Abstract
Long standing interest in the impact of gonadal steroid hormones on fluid and electrolyte balance has led to a body of literature filled with conflicting reports about gender differences, the effects of gonadectomy, hormone replacement, and reproductive cycles on plasma vasopressin (VP), VP secretion, and VP gene expression. This reflects the complexity of gonadal steroid hormone actions in the body resulting from multiple sites of action that impact fluid and electrolyte balance (e.g. VP target organs, afferent pathways regulating the VP neurons, and the VP secreting neurons themselves). It also reflects involvement of multiple types of estrogen receptors (ER) in these diverse sites including ERs that act as transcription factors regulating gene expression (i.e. the classic ERα as well as the more recently discovered ERβ) and potentially G-protein coupled, membrane localized ERs that mediate rapid non-genomic actions of estrogen. Furthermore, altered expression of these receptors in physiologically diverse conditions of fluid and electrolyte balance contributes to the difficulty of using simplistic approaches such as gender comparisons, gonadectomy, and hormone replacement to assess the role of gonadal steroids in regulation of VP secretion for maintenance of fluid and electrolyte homeostasis. This review catalogs these inconsistencies and provides a frame work for understanding them by describing: 1) the effect of gonadal steroids on target organ responsiveness to VP; 2) the expression of multiple types of estrogen receptors in the VP neurons and in brain regions monitoring feedback signals from the periphery; and 3) the impact of dehydration and hyponatremia on expression of these receptors.
1. Introduction
Women experience fluid retention during pregnancy and the luteal phase of the menstrual cycle. In addition, fluid retention is frequently an unpleasant side effect of the use of gonadal steroids for contraception or hormone replacement therapy. It is well established that the osmotic threshold for vasopressin (VP) secretion is reset during pregnancy and during the luteal phase of the menstrual cycle [13, 15, 80, 81, 94]. Furthermore, women astronauts are more susceptible to orthostatic hypotension following extended weightlessness [99]. Since VP (also known as antidiuretic hormone) acts on the kidneys to regulate water excretion and is a potent vasoconstrictor agent important for preventing decreases in blood pressure, it was natural to hypothesize that gonadal steroids might influence VP secretion. The first questions many neuroendocrinologists asked to address this possibility included: Are plasma VP levels or VP responses to osmotic and cardiovascular stimuli different in males and females? What is the impact of reproductive cycles on VP secretion? What happens to plasma VP levels following gonadectomy and/or hormone administration? Unfortunately, experiments to answer these questions have not provided consistent results. This is probably due to the fact that gonadal steroids act on components of the homeostatic system involved in regulation of water and electrolyte balance (Figure 1), and which one of these actions predominate is modified by a variety of factors including species, gender, diet, and reproductive and fluid balance status. VP action on the kidneys as well as its effect on blood pressure are modified by estrogen and show gender differences [46, 63, 96, 97]. This in turn modifies feedback signals that regulate VP secretion. Furthermore, estrogen receptors (ERs) are expressed not only in VP target tissues, but also in the VP neurons themselves, in osmoreceptive areas of the brain that control VP secretion, and in pathways transmitting information about blood pressure and blood volume to the VP neurons. This complexity is compounded by the fact that different types of ERs are expressed in these regions. These include the classical ERα and the more recently discovered ERβ both of which are involved in regulating gene expression. In addition, membrane estrogen receptors may also participate in the effect of the steroid hormones on VP secretion, because rapid, non-genomic actions of estrogen have been reported in VP and oxytocin (OT) neurons [29, 95], and G-protein receptor 30 (GPR30), a non-genomic estrogen receptor [17, 54, 89], was recently found in VP and OT neurons of the SON and PVN [4]. These aspects will be discussed in this review with an emphasis on the role of ERβ in the VP neurons themselves. Although ERs also play critically important roles in regulation of OT secretion, the other neurohypophyseal hormone, and modify its action on the uterus and mammary glands, the role of estrogens in regulating OT secretion is a similarly complex topic deserving of a separate review.
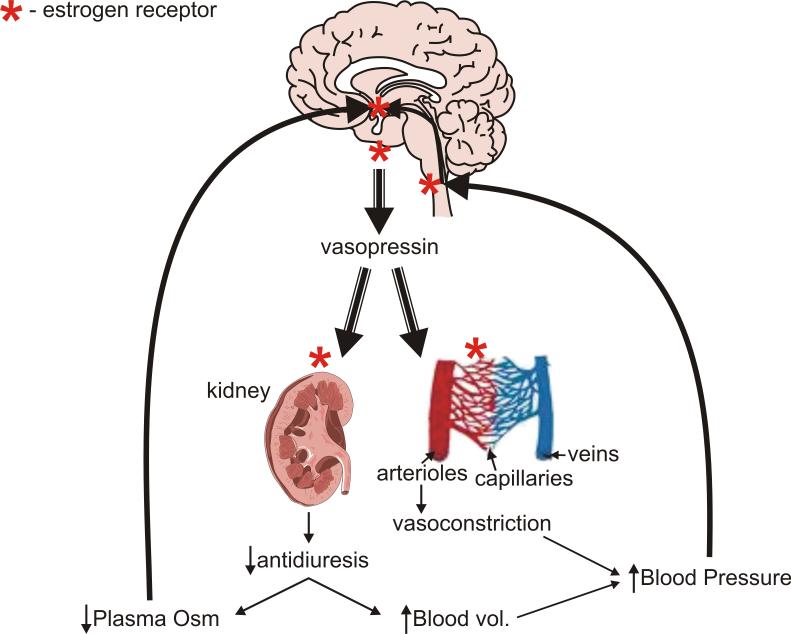
Figure 1.
Sites of ER expression (either ERα or ERβ) in components of the system for maintaining fluid homeostasis. ER (*) is expressed in osmosensor regions of the anterior hypothalamus, the VP producing neurons of SON and PVN, the brainstem areas transmitting cardiovascular feedback information, and the VP target organs, the kidneys and arterioles. See text for discussion and references. See Table 2 for information on the type of ER at each location.
2. Effect of gonadal steroids on plasma VP (pVP)
Let us start by recapping the extensive literature that attempted to elucidate the role of the gonadal steroids in regulation of VP secretion in whole animal and human studies (See Table 1, [71]). In 1979, Skowsky et al reported an increase in pVP two weeks after male rats were castrated that was reversed by testosterone administration [68]. However, they observed the opposite in females with estrogen treatment of ovariectomized rats increasing pVP [68]. Thus, they concluded that androgens inhibit and estrogen stimulates VP release. In contrast, Crofton et al reported a decrease in pVP following castration of male rats and an increase in pVP with testosterone treatment [7]. However, in female rats, Crofton, et al reported no change in pVP with estrogen treatment alone, but a decrease in pVP following combined administration of estrogen and progesterone [7, 68]. Peysner and Forsling reported that ovariectomy caused a decrease in pVP and a suppression of the diurnal variation in pVP [51]. They observed a dose dependent effect of estrogen replacement with a high dose decreasing pVP and a low dose increasing pVP [51]. Similarly divergent results were reported in women with estrogen administration to anovulatory or postmenopausal women having no effect or causing an increase in pVP [19, 101]. Reports on fluctuations in pVP associated with the estrus or menstrual cycle also yielded inconsistent results. Although Skowsky observed fluctuations in VP during the estrus cycle [68], others reported no effect [7] or reported that the magnitude of the diurnal fluctuation in pVP was influenced by the estrus cycle [51]. In women, an early study reported midcycle increases in pVP, but this was not confirmed in other studies [81, 91, 94, 101]. If, as suggested above, the inconsistency of these reports reflects multiple sites of action of gonadal steroids and activation of multiple ERs, dissecting the system into component parts is appropriate. Thus we will consider the effects of gonadal steroids on VP target tissues, on afferents to the VP neuron, and on the VP neuron itself. The involvement of both testosterone and estrogen will be discussed, because metabolites of testosterone (androgenic metabolites) as well as estrogen can activate ERβ [48, 49].
Table 1.
