Abstract
We have previously demonstrated that α-synuclein (Snca) gene ablation reduces brain arachidonic acid (20:4n-6) turnover rate in phospholipids through modulation of endoplasmic reticulum-localized acyl-CoA synthetase activity. Although 20:4n-6 is a precursor for prostaglandin (PG), Snca effect on PG levels is unknown. In the present study, we examined the effect of Snca ablation on brain PG level at basal conditions and following 30 sec of global ischemia. Brain PG were extracted with methanol, purified on C18 cartridges, and analyzed by LC-MS/MS. We demonstrate, for the first time, that Snca gene ablation did not affect brain PG mass under normal physiological conditions. However, total PG mass and masses of individual PG were elevated ∼2-fold upon global ischemia in the absence of Snca. These data are consistent with our previously observed reduction in 20:4n-6 recycling through endoplasmic reticulum-localized acyl-CoA synthetase in the absence of Snca, which may result in the increased 20:4n-6 availability for PG production in the absence of Snca during global ischemia and suggest a role for Snca in brain inflammatory response.
Keywords: alpha-synuclein, prostaglandin, brain fatty acids, arachidonic acid, acyl-CoA synthetase, neuroinflammation
INTRODUCTION
α-Synuclein (Snca) is widely distributed in neurons [22;24;28;31], astrocytes [9;33], oligodendroglia [33;43], and microglia [3;36] and accounts for 0.1 - 1% of neuronal cytosolic protein in nervous system [21;45]. Snca overexpression and mutations are associated with familial Parkinson disease [25;38;46;54], although aggregates containing Snca are hallmark of a number of neurodegenerative disorders [19;27;47;48;50]. Despite the close association with neurodegenerative diseases, the physiological function of Snca is poorly defined.
While Snca may have a number of diverse roles in the nervous system, a number of studies suggests its role in brain fatty acid metabolism. Snca facilitates palmitic acid and arachidonic acid (20:4n-6) uptake in astrocytes [9] and in brain [14;15], although it has no affect on docosahexaenoic acid (22:6n-3) uptake both in astrocytes and brain [9;16], indicating a fatty acid selective affect. More importantly, we have recently demonstrated a functional interaction of Snca with microsomal acyl-CoA synthetases in brain, accounting for the profound reduction in 20:4n-6 incorporation and turnover in phospholipids in Snca-/- mice [15]. Conversely, 22:6n-3 incorporation and turnover is increased in these mice as a result of metabolic compensation for the decrease in 20:4n-6 brain metabolism [16]. Because 20:4n-6 is a precursor for prostaglandins (PG), we hypothesize that reduced recycling of 20:4n-6 back into phospholipid pool may result in the increased availability of 20:4n-6 for PG formation upon 20:4n-6 release during ischemia, thus increasing brain PG mass upon stimulation.
To address the potential role for Snca in brain prostaglandin formation, we measured brain prostaglandin levels following global ischemia in Snca-/- and Snca+/+mice. Snca gene deletion did not alter basal brain PG levels, however all measured PG masses were increased ∼2-fold upon global ischemia as compared to wild-type animals. These data are consistent with our proposed hypothesis and demonstrate that Snca has a key role in modulating PG formation, suggesting a role in brain inflammatory response.
This study was conducted in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals (NIH publication 80-23) and under an animal protocol approved by the IACUC at the University of North Dakota (Protocol #407-9). α-Synuclein gene-ablated mice (Snca-/-) were generated from 129/SvEv strain by gene targeted deletion [8]. Male mice (25-30 g) were maintained on standard laboratory chow diet and water ad libitum. In both groups, the ages of the mice were between 9-11 months.
Fasted, male mice were anesthetized with halothane (1-3%) and killed either by decapitation or by head-focused microwave irradiation (2.8 kW, 1.35 s; Cober Electronics, Inc, Norwalk, CT) to heat denature enzymes in situ. The whole brain was removed, frozen in liquid nitrogen, and pulverized under liquid nitrogen temperatures to a fine, homogeneous powder. The total time of global brain ischemia was 30 s in non - microwaved brains, while brain basal levels were assessed in mice immediately killed using head-focused microwave irradiation.
