1991;122:37–40. doi: 10.1016/0304-3940(91)90187-x. [DOI] [PubMed] [Google Scholar]
- 18.Neve K A, Neve R L, Fidel S, Janowski A, Higgins G A. Proc Natl Acad Sci USA. 1991;88:2802–2806. doi: 10.1073/pnas.88.7.2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gerfen C R, Engber T M, Mahan L C, Susel Z, Chase T N, Monsma F J, Sibley D R. Science. 1991;250:1429–1432. doi: 10.1126/science.2147780. [DOI] [PubMed] [Google Scholar]
- 20.Parent A, Hazrati L N. Brain Res Rev. 1995;20:128–154. doi: 10.1016/0165-0173(94)00008-d. [DOI] [PubMed] [Google Scholar]
- 21.Ikarashi Y, Takahashi A, Ishimaru H, Arai T, Maruyama Y. Brain Res Bull. 1997;43:107–115. doi: 10.1016/s0361-9230(96)00351-6. [DOI] [PubMed] [Google Scholar]
- 22.Dougherty P M, Li Y J, Lenz F A, Rowland L, Mittman S. Brain Res. 1996;278:267–273. doi: 10.1016/0006-8993(96)00550-1. [DOI] [PubMed] [Google Scholar]
- 23.Mrzljak L, Bergson C, Pappy M, Huff R, Levenson R, Goldman-Rakic P S. Nature (London) 1996;381:245–248. doi: 10.1038/381245a0. [DOI] [PubMed] [Google Scholar]
- 24.Lidow M S, Goldman-Rakic P S. Proc Natl Acad Sci USA. 1994;91:4353–4356. doi: 10.1073/pnas.91.10.4353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kelly M A, Rubinstein M, Asa S L, Zhang G, Saez C, Bunzow J R, Allen R G, Hnasko R, Ben-Jonathan N, Grandy D K, Low M J. Neuron. 1997;19:103–113. doi: 10.1016/s0896-6273(00)80351-7. [DOI] [PubMed] [Google Scholar]
- 26.Harris G C, Aston-Jones G. Nature (London) 1994;371:155–157. doi: 10.1038/371155a0. [DOI] [PubMed] [Google Scholar]
- 27.Goldman-Rakic P S, Bergson C, Mrzljak L, William G V. In: The Dopamine Receptors. Neve K A, Neve R L, editors. Totowa, NJ: Humana ; 1996. pp. 499–522. [Google Scholar]
- 28.White F J. Annu Rev Neurosci. 1996;19:405–436. doi: 10.1146/annurev.ne.19.030196.002201. [DOI] [PubMed] [Google Scholar]
- 29.Goldman-Rakic P S. Adv Pharmacol. 1998;42:707–711. doi: 10.1016/s1054-3589(08)60846-7. [DOI] [PubMed] [Google Scholar]