Reported effects of gonadal steroids on VP secretion and action.
| Manipulation | Species | Sex | Plasma VP | VP mRNA | Antidiuretic Action ERα>ERβ[33]‡ | Vasoconstrictor/Vasopressor*Action ERα or ERβ‡ |
|---|---|---|---|---|---|---|
| Gender Differences | Rat | See below | Yes[12] | Male>Female[96] | Female>Male (constriction)[1] Male>Female (pressor)[8, 9] | |
| Gonadectomy | Rat | Male |
 [68] [68]  [7] [7] |
No Δ[6, 10] | No Δ[97] | ND |
| Female |
 [68] [68]  [7, 51] [7, 51] |
No Δ[10] |
 [97] [97] |
 pressor [8, 9] pressor [8, 9] |
||
| Hormone Replacement | Rat | Male Test. Estrogen |
 [68], [68],  [7] ND [7] ND |
See[10] See[45] | No Δ[97] ND | ND  constriction [1] constriction [1] |
| Female Estrogen |
 [68] No Δ[7] Dose dependent: •High [68] No Δ[7] Dose dependent: •High •Low •Low  [51] [51] |
ND |
 [97] [97] |
ND | ||
| Est.+Pro. |
 [7] [7] |
ND | ND | ND | ||
| Human | Female Anovul Postmen. | No Δ[19]  [101] [101] |
ND | ND | ND | |
| Rhesus | Female | ND | NoΔ[58] | ND | ND | |
| Reproductive Cycle | Rat | Female | Δ[68] NoΔ[7]  diurnal Δ [51] diurnal Δ [51] |
ND |
 @ estrus[97] @ estrus[97] |
 @ estrus [8, 9] @ estrus [8, 9] |
| Human | Female | No Δ[81, 91, 94, 101] ΔinOsmotic thresh.[94] | ND | ND | ND |
Differences in pressor effects may reflect either direct vasoconstrictor actions or modification of the baroreflex.
Both ERα and ERβ are expressed in the kidney and vasculature (See Table 2).
Δ=change. ND=no data.
3. Gonadal steroid effects on VP target tissues
Antidiuresis and vasoconstriction are the primary actions of VP as a peripheral hormone. Thus, the primary peripheral target organs for VP are the kidney and vasculature. Estrogen has been shown to influence the responses of these target tissues to VP (Fig 1, Table 1).
Effects on VP-induced antidiuresis
The ability of VP to induce antidiuresis is greater in male than in female rats [96, 97]. It is greater in females during estrus (the phase of the estrus cycle in which circulating estrogen is lowest) than in females in other phases of the estrus cycle [97]. Ovariectomy increases the antidiuretic response to VP to be comparable to males, and estradiol replacement in ovariectomized rats reduces the antidiuretic action of VP to that found in nonestrus females [97]. In contrast, gonadectomy and testosterone replacement in males did not alter the antidiuretic efficacy of VP [97]. Thus, estrogen attenuates the antidiuretic effect of VP. This may reflect activation of ERα, because although both ERα and ERβ have been localized to the kidney [100], ERα is most prominent [33] (See Table 2). ERα is present in the collecting duct in rat kidney [100], the antidiuretic site of VP, and estrogen regulation of gene expression is dependent on ERα in the kidney [30]. Although a role for ERβ in altering renal responsiveness to VP was suggested by studies in which rats fed ERβ agonist isoflavones (genistein+daidzein) for 2 weeks had increased plasma VP without altered fluid balance [18], one of these, daidzein, can give rise to equol, a nonsteroidal estrogen that binds both ERα and ERβ [57]. Thus, this effect of isoflavones might be mediated by ERα-induced changes in renal responsiveness to VP. Effects of estrogen on the renal response to VP can alter its impact on plasma osmolality and blood volume thereby altering feedback signals regulating VP secretion. Thus, renal activation of ERs could contribute to the impact of gonadectomy, hormone replacement, and endogenous fluctuations in plasma estradiol during reproductive cycles on VP secretion.
Table 2.
Differential Locations of Estrogen Receptor Subtype
| Organ | Structure or Region¶ | Receptor Subtype§ | Receptor Regulation | |
|---|---|---|---|---|
| Kidney | Collecting Duct | ERα[33, 100] | ND | |
| Vasculature | Endothelium or smooth muscle | ERα and ERβ[39] | ND | |
| Brain: | ||||
| Osmoreceptive regions | MCNs (VP) | rat | ERβ[28, 32, 65, 78, 98] |
 dehydration[79] dehydration[79] salt loading[78] salt loading[78] hyponatremia[78] hyponatremia[78] ovex+ E2[86] ovex+ E2[86] lactation[22, 74] lactation[22, 74] adrenalectomy[75] adrenalectomy[75] |
| mouse | Neither ER in SON[42, 66] | ND | ||
| GP | ERα[98] | ND | ||
| Human | ERα and ERβ[28, 32] | ND | ||
| Sheep | ERα and ERβ[61] | ND | ||
| OVLT | ERα[76, 93] | ND | ||
| SFO | ERα[76, 93] |
 hyperosmolar[76] hyperosmolar[76] |
||
| MnPO | ERα[76, 93] | ND | ||
| Baroreflex regions | NTS | ERα: 32−59% THneurons[11]* 48−62% ERα not TH[11]* |
Δ w/estrus cycle[25] | |
| ERβ: 13−20% TH neurons[11] | ND | |||
| Caudal VLM | ERα: 77% TH neurons[11]; 54% ERα not TH[11]* |
No Δ w/estrus cycle[25] |
||
| ERβ: 3% TH neurons[11] | ND | |||
Data are from rat except where noted differently.
Only ERα and ERβ are considered here. Other ERs (e.g. GPR30) may also contribute.
ERα positive neurons that project to SON are not TH positive (see text) [93]. Δ=change.
Abbreviations: A1-catecholamine neurons in VLM; A2-Catecholamine neurons in NTS; GP-guinea pig; MCN-magnocellular neurons in SON and PVN; ND-not done; NTS-nucleus tractus solitarius; ovex-ovariectomyOVLT-organum vasculosum of lamina terminalis; PCNs-parvocellular neurons; PVN-paraventricular nucleus; SFO-subfornical organ; SON-supraoptic nucleus; TH-tyrosine hydroxylase; VLM-ventrolateral medulla
Effects on VP-induced vasoconstriction
Gender differences also exist in the pressor effects of VP. VP induces vasoconstriction via activation of V1a VP receptors on vascular smooth muscle. Although sensitivity to the vasoconstrictor effects of VP varies between vascular beds, both large vessels (e.g. the aorta) and arterioles respond to VP, and in the mesenteric bed vasoconstriction in response to VP occurs at concentrations that are orders of magnitude below that required for angiotensin II or norepinephrine, other potent vasoconstrictor agents [2]. Isolated mesenteric vascular preparations from females were more sensitive to VP than those from males, and pretreatment of male rats with exogenous estrogen enhanced the constrictor response to VP [1] (See Table 1). Both ERα and ERβ are expressed in the vasculature and have been implicated in both fast nitric oxide-mediated vasodilation and genomic alterations in gene and protein expression [39]. Thus, estrogen modulation of the vasoconstrictor action of VP could be mediated by ERα and/or ERβ expression in either endothelial cells or smooth muscle of the vasculature (See Table 2). In contrast to these estrogen-mediated gender differences in the direct vascular effects of VP, the magnitude of the VP-induced increase in blood pressure is greater in male than female rats except at estrus, and ovariectomy increases the pressor response in females to be comparable to males [8, 9]. Since these effects are opposite to the direct effects of estrogen on the vasculature, this likely reflects actions of estradiol on the baroreflex as well as the other systems involved in blood pressure regulation (i.e. the renin-angiotensin and sympathetic nervous systems) and demonstrates the complexity of gonadal steroid actions that impact VP secretion.