Brain PG were extracted with methanol and purified on a C18 column as described previously [29;39]. Briefly, 20 mg of non-microwaved or 100 mg of microwaved brain tissue powder was homogenized in 3 ml of 15% methanol at pH=3 containing 0.005% of butylated hydroxytoluene (BHT), and PGE2d4 and 6-keto-PGF1αd4 as internal standards. The tissue debris were removed by centrifugation and supernatant was loaded onto C18 Sep-Pak classic cartridges (Waters, Corporation, Milford, MA) that were prewashed with methanol and water. The cartridges were then washed with 20 mL of 15% methanol following with 20 mL of water, and then the PG were eluted with 10 mL of methyl formate (spectral grade, Acros Organics, Pittsburg, PA). The methyl formate was removed under a stream of nitrogen and PG were then dissolved in acetonetrile for analysis.
Reverse-phase LC electrospray ionization mass spectrometry was used for PG analysis. The PG were separated on a Luna C-18(2) (3 μm column, 100 A pore diameter, 150 × 2.0 mm) (Phenomenex, Torrance, CA, USA) with a stainless steel frit filter (0.5 μm) and security guard cartridge system (C-18) (Phenomenex, Torrance, CA, USA). The LC system consisted of an Agilent 1100 series LC pump with a wellplate autosampler (Agilent Technologies, Santa Clara, CA). The solvent system was composed of 0.1% formic acid in water (solvent A) and 0.1% formic acid in acetonitrile (solvent B). The flow rate was 0.2 ml/min. The separation program started with 10% of solvent B. At 2 min, the percentage of B was increased to 65% over 8 min, at 15 min the percentage of B was increased to 90% over 5 min, and at 35 min it was reduced to 10% over 2 min. Equilibration time between runs was 13 min.
MS analysis was performed using a quadrapole mass spectrometer (API3000, Applied Biosystem, Foster City, CA, USA) equipped with a TurboIonSpray ionization source. Analyst software version 1.4.2 (Applied Biosystem,) was used for instrument control, data acquisition, and data analysis. The mass spectrometer was optimized in the multiple reaction-monitoring mode. The source was operated in negative ion electrospray mode at 450 °C, electrospray voltage was -4250 V, nebulizer gas was 8 L/min and curtain gas was 11 L/min. Declustering potential, focusing potential, and entrance potential were optimized individually for each analyte. The quadrupole mass spectrometer was operated at unit resolution. PGE2, PGD2, PGF2α, and TXB2 were quantified using PGE2d4 as the internal standard and 6-keto-PGF1α was quantified using 6-keto-PGF1αd4 as the internal standard.
All statistical comparisons were calculated using a one-way ANOVA followed by a Tukey-Kramer post-hoc test using Instat II (Graphpad, San Diego, CA). Statistical significance was defined as <0.05. All values are expressed as mean ± SD.
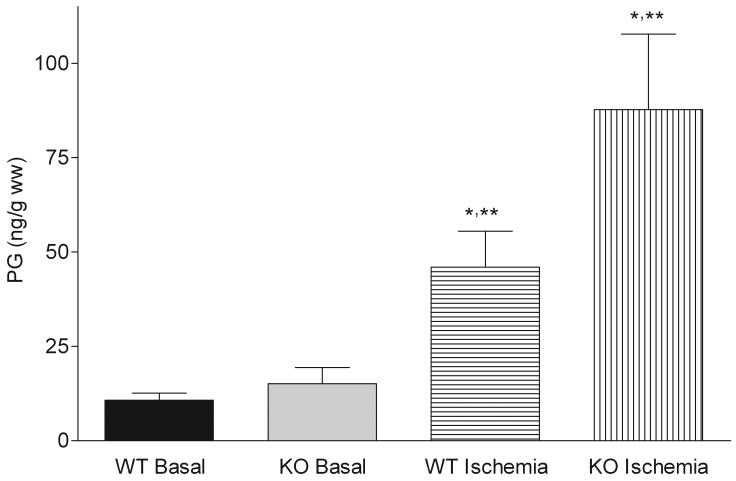
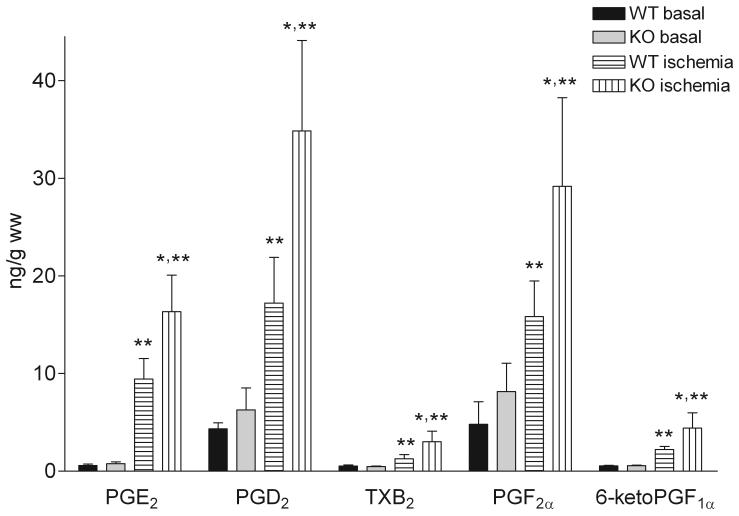
Snca gene ablation did not affect basal levels of total (Figure 1) and individual PG (Figure 2). However, the total and individual PG mass in Snca-/- brains was elevated ∼2-fold as compared to wild-type brains upon stimulation with 30 s of global ischemia (Figure 1 and 2).