4. Gonadal steroid effects on afferents to the VP neurons
Osmoreceptive afferents
The antidiuretic effect of VP on the kidney results in increased water reabsorption from the renal filtrate resulting in a decrease in plasma osmolality. Extracellular fluid osmolality is monitored by osmosensitive neurons located in two circumventricular organs in the preoptic/anterior hypothalamus, the subfornical organ (SFO) and organum vasculosum of the lamina terminalis (OVLT; [38]) as well as peripheral osmoreceptors [83] and the VP neurons themselves [37]. As shown in Fig 2, osmoresponsive neurons in both SFO and OVLT express ERα [76] (See Table 2), and the ERα expressing neurons in OVLT, SFO, and median preoptic nucleus project to SON [93]. Thus these ERs are strong candidates for mediating estrogen effects on osmotic regulation of VP secretion. These ERα expressing neurons may also mediate responses to other peripheral hormones important in maintaining fluid balance. The ERα expressing neurons in these regions express AT1 angiotensin receptors [56], and the neurons in these regions that project to SON and PVN are responsive to relaxin [85]. Both of these hormones stimulate VP secretion, and their potent dipsogenic actions are mediated by the SFO [38]. Thus, activation of ERα in SFO, OVLT, and MnPO has the potential for modulating both fluid intake and output (see [74] for further discussion of estrogen effects on thirst). Although the impact on VP secretion of activation of these specific ERs has not been evaluated, a consistent finding reported in the literature is that the osmotic threshold for VP release and thirst fluctuates with physiological changes in circulating estrogen. The osmotic threshold for VP release is lower in the midluteal phase compared to the midfollicular phase of the menstrual cycle [80, 81, 94], and in both humans and rats the osmotic threshold for VP secretion is lower during gestation [15]. In rats, ovariectomy reduced the VP response to a hypertonic stimulus and the response was restored by estrogen treatment [24]. The expression of ERα in these osmosensitive areas could provide the basis for estrogen-induced alterations in the osmotic threshold for VP release, and could contribute to observed gender differences in VP secretion.
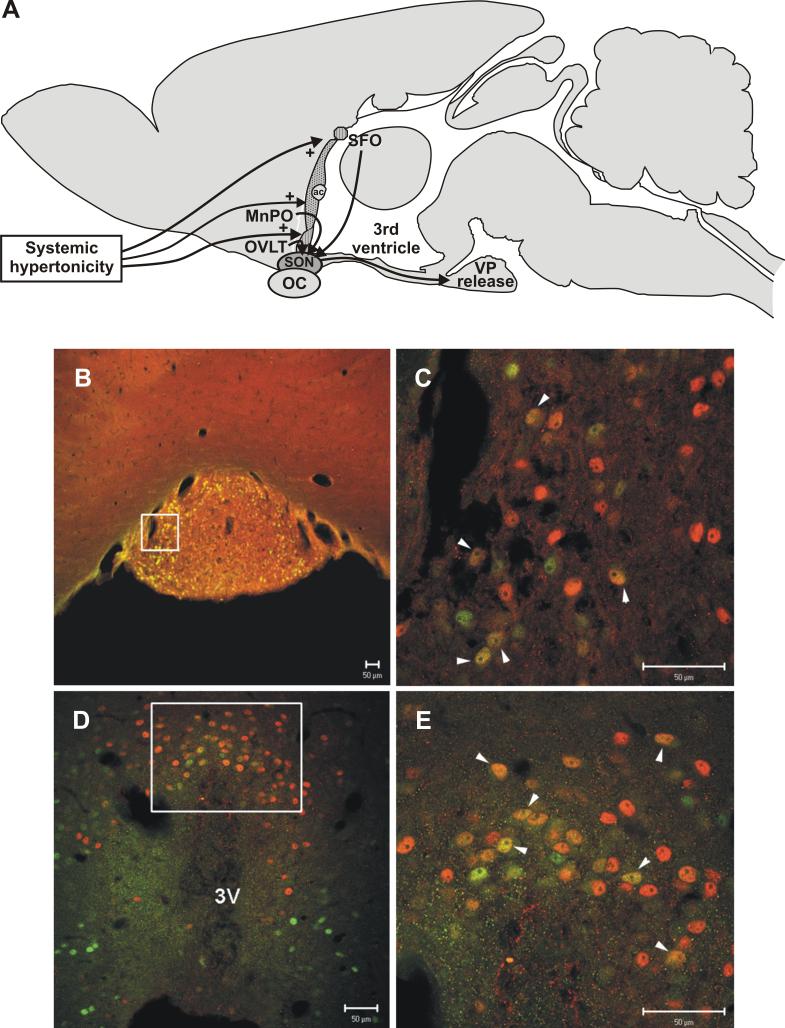
Figure 2.
ERα expression in the osmosensitive components of the lamina terminalis. A. Diagram showing location of subfornical organ (SFO) and organum vasculosum of the lamina terminalis (OVLT), the two circumventricular organs in the anterior hypothalamus that monitor extracellular fluid osmolality. B.-E. Sections through SFO (B,C) and OVLT (D,E) from 48 hr water deprived rats were double stained for ERα (green) and Fos (red). Note the nuclear localization of both ERα and Fos that is indicative of their roles as transcription regulatory factors. Unlike some steroid receptors (e.g. glucocorticoid receptors), the nuclear localization of ERs is not dependent on a steroid ligand binding to the receptor, and evidence exists for ligand-independent, gene regulatory effects of ERs [50, 60]. The rectangles in B and D indicate regions shown at higher magnification in C and E respectively. Fos staining is indicative of neurons activated by the dehydration protocol, and numerous ERα positive neurons show Fos activation (yellow/orange, some indicted by white arrowheads in C and E). Scale bars, 50μm. Modified from [38, 76].
Cardiovascular afferents
Blood volume is monitored by stretch receptors in the cardiac atria that respond to the degree of filling of the right atria. Blood pressure is monitored by stretch receptors in the carotid sinus. This information is relayed to neurons in the nucleus tractus solitarius (NTS) and dorsal vagal complex (DVC) via afferent fibers in the IXth and Xth cranial nerves (glossopharyngeal and vagus; [35]). It is then transmitted to the VP neurons over multi-synaptic pathways determined in part by the nature of the stimulus. Information encoding decreases in blood volume and pressure is transmitted from the NTS to the A1 catecholamine neurons in the ventrolateral medulla (VLM) which in turn innervate the VP neurons in SON and PVN [53, 72]. ERs have been localized in all subnuclei of the NTS including 60% of the caudal A2 catecholamine neurons [11, 25, 67, 102] (See Table 2). Both ERα and ERβ are expressed in NTS [65], and the expression of ERα fluctuates with the estrus cycle [25]. The A1 catecholamine neurons also express both ERα and ERβ with ERα expressing neurons being more numerous (Table 2; [11, 25, 67]). ER expressing neurons in NTS and the VLM project to SON [93], but at least the ERα neurons that project to SON are not catecholamine positive [93]. Therefore, the transmitter phenotype of the ERα and ERβ neurons in this region the innervate the magnocellular neurons in SON and PVN remains to be determined. Thus, estrogen has the potential to modulate afferent pathways from NTS and VLM to SON and PVN. The report that estrogen restored the VP response to a hypovolemic challenge in ovariectomized rats is consistent with estrogen modulation of this pathway [24]. Since expression of ERα receptors in A2 neurons fluctuates with the estrus cycle [25], the impact of estrogen on these pathways may vary with the reproductive cycle. This could contribute to inconsistencies in reports about estrogen regulation on VP secretion.
Since VP secretion is regulated by these two essentially independent afferent mechanisms (i.e. osmolality versus blood volume/pressure), similar alterations in the response to both regulatory pathways by estrogen might be construed as evidence that the site of estrogen action is on the ‘final common pathway’, the VP neuron itself. However, as described above both the afferents carrying osmotic information and those carrying cardiovascular information can be modulated by estrogen. Thus, it is feasible that the site of estrogen action is on the afferent pathways delivering feedback information to the VP neurons.
5. Gonadal steroid effects on the VP neurons
Direct effects of estrogen on the VP neurons are also possible, because the VP magnocellular neurons in SON and PVN express ERs. However, species differences exist relative to both the presence of ERs in magnocellular neurons and the type of ER (Table 2). ERβ is prominent in VP magnocellular neurons in rats and humans [28, 32, 65], guinea pigs express only ERα in magnocellular neurons [98], sheep and humans express both ERα and ERβ [28, 32, 61], and neither receptor is present in mouse SON [42, 64, 66]. As discussed below, physiological regulation of ER expression might underlie these species differences, but since ERα and ERβ often have opposite effects on gene expression [59] and may regulate different genes, this could contribute to variability in the reported effect of estrogen on VP secretion.
In addition to the classical ERs, VP neurons may be regulated by other types of estrogen receptors, because evidence for fast actions of estrogen on SON neurons exists [29, 95]. Specifically, estrogen has been shown to stimulate dendro/somatic release of VP and OT from magnocellular neurons [95], and in lactating rats, estrogen modulates electrical properties and responses to kainic acid of OT neurons [29]. The recent report that GPR30, the G-protein coupled, estrogen regulated receptor is expressed in SON and PVN provides one potential candidate for the receptor responsible for these actions [4]. This topic has been dealt with in more detail in a recent review [74].
Estrogen effects on VP release
Explants of the hypothalamo-neurohypophyseal system (HNS) were used to evaluate the effect of estradiol on VP release from the neural lobe. These explants include SON with its axonal projections extending through the median eminence and terminating in the neural lobe. In addition, this preparation contains the suprachiasmatic, arcuate, ventromedial, preoptic, and periventricular nuclei as well as the OVLT, but PVN is excluded. Inclusion of OVLT is essential for osmotic stimulation of VP release [70]. Although in these explants, VP is also released from dendrites of the magnocellular VP neurons and parvocellular neurons in the suprachiasmatic nucleus [16], the VP release measured reflects axonal release from neural lobe, because this exceeds all hypothalamic sources in the explant by 10-fold [23]. In HNS explants, VP release in response to NMDA (N-methyl-D-aspartic acid) and hypertonicity is inhibited by estradiol [87, 88]. Since the OVLT is required for osmotic stimulation of VP release both in vivo and in these HNS explants [70], it is possible that the inhibitory effect of estradiol on osmotic stimulation of VP release is mediated by ERα in the OVLT. However, since the OVLT projections to SON are glutamatergic [55] and glutamatergic transmission is required for osmotic stimulation of VP release [43, 69, 87], the inhibitory effect of estradiol on NMDA-stimulated VP secretion may mediate estrogen's inhibitory effect on osmotic stimulation. Since ERβ is present in the SON VP neurons, the role of ERα and ERβ in estradiol inhibition of NMDA-stimulated VP release was evaluated using genistein, an agonist with higher affinity for ERβ than ERα, and R,R-THC, a nonsteroidal molecule with antagonist activity on ERβ and agonist activity on ERα [84]. Genistein mimicked the effect of estrogen on NMDA-stimulated VP secretion, while R,R-THC prevented estrogen-inhibition of NMDA-stimulated VP release rather than mimicking the effect of estrogen [73]. These observations suggest that ERβ mediates the inhibitory effects of estrogen on NMDA-stimulation of VP release, and provide evidence for a functional role for ERβ in SON VP neurons. ERβ-mediated inhibition of VP release is consistent with the report by Skowsky et al [68] that gonadectomy increased pVP in male rats and testosterone replacement decreased pVP.
Effects on neurohypophyseal peptide gene expression
Studies with HNS explants also implicated estrogen in osmotic stimulation of VP gene expression. Chronic osmotic stimulation in vivo and extended exposure of HNS explants to hypertonicity results in increased VP mRNA in SON [5, 103, 107]. This reflects increased VP gene transcription as indicated by increases in VP heteronuclear RNA [26, 104]. Estrogen prevented the increase in VP mRNA in osmotically-stimulated HNS explants [88] suggesting an inhibitory role for ERβ in regulation of VP gene expression in VP magnocellular neurons. This possibility is supported by luciferase reporter assay studies in which estrogen inhibited VP promoter activity in cells transfected with ERβ [62]. In contrast, VP promoter activity was increased by estrogen in cells transfected with ERα [62]. This differential regulation of VP transcription by ERα and ERβ is important relative to understanding the impact of estrogen on VP expression in functionally distinct VP neurons (e.g. magnocellular vs the sexually dimorphic neurons in the bed nucleus of the stria terminalis that express both ERα and ERβ [14, 32, 41, 65]). Further evidence that estradiol activation of ERβ can inhibit VP gene expression was obtained in studies on the mouse PVN in which estrogen treatment resulted in a decrease in VP expression that was absent in ERβ knock out mice [44]. Although this probably represents estrogenic regulation of VP expression in parvocellular rather than magnocellular neurons, it demonstrates ERβ–mediated negative regulation of VP gene expression by estrogen.
Effects of androgens
To this point, we have focused on the effect of estrogen on VP gene expression, because that is the ligand equated with activation of ERs. However, there is considerable evidence that testosterone, in addition to estrogen, can alter VP secretion, and this is important relative to understanding reports of gender differences in regulation of VP secretion and fluid and electrolyte balance. In fact, the original inspiration for studying the effect of gonadal steroids on VP release from HNS explants was the report by Crowley and Amico [10] that gonadectomy prevented the dehydration-induced increase in VP mRNA and administration of testosterone to male rats restored the response. Since the perifusion medium for the HNS explants was not supplemented with steroids and the HNS explants were from male rats, it was anticipated that the addition of testosterone to the medium would augment the VP response to an osmotic stimulus. To our surprise, the opposite was observed. Testosterone and its androgenic metabolite, dihydrotestosterone (DHT), were as effective as estradiol in inhibiting both NMDA- and osmotically-stimulated VP release from HNS explants [87, 88].
The question is: Do the androgenic steroids act via the same mechanism as estrogen? The classic intracellular androgen receptor has not been demonstrated in magnocellular VP neurons, but in rats, some neurons in the perinuclear zone of SON do express androgen receptors [106]. Thus, these receptors are potential mediators of the androgenic actions. However, another possibility is that the actions of testosterone and DHT are mediated by ERβ, because some metabolites of testosterone such as 5α-androstane-3β, 17β-diol (3β-diol) have affinity for ERβ [34]. In a neuronal cell line, 3β-diol altered ERβ regulated gene transcription with a potency equivalent to that of estradiol [49]. As shown in Figure 3, 3β-diol prevented NMDA-induced VP release from HNS explants in a manner similar to that observed for estradiol, DHT, and genistein [73]. This supports the hypothesis that both estrogenic and androgenic metabolites of testosterone can activate ERβ-mediated inhibition of VP release. Further support for such a hypothesis is provided by evidence that the enzymes required to metabolize testosterone to estrogen (aromatase), DHT (5α-reductase), and DHT to 3β-diol [17β hydroxysteroid dehydrogenase (17β-HSD) or 3α HSD [20, 82, 90]] are expressed in the hypothalamus [36]. [Note: The report that VP immunoreactivity was not altered in SON of aromatase knock out mice [52] does not detract from this hypothesis, because as mentioned previously ERβ is not present in the mouse SON [42]]. To our knowledge, the impact of alterations in fluid balance on the expression of these enzymes has not been investigated.
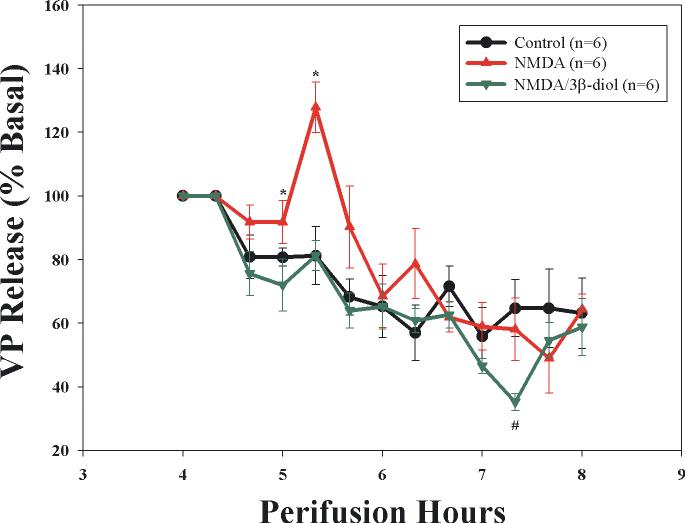
Figure 3.
Effect of 3-β-diol, an androgenic metabolite of testosterone that acts as an ERβ agonist, on NMDA-stimulated VP release from explants of the hypothalamo-neurohypophyseal system (HNS). NMDA (50 μM) induced a significant, but transient increase in VP secretion (red triangle) that was blocked by inclusion of 3-β-diol (10nM) in the perifusate (green inverted triangle). [Two-way repeated measure ANOVA F=5.07, p=0.02; individual mean comparison at 5.3 hrs *p<0.05 versus time control (black circles) and NMDA alone]. Basal release (pg/ml was as follows: Time control, 142±23; NMDA, 163±17; NMDA+3-β-diol, 201±34.