Figure 1. α-Synuclein gene ablation increases total prostaglandin mass following 30 sec of global ischemia.
Wild type and α-synuclein gene ablated mice were subjected to either 30 seconds of global ischemia or the brains were fixed in situ using head-focused microwave irradiation (basal PG levels). Brain PG were extracted with methanol, purified on C18 cartridges, and analyzed by LC-MS/MS. Values are means ± SD. PG-prostaglandins; WT-wild type mice; KO-α-synuclein gene ablated mice; * - significantly different from WT, p< 0.05; ** - significantly different from WT and KO basal levels, p< 0.05.
Figure 2. α-Synuclein gene ablation increases prostaglandin mass following 30 sec of global ischemia.
Wild type and α-synuclein gene ablated mice were subjected to either 30 seconds of global ischemia or the brains were fixed in situ using head-focused microwave irradiation (basal PG levels). Brain PG were extracted with methanol, purified on C18 cartridges, and analyzed by LC-MS/MS. Values are means ± SD. PG-prostaglandins; WT-wild type mice; KO-α-synuclein gene ablated mice; * - significantly different from WT, p< 0.05; ** - significantly different from WT and KO basal levels, p< 0.05.
The observed 4-to 20- fold elevation of brain PG levels upon a global ischemia modeled by decapitation is consistent with previously reported values [2;7]. Increased PG formation is the result of dramatic 20:4n-6 release from phospholipids following cerebral ischemia [2;5-7;10;13] through activation of phospholipases and diacylglycerol lipases [18;26;34;53]. This released 20:4n-6 is used by COX1 and COX2 for PG formation, thereby acting as a proinflammatory mediator. As a possible protective mechanism against neuroinflammation following ischemia, 20:4n-6 is recycled back into brain phospholipid pool via its initial conversion to acyl-CoA by acyl-CoA synthetases [41;42]. As the result of acceleration of 20:4n-6 recycling following ischemia [41], brain 20:4n-6-CoA mass is increased, while 22:6n-3-CoA mass is decreased after decapitation [12;42], indicating fatty acid selectivity of the recycling mechanism following brain ischemia. Because Snca specifically stimulates 20:4n-6 recycling by activation 20:4n-6-CoA formation through acyl-CoA synthetases mechanism [15;16], the recycling of a released 20:4n-6 following ischemia would be depressed in the Snca-/- brains. The reduced recycling of 20:4n-6 in Snca-/- brains would lead to increased substrate availability for PG formation, thereby leading to the observed increase in the PG levels in Snca-/- brains (Figure 1 and 2). Importantly, all of the PG analyzed were increased to the same extent in Snca-/- brains, further supporting our assumption that the increased PG levels in Snca-/- brains was the result of increased substrate availability for COX rather than modulation of specific PG-synthetases by Snca.