6. Impact of Fluid Balance on ER expression
ERα expression in osmoreceptive regions (SFO/OVLT)
As described above, osmosensitive neurons in the SFO, MnPO, and OVLT express ERα (Fig 2, Table 2). Since afferents from these regions are required for osmotic regulation of VP release, this provides a likely site for estrogen modulation of osmoregulation of VP release. Furthermore, expression of ERα in these regions may be regulated in response to chronic hyperosmolality. As shown in Figure 4, ERα is dramatically upregulated in the outer rim of the SFO in animals made extremely hyperosmolar by 48 h water deprivation following lesion of the region anterior and ventral to the 3rd ventricle (AV3V) [76]. Both the number of neurons expressing ERα and the density of ERα staining in SFO is increased [Figure 4, [76]]. ERα expression in these neurons is also modestly increased by the more physiological increase in osmolality induced by 48 hr water deprivation in intact rats [76]. This could increase the impact of circulating estrogens on these osmoreceptive cells. Since circulating steroids were reduced by dehydration [77, 78], the increase in ERα expression in SFO may be a mechanism for maintaining the influence of circulating gonadal steroids during circumstances of reduced ligand availability. Thus this may be important for maintaining fluid homeostasis by regulating both water excretion via VP secretion and water intake by regulating thirst [38]. The impact of estrogen on thirst has been considered in more detail in a recent review [74].
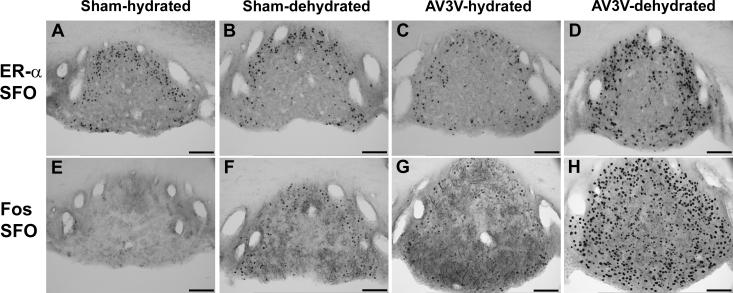
Figure 4.
ERα and Fos immunoreactivity in SFO of hydrated (sham-hydrated), 48 hr water deprived (sham-dehydrated), and hydrated or water deprived AV3V lesions rats (AV3V+hydrated and AV3V-dehydrated respectively) rats. Water deprivation induced a significant increase in Fos expression in SFO which was markedly exaggerated in the AV3V lesioned animals. Due to the impairment of osmotically stimulated VP secretion in these animals, they experience an extreme increase in plasma osmolality during water deprivation (380±10 vs 304±1 mOsm/kg H2O in sham-dehydrated). The density of ERα staining was greater in the periphery of SFO in the sham-dehydrated rats compared to hydrated rats (panel B; p<0.05), and both the density and number of ERα positive neurons increased in the AV3V+dehydrated rats with the increase in ERα positive neurons occurring in both the periphery and core of SFO (panel D; p<0.05). In spite of this dramatic increase in ERα expression, not all Fos positive cells (panel H) became ERα positive. Modified from [76].
ERβ expression in magnocellular VP neurons
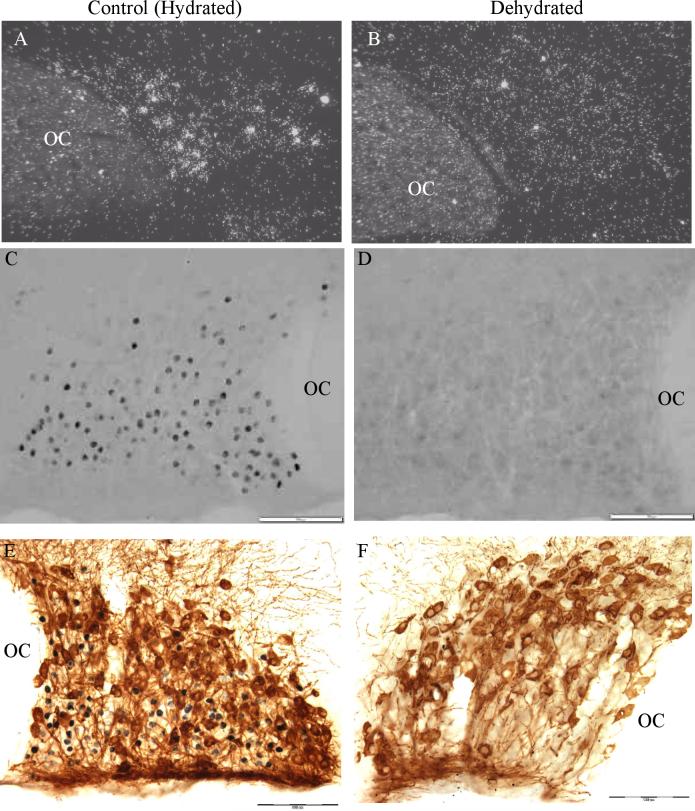
is also dramatically impacted by dehydration [78], but as shown in Figure 5, instead of increasing, it disappears. In euhydrated rats, ERβ immunoreactivity is strong in magnocellular VP neurons in SON and PVN with faint or absent ERβ staining in magnocellular OT neurons [78]. This observation was replicated by Suzuki and Handa [86] who reported that in SON, 72% of VP neurons co-expressed ERβ while the expression in OT neurons was low. Seventy-two hours of drinking 2% saline induced a significant decrease in ERβ mRNA evaluated by in situ hybridization and a disappearance of immunoreactive ERβ staining in the SON [78]. In the PVN the disappearance of ERβ immunoreactivity was limited to the magnocellular division of the nucleus while ERβ staining was not altered in the parvocellular regions [78]. Also, ERβ mRNA was increased in SON in animals made chronically hyponatremic by consuming a liquid diet during treatment with d-amino-D-arginine VP [78]. Thus, ERβ expression in magnocellular VP neurons is inversely correlated with changes in plasma osmolality, and this requires monitoring of changes in plasma osmolality by the osmoreceptive neurons in the lamina terminalis, i.e. those in SFO, MnPO, and OVLT [77]. Hypovolemia, another potent stimulus for VP secretion, also induces a decrease in ERβ expression in magnocellular VP neurons [79]. The time course of the changes in ERβ expression in magnocellular VP neurons in response to water deprivation, a physiologically important stimulus for VP secretion, is compatible with this being a physiologically relevant component of regulation of VP secretion in response to alterations in fluid balance and hypovolemia. ERβ immunoreactivity was significantly decreased in magnocellular VP neurons of SON and PVN following 20 hrs of water deprivation in rats [79]. Rehydration of rats subjected to 26 hours of water deprivation resulted in a significant although not complete restoration of ERβ immunoreactivity within 6 hours [79]. Since ERβ mediates inhibition of VP release by estrogen and probably testosterone, these findings support the conclusion that decreased expression of ERβ during dehydration or hypovolemia may contribute to the upregulation of VP secretion that is prominent in these conditions.
Figure 5.
ERα expression in SON in hydrated and dehydrated rats. A. and B. In situ hybridization for ERα mRNA reveals a decrease in ERβ mRNA following 72 hrs of 2% saline ingestion (see [78] for details). C. and D. ERβ immunohistochemistry in SON reveals a disappearance of ERβ protein in SON following 48 hrs of water deprivation. Note in the hydrated section that dense ERβ immunoreactivity is seen predominantly in the neurons positioned in the ventral portion of SON corresponding to the location of VP neurons. However, faint immunoreactivity is also present in neurons located more dorsally (e.g. in the region corresponding to OT neurons). E. and F. Double immunohistochemistry for OT (brown) and ERβ (black). Note the cytoplasmic localization of the OT immunoreactivity versus the nuclear localization of the ERβ immunoreactivity. In the hydrated example, dense ERβ immunoreactivity is not present in the OT neurons indicating it is primarily expressed in VP neurons in SON (see [78] for details and for pictures of VP/ERβ double immunostaining). Scale bar, 100 μm. OC, optic chiasm.