The effect of Snca on PG formation following ischemia suggests that Snca has a role in the brain physiological response to injury and downstream processes such as neuroinflammation. The proposed role for Snca in suppressing neuroinflammatory response is not without evidence. First, Snca-/- microglia have an activated phenotype that secretes elevated levels of proinflammatory cytokines upon stimulation with proinflammatory stimuli [3]. This phenotype may be the result of elevated phospholipase D (PLD) activity because PLD is involved in promoting a reactive state in microglia [4;11;32;40;44] and because Snca tonically inhibits PLD activity in vitro [1;23;37]. We have demonstrated an increase in palmitic acid (16:0) turnover in brain phosphatidylcholine pools in Snca-/- mice [14], consistent with an increase in turnover due to the absence of PLD inhibition by Snca. The increase in TNFα secretion in cultured Snca-/- microglia [3] may result in increased astrocyte PG formation because TNFα increases astrocyte 20:4n-6 release and downstream PG formation [51]. In addition, Parkinsonism is associated with a maintained presence of reactive microglia [30;35;49]. Importantly, mutant forms of Snca, that are associated with familial forms of Parkinsonism [25;38;54], do not restore 20:4n-6-CoA synthetase activity in Snca ablation which we show is critical for 20:4n-6 recycling [15]. Collectively, this suggests a link between the functions of Snca and neuroinflammation associated with Parkinsonism. Second, Snca expression is significantly increased during cerebral ischemia and hypoxia [17;20;52]. Although this is not direct evidence, our proposed role for Snca in regulating brain 20:4n-6 metabolism and downstream of PG is consistent with this observation. Ischemic/hypoxic conditions are characterized by increased PG formation; hence ischemia-induced increases in Snca levels may serve as a protective mechanism to down regulate brain PG levels. Third, the level of Snca is upregulated in neurons, astrocytes and oligodendrocytes via induced transcription of mRNA in a model of multiple sclerosis [36]. This is important because inflammation is increased during this disease process, once again indicating a link between Snca and neuroinflammatory response.
Taken together, our results indicate that Snca gene deletion increases brain PG formation following 30 s of global ischemia. This is consistent with our previously observed reduction in 20:4n-6 recycling through endoplasmic reticulum-localized acyl-CoA synthetase in the absence of Snca, which would result in the increased 20:4n-6 availability for PG production in the absence of Snca. During pathological events such as ischemia where 20:4n-6 level is increased, the absence of Snca would provide more substrate for downstream PG formation as observed herein, suggesting Snca is an important regulator of brain PG formation during such events. This impact of Snca may be exacerbated in its absence via a dysregulation of PLD-mediated signaling in microglia, leading to increased cytokine release [3], resulting in a downstream elevation in PG formation in astrocytes [51]. More than likely, in the absence of Snca, multiple lipid-mediated signaling cascades in different cell populations in the brain are altered, resulting in our observed increase in PG formation during ischemia.
Acknowledgments
We thank Dr Carole Haselton for her excellent technical assistance. We thank Cindy Murphy for the typed preparation of the manuscript. This work was supported by a project (EJM) on a COBRE grant from NIH, P20 RR17699.
Abbreviations
- Snca
alpha-synuclein
- 20:4n-6
arachidonic acid
- 22:6n-3
docosahexaenoic acid
- PG
prostaglandins
- CoA
coenzyme A
- BHT
butylated hydroxytoluene
- COX
cyclooxygenase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Ahn BH, Rhim H, Kim SY, Sung Y-M, Lee M-Y, Choi J-Y, Wolozin B, Chang J-S, Lee YH, Kwon TK, Chung KC, Yoon S-H, Hahn SJ, Kim M-S, Jo Y-H, Min DS. α-Synuclein interacts with phospholipase D isozymes and inhibits pervanadate-induced phospholipase D activation in human embryonic kidney-293 cells. Journal of Biological Chemistry. 2002;277(14):12334–12342. doi: 10.1074/jbc.M110414200. [DOI] [PubMed] [Google Scholar]
- [2].Anton RF, Wallis C, Randall CL. In vivo regional levels of PGE and thromboxane in mouse brain: Effect of decapitation, focused microwave fixation, and indomethacin. Prostaglandins. 1983;26(3):421–429. doi: 10.1016/0090-6980(83)90177-6. [DOI] [PubMed] [Google Scholar]
- [3].Austin SA, Floden AM, Murphy EJ, Combs CK. α-Synuclein expression modulates microglial activation phenotype. Journal of Neuroscience. 2006;26(4):10558–10563. doi: 10.1523/JNEUROSCI.1799-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Balsinde J, Balboa MA, Insel PA, Dennis EA. Differential regulation of phospholipase D and phospholipase A2 by protein kinase C in P388D1 macrophages. Biochemical Journal. 1997;321:805–809. doi: 10.1042/bj3210805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Bazan NG. Effects of ischemia and electroconvulsive shock on free fatty acid pool in the brain. Biochimica et Biophysica Acta. 1970;218:1–10. doi: 10.1016/0005-2760(70)90086-x. [DOI] [PubMed] [Google Scholar]
- [6].Bazan NG, Aveldaño de Caldironi MI, Rodríguez de Turco EB. Rapid release of free arachidonic acid in the central nervous system due to stimulation. Prog.Lipid Res. 1981;20:523–529. doi: 10.1016/0163-7827(81)90092-8. [DOI] [PubMed] [Google Scholar]
- [7].Bosisio E, Galli C, Galli G, Nicosia S, Spagnuolo C, Tosi L. Correlation between release of free arachidonic acid and prostaglandin formation in brain cortex cerebellum. Prostaglandins. 1976;11(5):773–781. doi: 10.1016/0090-6980(76)90186-6. [DOI] [PubMed] [Google Scholar]
- [8].Cabin DE, Shimazu K, Murphy D, Cole NB, Gottschalk W, McIlwain KC, Orrison B, Chen A, Ellis CE, Paylor R, Lu B, Nussbaum RL. Synaptic vesicle depletion correlates with attenuated synaptic responses to prolonged repetitive stimulation in mice lacking α-synuclein. Journal of Neuroscience. 2002;22(20):8797–8807. doi: 10.1523/JNEUROSCI.22-20-08797.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Castagnet PI, Golovko MY, Barceló-Coblijn G, Nussbaum RL, Murphy EJ. Fatty acid incorporation is decreased in astrocytes cultured from α-synuclein gene-ablated mice. Journal of Neurochemistry. 2005;94(3):839–849. doi: 10.1111/j.1471-4159.2005.03247.x. [DOI] [PubMed] [Google Scholar]
- [10].Chen J, Weinstein PR, Graham SH. Attenuation of postischemic brain hypoperfusion and reperfusion injury by the cycloxygenase-lipoxygenase inhibitor BW755C. Journal of Neurosurgery. 1995;83(1):99–104. doi: 10.3171/jns.1995.83.1.0099. [DOI] [PubMed] [Google Scholar]
- [11].De Valck D, Beyaert R, Van Roy F, Fiers W. Tumor necrosis factor cytotoxicity is associated with phospholipase D activation. European Jouranl of Biochemistry. 1993;212(2):491–497. doi: 10.1111/j.1432-1033.1993.tb17686.x. [DOI] [PubMed] [Google Scholar]
- [12].Deutsch J, Rapoport SI, Purdon AD. Relation between free fatty acid and acyl-CoA concentrations in rat brain following decapitation. Neurochemical Research. 1997;22(7):759–765. doi: 10.1023/a:1022030306359. [DOI] [PubMed] [Google Scholar]
- [13].Galli C, Racagni G. Use of microwave techniques to inactivate brain enzymes rapidly. In: Lands WEM, Smith WL, editors. Prostaglandins and Arachidonate Metabolites, Methods in Enzymology. 86 Academic Press; New York: pp. 635–642. [DOI] [PubMed] [Google Scholar]
- [14].Golovko MY, Færgeman NJ, Cole NB, Castagnet PI, Nussbaum RL, Murphy EJ. α-Synuclein gene-deletion decreases brain palmitate uptake and alters the palmitate metabolism in the absence of α-synuclein palmitate binding. Biochemistry. 2005;44(23):8251–8259. doi: 10.1021/bi0502137. [DOI] [PubMed] [Google Scholar]