Other regulators of ERβ expression in magnocellular VP neurons
ERβ expression in magnocellular VP neurons is regulated in response to gonadal and adrenal hormones. Estradiol replacement caused a decrease in ERβ in magnocellular neurons of ovariectomized rats [86]. Based on our evidence that ERβ expression in SON mediates inhibition of VP secretion from HNS explants, a decrease in ERβ expression induced by treating ovariectomized female rats with estradiol would be expected to cause an increase in plasma VP as reported by Skowsky et al [68] and Peysner and Forsling [51] (Table 1). The question then arises, are the dehydration-induced changes in ERβ secondary to dehydration-induced changes in circulating gonadal steroids? In fact, testosterone was suppressed by both hyper- and hypo-osmolality [78]. Therefore, the dehydration-induced changes in ERβ expression in magnocellular neurons were not correlated with changes in plasma testosterone and circulating estrogen did not change significantly [78] suggesting that dehydration induced changes in circulating gonadal steroids are not responsible for the changes in ERβ observed in SON and PVN magnocellular neurons during dehydration. Adrenalectomy caused a greater than 2-fold increase in ERβ mRNA in magnocellular neurons in SON and PVN which was partially prevented by corticosterone replacement [75]. However, this may reflect the hydromineral derangement consequent to adrenalectomy, rather than direct effect of glucocorticoids on ERβ expression [75]. The absence of glucocorticoid receptor expression in SON in normally hydrated rats supports this view [3, 31, 92]. Similarly changes reported during lactation in ERβ mRNA in SON VP neurons may reflect the impact of milk production on fluid balance. Although an overall increase in the number of cells expressing ERβ mRNA was reported in SON neurons in one study [22], ERβ mRNA per cell actually decreases in the ventral portion of the nucleus where the VP neurons are concentrated [74]. Thus, ERβ expression in VP magnocellular neurons appears to be most prominently regulated by parameters related to fluid and electrolyte balance rather than fluctuations in circulating steroids.
7. Conclusions and future research
Changes in fluid and electrolyte balance associated with pregnancy and different stages of the reproductive cycle as well as the prominent expression of ERβ in magnocellular VP neurons has prompted considerable interest in the role of estrogen in regulation of VP secretion. However, what appears simple on the surface is complicated by the fact that the effect of estrogen or testosterone on plasma VP reflects the summation of the multifaceted effects of gonadal steroids on the complex system involved in regulation of fluid and electrolyte homeostasis. In addition, the relative contribution of each of these effects can be altered as a result of the ability of the circulating hormones as well as fluid and electrolyte balance, gender, and reproductive status to regulate the expression of these receptors. Differences in dietary sources of steroid ligands for the receptors introduces yet another variable with the potential to alter the balance of these various components [18]. Thus the discordance in reports in the literature can be understood based on differences in the status of experimental subjects in each study relative to these variables that were largely unrecognized for their importance at the time the studies were performed.
The importance of the involvement of multiple types of gonadal steroid receptors is beginning to be appreciated, and emerging evidence that the expression of these receptors undergoes dramatic changes with alterations in reproductive and fluid balance status is important for deciphering the effects of gonadal steroids on VP secretion. Changes in ER expression can alter the impact of gonadal steroids by amplifying or diminishing the effect of increases in the ligand such as occurs with estradiol during pregnancy and the luteal phase of the menstrual cycle or decreases as occurs during dehydration. Thus, as described herein, ERα expression increases in SFO during chronic hypertonicity. Since ERα is expressed in the osmoreceptive neurons in SFO, an increase in ERα expression in concert with increased circulating estrogen such as occurs during pregnancy would be expected to alter responses of these neurons to osmotic stimuli. In fact, the decrease in the osmotic threshold for VP secretion (and thirst; see [74] for more details) observed during pregnancy and in the luteal phase of the menstrual cycle is consistent with ERα-mediated increases in sensitivity of the osmoreceptors. In contrast, ERβ expression in the magnocellular VP neurons of SON and PVN decreases dramatically during dehydration or in response to hypertonicity induced by saline drinking. Since ERβ mediates estrogen inhibition of VP release, the decrease in ERβ expression removes an inhibitory influence allowing the prominent increase in VP secretion that characterizes responses to dehydration. Thus, the opposite effect of hypertonicity on ERα expression in SFO and ERβ expression in SON is consistent with achieving maximal secretion of VP during hyperosmolar challenges. Furthermore, this dichotomy in expression and direction of action of ERα and ERβ may explain the diverse reports in the literature as well (Table 1). For example, the E2-induced increase in pVP reported by Skowsky et al [68] in female rats could reflect predominance of ERα-mediated enhanced osmosensitivity while the lack of effect of estrogen reported by Crofton et al [7] could reflect a balance between ERα enhanced osmosensitivity and ERβ-mediated inhibition at the VP neurons. Similarly, the biphasic dose response reported by Peysner and Forsling [51] may reflect differences in the effective concentration of estrogen at ERα in SFO, OVLT, and MnPO versus ERβ in SON and PVN magnocellular VP neurons, and therefore a shift in the balance between positive and negative estrogen effects on VP secretion. However, it could also reflect differences in the effect of estrogen on target tissues and therefore, the feedback signals regulating VP secretion.
The lack of ERβ expression in species such as mice and guinea pigs suggests that it is not evolutionarily conserved and thus, perhaps, lacks physiological importance. However, an interesting possibility is that the observed regulation of ER expression may underlie apparent species differences. Specifically, the disappearance of ERβ immunoreactivity in the rat SON with dehydration and the increase in ERβ mRNA with hyponatremia suggests that the reported absence of ERβ expression in SON neurons of the mouse and guinea pig might reflect differences between these species and rats in the balance between positive and negative factors regulating ERβ under basal conditions. The higher basal plasma osmolality in mice (303−355 mOsm/kg) versus rats (280−295 mOsm/kg) [40] is consistent with this possibility. Additional ERβ regulatory candidates that might differ between species include gonadal and adrenal steroid hormones as well as the neurotransmitters carrying information about osmolality (glutamate, GABA, angiotensin) and blood volume (norepinephrine, ATP, neuropeptide Y, others?). Thus, further clarification of the mechanisms regulating expression of the ERs in various neuronal populations remains important for dissecting the role of estrogen and testosterone in regulating VP secretion.
Identification of the specific target genes regulated by ERα and ERβ is also required to elucidate the function of these receptors in the osmosensitive neurons in SFO, OVLT and MnPO and the magnocellular VP neurons. Numerous genes are up regulated in SON during dehydration [21, 27, 105]. The concurrent decrease in ERβ, a potentially inhibitory transcription factor, identifies genes up-regulated by dehydration as candidates for ERβ inhibition. The ability of ERβ to inhibit genes expressing classical estrogen response elements [62] as well as genes expressing AP1 elements [47] suggests that down regulation of ERβ in dehydration could influence a wide variety of genes contributing to the maintenance of fluid homeostasis by increasing VP secretion.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This work was supported by NIH Grant RO1 NS27975 (C.D.S.) and an AHA Midwest Affiliate Postdoctoral Fellowship (S.J.S.). The authors acknowledge the contributions of Brian Gulbransen to Figure 3.