- [15].Golovko MY, Rosenberger TA, Færgeman NJ, Feddersen S, Cole NB, Pribill I, Berger J, Nussbaum RL, Murphy EJ. Acyl-CoA synthetase activity links wild-type but not mutant α-synuclein to brain arachidonate metabolism. Biochemistry. 2006;45:6956–6966. doi: 10.1021/bi0600289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Golovko MY, Rosenberger TA, Feddersen S, Færgeman NJ, Murphy EJ. α-Synuclein gene ablation increases docosahexaenoic acid incorporation and turnover in brain phospholipids. Journal of Neurochemistry. 2007;101:201–211. doi: 10.1111/j.1471-4159.2006.04357.x. [DOI] [PubMed] [Google Scholar]
- [17].Hu X, Rea HC, Wiktorowicz JE, Perez-Polo JR. Proteomic analysis of hyppoxia/ischemia-induced alteration of cortical development and dopamine neurotransmission in neonatal rat. Journal of Proteome Research. 2006;5(2396):2404. doi: 10.1021/pr060209x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Ikeda M, Yoshida S, Busto R, Santiso M, Ginsberg MD. Polyphosphoinositides as a probable source of brain free fatty acids accumulated at the onset of ischemia. Journal of Neurochemistry. 1986;47:123–132. doi: 10.1111/j.1471-4159.1986.tb02839.x. [DOI] [PubMed] [Google Scholar]
- [19].Iseki E, Marui W, Kosaka K, Akiyama H, Ueda K, Iwatsubo T. Degenerative terminals of the perforant pathway are human alpha-synuclein-immunoreactive in the hippocampus of patients with diffuse Lewy body disease. Neuroscience Letters. 1998;258(2):81–84. doi: 10.1016/s0304-3940(98)00856-8. [DOI] [PubMed] [Google Scholar]
- [20].Ishimaru H, Uéda K, Takahashi A, Maruyama Y. Changes in presynaptic protein NACP/α-synuclein in an ischemic gerbil hippocampus. Brain Research. 1998;788:311–314. doi: 10.1016/s0006-8993(98)00033-x. [DOI] [PubMed] [Google Scholar]
- [21].Iwai A, Masliah E, Yoshimoto M, Ge N, Flanagan L, de Silva HA, Kittel A, Saitoh T. The precursor protein of non-A beta component of Alzheimer’s disease amyloid is a presynaptic protein of the central nervous system. Neuron. 1995;14(2):467–475. doi: 10.1016/0896-6273(95)90302-x. [DOI] [PubMed] [Google Scholar]
- [22].Jakes R, Spillantini MG, Goedert M. Identification of two distinct synucleins from human brain. FEBS Letters. 1994;345:27–32. doi: 10.1016/0014-5793(94)00395-5. [DOI] [PubMed] [Google Scholar]
- [23].Jenco JM, Rawlingson A, Daniels B, Morris AJ. Regulation of phospholipase D2: Selective inhibition of mammalian phospholipase D isoenzymes by alpha- and beta-synucleins. Biochemistry. 1998;37(14):4901–4909. doi: 10.1021/bi972776r. [DOI] [PubMed] [Google Scholar]
- [24].Jo E, McLaurin J, Yip CM, St.George-Hyslop P, Fraser PE. α-Synuclein membrane interactions and lipid specificity. Journal of Biological Chemistry. 2000;275:34328–34334. doi: 10.1074/jbc.M004345200. [DOI] [PubMed] [Google Scholar]
- [25].Kruger R, Kuhn W, Muller T, Woitalla D, Graeber S, Kosel S, Przuntek H, Epplen JT, Schols L, Reiss O. Ala30Pro mutation in the gene encoding alpha-synuclein in Parkinson’s disease. Nature Genetics. 1998;18:106–108. doi: 10.1038/ng0298-106. [DOI] [PubMed] [Google Scholar]
- [26].Lin TN, Liu TH, Xu J, Hsu CY, Sun GY. Brain polyphosphoinositide metabolism during focal ischemia in rat cortex. Stroke. 1991;22:495–498. doi: 10.1161/01.str.22.4.495. [DOI] [PubMed] [Google Scholar]
- [27].Lippa CF, Schmidt ML, Lee VM, Trojanowski JQ. Antibodies to alpha-synuclein detect Lewy bodies in many Down’s syndrome brains with Alzheimer’s disease. Ann.Neurol. 1999;45(3):353–357. doi: 10.1002/1531-8249(199903)45:3<353::aid-ana11>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- [28].Maroteaux L, Campanelli JT, Scheller RH. Synuclein: A neuron-specific protein localized in the nucleus and presynaptic nerve terminal. Journal of Neuroscience. 1988;8(8):2804–2815. doi: 10.1523/JNEUROSCI.08-08-02804.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Masoodi M, Nicolaou A. Lipidomic analysis of twenty-seven prostanoids and isoprostanes by liquid chromatography/electrospray tandem mass spectrometry. Rapid Communications in Mass Spectrometry. 2006;20(3023):3029. doi: 10.1002/rcm.2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].McGeer PL, McGeer EG. Inflammation and neurodegeneration in Parkinson’s disease. Parkinsonism and Related Disorders. 2004;10:S3–S7. doi: 10.1016/j.parkreldis.2004.01.005. [DOI] [PubMed] [Google Scholar]
- [31].McLean PJ, Ribich S, Hyman BT. Subcellular localization of alpha-synuclein in primary neuronal cultures: Effect of missense mutations. Journal of Neural Transm Supplement. 2000;58:53–63. doi: 10.1007/978-3-7091-6284-2_5. [DOI] [PubMed] [Google Scholar]
- [32].Meats JE, Steele L, Bowen JG. Identification of phospholipase D (PLD) activity in mouse peritoneal macrophages. Agents Actions. 1993;39:C14–C16. doi: 10.1007/BF01972706. [DOI] [PubMed] [Google Scholar]
- [33].Mori F, Tanji K, Yoshimoto M, Takahashi H, Wakabayashi K. Demonstration of α-synuclein immunoreactivity in neuronal and glial cytoplasm in normal human brain tissue using proteinkinase K and formic acid pretreatment. Experimental Neurology. 2002;176:98–104. doi: 10.1006/exnr.2002.7929. [DOI] [PubMed] [Google Scholar]
- [34].Murphy EJ, Haun SE, Rosenberger TA, Horrocks LA. Altered lipid metabolism in the presence and absence of extracellular Ca2+ during combined oxygen-glucose deprivation in primary astrocyte cell cultures. Journal of Neuroscience Research. 1995;42:109–116. doi: 10.1002/jnr.490420112. [DOI] [PubMed] [Google Scholar]
- [35].Nagatsu T, Sawada M. Inflammatory process in Parkinson’s disease: Role for cytokines. Current Pharmaceutical Design. 2005;11(8):999–1016. doi: 10.2174/1381612053381620. [DOI] [PubMed] [Google Scholar]
- [36].Papadopoulos D, Ewans L, Pham-Dinh D, Knott J, Reynolds R. Upregulation of α-synuclein in neurons and glia in inflammatory demyelinating disease. Mol.Cell.Neurosci. 2006;31:597–612. doi: 10.1016/j.mcn.2006.01.007. [DOI] [PubMed] [Google Scholar]
- [37].Payton JE, Perrin RJ, Woods WS, George JM. Structural determinants of PLD2 inhibition by alpha-synuclein. J.Mol.Biol. 2004;337(4):1001–1009. doi: 10.1016/j.jmb.2004.02.014. [DOI] [PubMed] [Google Scholar]
- [38].Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, Stenroos ES, Chandrasekharappa S, Athanassiadou A, Papapetropoulos T, Johnson WG, Lazzarini AM, Duvoisin RC, DiIorio G, Golbe LI, Nussbaum RL. Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science. 1997;276:2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- [39].Powell WS. Precolumn extraction and reversed-phase high-pressure liquid chromatography of prostaglandins and leukotrienes. Analytical Biochemistry. 1987;164(1):117–131. doi: 10.1016/0003-2697(87)90375-7. [DOI] [PubMed] [Google Scholar]
- [40].Powner DJ, Payne RM, Pettitt TR, Guidici ML, Irvine RF, Wakelam MJ. Phospholipase D2 stimulates integrin-mediated adhesion via phosphatidylinositol 4-phosphate 5-kinase l Γ b. J.Cell Sci. 2005;118(Pt 13):2975–2986. doi: 10.1242/jcs.02432. [DOI] [PubMed] [Google Scholar]
- [41].Rabin O, Chang MCJ, Grange E, Bell J, Rapoport SI, Deutsch J, Purdon AD. Selective acceleration of arachidonic acid reincorporation into brain membrane phospholipid following transient ischemia in awake gerbil. Journal of Neurochemistry. 1998;70:325–334. doi: 10.1046/j.1471-4159.1998.70010325.x. [DOI] [PubMed] [Google Scholar]
- [42].Rabin O, Deutsch J, Grange E, Pettigrew KD, Chang MCJ, Rapoport SI, Purdon AD. Changes in cerebral acyl-CoA concentrations following ischemia - reperfusion in awake gerbils. Journal of Neurochemistry. 1997;68:2111–2118. doi: 10.1046/j.1471-4159.1997.68052111.x. [DOI] [PubMed] [Google Scholar]