References
- 1.Altura BM. Sex and estrogens and responsiveness of terminal arterioles to neurohypophyseal hormones and catecholamines. J Pharmacol Exp Ther. 1975;193(2):403–412. [PubMed] [Google Scholar]
- 2.Altura BM, Altura BT. Vascular smooth muscle and neurohypophyseal hormones. Federation Proceedings. 1977;36:1853–1860. [PubMed] [Google Scholar]
- 3.Berghorn KA, Knapp LT, Hoffman GE, Sherman TG. Induction of glucocorticoid receptor expression in hypothalamic magnocellular vasopressin neurons during chronic hypoosmolality. Endocrinology. 1995;136:804–811. doi: 10.1210/endo.136.2.7835313. [DOI] [PubMed] [Google Scholar]
- 4.Brailoiu E, Dun SL, Brailoiu GC, Mizuo K, Sklar LA, Oprea TI, Prossnitz ER, Dun NJ. Distribution and characterization of estrogen receptor G protein-coupled receptor 30 in the rat central nervous system. J Endocrinol. 2007;193(2):311–321. doi: 10.1677/JOE-07-0017. [DOI] [PubMed] [Google Scholar]
- 5.Burbach JPH, DeHoop MJ, Schmale H, Richter D, DeKloet ER, TenHaaf JA, DeWied D. Differential responses to osmotic stress of vasopressin-neurophysin mRNA in hypothalamic nuclei. Neuroendocrinology. 1984;39:582–584. doi: 10.1159/000124040. [DOI] [PubMed] [Google Scholar]
- 6.Carter DA, Pardy K, Murphy D. Regulation of vasopressin gene expression: changes in the level, but not the size, of vasopressin mRNA following endocrine manipulations. Cell Mol Neurobiol. 1993;13(1):87–95. doi: 10.1007/BF00712991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crofton JT, Baer PG, Share L, Brooks DP. Vasopressin release in male and female rats: Effects of gonadectomy and treatment with gonadal steroid hormones. Endocrinology. 1985;117:1195–1200. doi: 10.1210/endo-117-3-1195. [DOI] [PubMed] [Google Scholar]
- 8.Crofton JT, Ratliff DL, Brooks DP, Share L. The metabolic clearance rate of and pressor responses to vasopressin in male and female rats. Endocrinology. 1986;118(5):1777–1781. doi: 10.1210/endo-118-5-1777. [DOI] [PubMed] [Google Scholar]
- 9.Crofton JT, Share L, Brooks DP. Pressor responsiveness to and secretion of vasopressin during the estrous cycle. Am J Physiol. 1988;255:R1041–R1045. doi: 10.1152/ajpregu.1988.255.6.R1041. [DOI] [PubMed] [Google Scholar]
- 10.Crowley RS, Amico JA. Gonadal steroid modulation of oxytocin and vasopressin gene expression in the hypothalamus of the osmotically stimulated rat. Endocrinology. 1993;133:2711–2718. doi: 10.1210/endo.133.6.8243294. [DOI] [PubMed] [Google Scholar]
- 11.Curran-Rauhut MA, Petersen SL. Oestradiol-dependent and -independent modulation of tyrosine hydroxylase mRNA levels in subpopulations of A1 and A2 neurones with oestrogen receptor (ER)alpha and ER beta gene expression. J Neuroendocrinol. 2003;15(3):296–303. doi: 10.1046/j.1365-2826.2003.01011.x. [DOI] [PubMed] [Google Scholar]
- 12.Dai WJ, Yao T. Effects of dehydration and salt-loading on hypothalamic vasopressin mRNA level in male and female rats. Brain Res. 1995;676:178–182. doi: 10.1016/0006-8993(95)00112-4. [DOI] [PubMed] [Google Scholar]
- 13.Davison JM, Gilmore EA, Durr J, Robinson AG, Lindheimer MD. Altered osmotic thresholds for vasopressin secretion and thirst in human prenancy. Am. J. Physiol. Renal Fluid Electrolyte Physiol. 1984;246:F105–F109. doi: 10.1152/ajprenal.1984.246.1.F105. [DOI] [PubMed] [Google Scholar]
- 14.De Vries GJ, Wang Z, Bullock NA, Numan S. Sex differences in the effects of testosterone and its metabolites on vasopressin messenger RNA levels in the bed nucleus of the stria terminalis of rats. J.Neurosci. 1994;14:1789–1794. doi: 10.1523/JNEUROSCI.14-03-01789.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Durr JA, Stamoutsos B, Lindheimer MD. Osmoregulation during pregnancy in the rat: Evidence for resetting of the threshold for vasopressin secretion during gestation. J.Clin.Invest. 1982;68:336–346. doi: 10.1172/JCI110261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Earnest DJ, Sladek CD. Circadian vasopressin release from perifused rat suprachiasmatic explants in vitro: Effects of acute stimulation. Brain Research. 1987;422:398–402. doi: 10.1016/0006-8993(87)90952-8. [DOI] [PubMed] [Google Scholar]
- 17.Filardo E, Quinn J, Pang Y, Graeber C, Shaw S, Dong J, Thomas P. Activation of the Novel Estrogen Receptor G Protein-Coupled Receptor 30 (GPR30) at the Plasma Membrane. Endocrinology. 2007;148(7):3236–3245. doi: 10.1210/en.2006-1605. [DOI] [PubMed] [Google Scholar]
- 18.Forsling ML, Kallo I, Hartley DE, Heinze L, Ladek R, Coen CW, File SE. Oestrogen receptor-beta and neurohypophysial hormones: functional interaction and neuroanatomical localisation. Pharmacol Biochem Behav. 2003;76(3−4):535–542. doi: 10.1016/j.pbb.2003.09.013. [DOI] [PubMed] [Google Scholar]
- 19.Forsling ML, Stromberg P, Akerlund M. Effect of ovarian steroids on vasopressin secretion. J.Endocrinol. 1982;95:147–151. doi: 10.1677/joe.0.0950147. [DOI] [PubMed] [Google Scholar]
- 20.Gangloff A, Shi R, Nahoum V, Lin SX. Pseudo-symmetry of C19 steroids, alternative binding orientations, and multispecificity in human estrogenic 17beta-hydroxysteroid dehydrogenase. FASEB J. 2003;17(2):274–276. doi: 10.1096/fj.02-0397fje. [DOI] [PubMed] [Google Scholar]
- 21.Ghorbel MT, Sharman G, Hindmarch C, Becker KG, Barrett T, Murphy D. Microarray screening of suppression subtractive hybridization-PCR cDNA libraries identifies novel RNAs regulated by dehydration in the rat supraoptic nucleus. Physiol Genomics. 2006;24(2):163–172. doi: 10.1152/physiolgenomics.00229.2005. [DOI] [PubMed] [Google Scholar]
- 22.Greco B, Lubbers LS, Blaustein JD. Estrogen receptor β messenger ribonucleic acid expression in the forebrain of proestrous, pregnant, and lactating female rats. Endocrinology. 2003;144:1869–1875. doi: 10.1210/en.2002-220807. [DOI] [PubMed] [Google Scholar]
- 23.Gregg CM, Sladek CD. A compartmentalized, organ-cultured hypothalamo-neurohypophysial system for the study of vasopressin release. Neuroendocrinology. 1984;38:397–402. doi: 10.1159/000123924. [DOI] [PubMed] [Google Scholar]
- 24.Hartley DE, Dickson SL, Forsling ML. Plasma vasopressin concentrations and Fos protein expression in the supraoptic nucleus following osmotic stimulation or hypovolaemia in the ovariectomized rat: effect of oestradiol replacement. J Neuroendocrinol. 2004;16(3):191–197. doi: 10.1111/j.0953-8194.2004.01150.x. [DOI] [PubMed] [Google Scholar]
- 25.Haywood SA, Simonian SX, van der Beek EM, Bicknell RJ, Herbison AE. Fluctuating estrogen and progesterone receptor expression in brainstem norepinephrine neurons through the rat estrous cycle. Endocrinology. 1999;140(7):3255–3263. doi: 10.1210/endo.140.7.6869. [DOI] [PubMed] [Google Scholar]
- 26.Herman JP, Schafer MK-H, Watson SJ, Sherman TG. In situ hybridization analysis of arginine vasopressin gene transcription using intron-specific probes. Molecular Endocrinology. 1991;5:1447–1456. doi: 10.1210/mend-5-10-1447. [DOI] [PubMed] [Google Scholar]
- 27.Hindmarch C, Yao S, Beighton G, Paton J, Murphy D. A comprehensive description of the transcriptome of the hypothalamoneurohypophyseal system in euhydrated and dehydrated rats. Proc Natl Acad Sci U S A. 2006;103(5):1609–1614. doi: 10.1073/pnas.0507450103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ishunina TA, Kruijver FPM, Balesar R, Swaab DF. Differential expression of estrogen receptor α and β immunoreactivity in the human supraoptic nucleus in relation to sex and aging. J.Clin.Endocrinol.Metab. 2000;85:3283–3291. doi: 10.1210/jcem.85.9.6826. [DOI] [PubMed] [Google Scholar]