- [43].Richter-Landsberg C, Gorath M, Trojanowski JQ, Lee VM. Alpha-synuclein is developmentally expressed in cultured rat brain oligodendrocytes. Journal of Neuroscience Research. 2000;62:9–14. doi: 10.1002/1097-4547(20001001)62:1<9::AID-JNR2>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- [44].Serrander L, Fallman M, Stendahl O. Activation of phospholipase D is an early event in integrin-mediated signalling leading to phagocytosis in human neutrophils. Inflammation. 1996;20(4):439–450. doi: 10.1007/BF01486745. [DOI] [PubMed] [Google Scholar]
- [45].Shibayama-Imazu T, Okahashi I, Omata K, Nakajo S, Ochiai H, Nakai Y, Hama T, Nakamura Y, Nakaya K. Cell and tissue distribution and developmental change of neuron specific 14 kDa protein (phosphoneuroprotein 14) Brain Research. 1993;622(12):17–25. doi: 10.1016/0006-8993(93)90796-p. [DOI] [PubMed] [Google Scholar]
- [46].Singleton AB, Farrer M, Johnson J, Singleton A, Hague S, Kachergus J, Hulihan M, Peuralinna T, Dutra A, Nussbaum R, Lincoln S, Crawley A, Hanson M, Baptista M, Miller D, Blancato J, Hardy J, Gwinn-Hardy K. α-Synuclein locus triplication causes Parkinson’s disease. Science. 2003;302:841. doi: 10.1126/science.1090278. [DOI] [PubMed] [Google Scholar]
- [47].Spillantini MG, Crowther RA, Jakes R, Cairns NJ, Lantos PL, Goedert M. Filamentous alpha-synuclein inclusions link multiple system atrophy with Parkinson’s disease and dementia with Lewy bodies. Neuroscience Letters. 1998;251(3):205–208. doi: 10.1016/s0304-3940(98)00504-7. [DOI] [PubMed] [Google Scholar]
- [48].Takeda A, Hashimoto M, Mallory M, Sundsumo M, Hansen L, Sisk A, Masliah E. Abnormal distribution of the non-Abeta component of Alzheimer’s disease amyloid precursor/alpha-synuclein in Lewy body disease as revealed by proteinase K and formic acid pretreatment. Laboratory Investigation. 1998;78(9):1169–1177. [PubMed] [Google Scholar]
- [49].Teismann P, Schulz JB. Cellular pathology of Parkinson’s disease: Astrocytes, microglia and inflammation. Cell and Tissue Research. 2004;318:149–161. doi: 10.1007/s00441-004-0944-0. [DOI] [PubMed] [Google Scholar]
- [50].Trojanowski JQ, Goedert M, Iwatsubo T, Lee VM. Fatal attractions: Abnormal protein aggregation and neuron death in Parkinson’s disease and Lewy body dementia. Cell Death Differentiation. 1998;5(10):832–837. doi: 10.1038/sj.cdd.4400432. [DOI] [PubMed] [Google Scholar]
- [51].Xu J, Chalimoniuk M, Shu Y, Simonyi A, Sun AY, Gonzalez FA, Weisman GA, Wood WG, Sun GY. Prostaglandin E2 production in astrocytes: Regulation by cytokines, extracellular ATP, and oxidative agents. Prostagl.Leukotr.Essen.Fatty Acids. 2003;69:437–448. doi: 10.1016/j.plefa.2003.08.016. [DOI] [PubMed] [Google Scholar]
- [52].Yoon D-K, Hwang IK, Yoo K-Y, Lee Y-B, Lee J-J, Kim J-H, Kang T-C, Lee B-H, Sohn H-S, Won MH. Comparison of α-synuclein immunoreactivity and protein levels in ischemic hippocampal CA1 region between adult and aged gerbils and correlation with Cu,Zn-superoxide dismutase. Neuroscience Research. 2006;55:434–441. doi: 10.1016/j.neures.2006.04.014. [DOI] [PubMed] [Google Scholar]
- [53].Yoshida S, Ideda M, Busto R, Santiso M, Martinez E, Ginsberg MD. Cerebral phosphoinositide, triacylglycerol, and energy metabolism in reversible ischemia: Origin and fate of free fatty acids. Journal of Neurochemistry. 1986;47:744–757. doi: 10.1111/j.1471-4159.1986.tb00675.x. [DOI] [PubMed] [Google Scholar]
- [54].Zarranz JJ, Alegre J, Gómez-Esteban JC, Lezcano E, Ros R, Ampuero I, Vidal L, Hoenicka J, Rodriguez O, Atarés B, Llorens V, Gomez Tortosa E, del Ser T, Muñoz DG, de Yebenes JG. The new mutation, E46K, of α-synuclein causes Parkinson and Lewy Body dementia. Ann.Neurol. 2004;55:164–173. doi: 10.1002/ana.10795. [DOI] [PubMed] [Google Scholar]