- 29.Israel J-M, Poulain DA. 17 β-Oestradiol modulates in vitro electrical properties and responses to kainate of oxytocin neurones in lactating rats. Journal of Physiology. 2000;524.2:457–470. doi: 10.1111/j.1469-7793.2000.t01-2-00457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jelinsky SA, Harris HA, Brown EL, Flanagan K, Zhang X, Tunkey C, Lai K, Lane MV, Simcoe DK, Evans MJ. Global transcription profiling of estrogen activity: estrogen receptor alpha regulates gene expression in the kidney. Endocrinology. 2003;144(2):701–710. doi: 10.1210/en.2002-220728. [DOI] [PubMed] [Google Scholar]
- 31.Kiss JZ, Van Eekelen JAM, Reul JMHM, Westphal HM, DeKloet ER. Glucocorticoid receptor in magnocellular neurosecretory cells. Endocrinology. 1988;122:444–449. doi: 10.1210/endo-122-2-444. [DOI] [PubMed] [Google Scholar]
- 32.Kruijver FP, Balesar R, Espila AM, Unmehopa UA, Swaab DF. Estrogen-receptor-beta distribution in the human hypothalamus: similarities and differences with ER alpha distribution. J Comp Neurol. 2003;466(2):251–277. doi: 10.1002/cne.10899. [DOI] [PubMed] [Google Scholar]
- 33.Kuiper GGJM, Carlsson B, Grandien K, Enmark E, Haggblad J, Nilsson S, Gustafsson J-A. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors α and β. Endocrinology. 1997;138:863–870. doi: 10.1210/endo.138.3.4979. [DOI] [PubMed] [Google Scholar]
- 34.Kuiper GGJM, Lemmen JG, Carlsson B, Corton JC, Safe SH, van der Saag PT, van der Burg B, Gustafsson J-A. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor β. Endocrinology. 1998;139:4252–4263. doi: 10.1210/endo.139.10.6216. [DOI] [PubMed] [Google Scholar]
- 35.Kumada M, Terui N, Kuwaki T. Arterial baroreceptor reflex: Its central and peripheral neural mechanisms. Progress in Neurobiology. 1990;35:331–361. doi: 10.1016/0301-0082(90)90036-g. [DOI] [PubMed] [Google Scholar]
- 36.Lund TD, Hinds LR, Handa RJ. The androgen 5alpha-dihydrotestosterone and its metabolite 5alpha-androstan-3beta, 17beta-diol inhibit the hypothalamo-pituitary-adrenal response to stress by acting through estrogen receptor beta-expressing neurons in the hypothalamus. J Neurosci. 2006;26(5):1448–1456. doi: 10.1523/JNEUROSCI.3777-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mason WT. Supraoptic neurons of rat hypothalamus are osmosensitive. Nature. 1980;287:154–157. doi: 10.1038/287154a0. [DOI] [PubMed] [Google Scholar]
- 38.McKinley MJ, Johnson AK. The physiological regulation of thirst and fluid intake. News Physiol Sci. 2004;19:1–6. doi: 10.1152/nips.01470.2003. [DOI] [PubMed] [Google Scholar]
- 39.Mendelsohn ME. Genomic and nongenomic effects of estrogen in the vasculature. Am J Cardiol. 2002;90(1A):3F–6F. doi: 10.1016/s0002-9149(02)02418-9. [DOI] [PubMed] [Google Scholar]
- 40.Meneton P, Ichikawa I, Inagami T, Schnermann J. Renal physiology of the mouse. Am J Physiol Renal Physiol. 2000;278(3):F339–351. doi: 10.1152/ajprenal.2000.278.3.F339. [DOI] [PubMed] [Google Scholar]
- 41.Miller MA, Urban JH, Dorsa DM. Steroid dependency of vasopressin neurons in the bed nucleus of the stria terminalis by in situ hybridization. Endocrinology. 1989;125:2335–2340. doi: 10.1210/endo-125-5-2335. [DOI] [PubMed] [Google Scholar]
- 42.Mitra SW, Hoskin E, Yudkovitz J, Pear L, Wilkinson HA, Hayashi S, Pfaff DW, Ogawa S, Rohrer SP, Schaeffer JM, McEwen BS, Alves SE. Immunolocalization of estrogen receptor β in the mouse brain: Comparison with estrogen receptor β. Endocrinology. 2003;144:2055–2067. doi: 10.1210/en.2002-221069. [DOI] [PubMed] [Google Scholar]
- 43.Morsette DJ, Sidorowicz HE, Sladek CD. Role of non-NMDA receptors in vasopressin and oxytocin release from rat hypothalamo-neurohypophyseal explants. Am.J.Physiol.Regul.Integr.Comp.Physiol. 2001;280:R313–R322. doi: 10.1152/ajpregu.2001.280.2.R313. [DOI] [PubMed] [Google Scholar]
- 44.Nomura M, McKenna E, Korach KS, Pfaff DW, Ogawa S. Estrogen receptor-b regulates transcript levels for oxytocin and argininge vasopressin i the hypothalamic paraventricular nucleus of male mice. Mol.Brain Res. 2002;109:84–94. doi: 10.1016/s0169-328x(02)00525-9. [DOI] [PubMed] [Google Scholar]
- 45.O'Keefe JA, Crowley RS, Hrivnak P, Kim NB, Amico JA. The effect of testosterone and its metabolites on the accumulation of vasopressin messenger ribonucleic acid in the hypothalamus of the osmotically stimulated male rat. Neuroendocrinology. 1995;61:405–411. doi: 10.1159/000126862. [DOI] [PubMed] [Google Scholar]
- 46.Ota M, Crofton JT, Share L. Hemorrhage-induced vasopressin release in the paraventricular nucleus measured by in vivo microdialysis. Brain Research. 1994;658:49–54. doi: 10.1016/s0006-8993(09)90009-9. [DOI] [PubMed] [Google Scholar]
- 47.Paech K, Webb P, Kuiper GGJM, Nilsson S, Gustafsson J-A, Kushner PJ, Scanlon TS. Differential ligand activation of estrogen receptors ERα and ERβ at AP1 sites. Science. 1997;277:1508–1510. doi: 10.1126/science.277.5331.1508. [DOI] [PubMed] [Google Scholar]
- 48.Pak TR, Chung WC, Hinds LR, Handa RJ. Estrogen Receptor-beta Mediates Dihydrotestosterone-Induced Stimulation of the Arginine Vasopressin Promoter in Neuronal Cells. Endocrinology. 2007;148(7):3371–3382. doi: 10.1210/en.2007-0086. [DOI] [PubMed] [Google Scholar]
- 49.Pak TR, Chung WC, Lund TD, Hinds LR, Clay CM, Handa RJ. The androgen metabolite, 5alpha-androstane-3beta, 17beta-diol, is a potent modulator of estrogen receptor-beta1-mediated gene transcription in neuronal cells. Endocrinology. 2005;146(1):147–155. doi: 10.1210/en.2004-0871. [DOI] [PubMed] [Google Scholar]
- 50.Pak TR, Chung WC, Roberts JL, Handa RJ. Ligand-independent effects of estrogen receptor beta on mouse gonadotropin-releasing hormone promoter activity. Endocrinology. 2006;147(4):1924–1931. doi: 10.1210/en.2005-1297. [DOI] [PubMed] [Google Scholar]
- 51.Peysner K, Forsling ML. Effect of ovariectomy and treatment with ovarian steroids on vasopressin release and fluid balance in the rat. J.Endocrinol. 1990;124:277–284. doi: 10.1677/joe.0.1240277. [DOI] [PubMed] [Google Scholar]
- 52.Plumari L, Viglietti-Panzica C, Allieri F, Honda S, Harada N, Absil P, Balthazart J, Panzica GC. Changes in the arginine-vasopressin immunoreactive systems in male mice lacking a functionall aromatase gene. J Neuroendocrinology. 2002;14:971–978. doi: 10.1046/j.1365-2826.2002.00866.x. [DOI] [PubMed] [Google Scholar]
- 53.Raby WN, Renaud LP. Dorsomedial medulla stimulation activates rat supraoptic oxytocin and vasopressin neurones through different pathways. Journal of Physiology. 1989;417:279–294. doi: 10.1113/jphysiol.1989.sp017801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science. 2005;307(5715):1625–1630. doi: 10.1126/science.1106943. [DOI] [PubMed] [Google Scholar]
- 55.Richard D, Bourque CW. Synaptic control of rat supraoptic neurones during osmotic stimulation of the organum vasculosum lamina terminalis in vitro. J.Physiol.(Lond.) 1995;489:567–577. doi: 10.1113/jphysiol.1995.sp021073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rosas-Arellano MP, Solano-Flores LP, Ciriello J. Co-localization of estrogen and angiotensin receptors within subfornical organ neurons. Brain Research. 1999;837:254–262. doi: 10.1016/s0006-8993(99)01672-8. [DOI] [PubMed] [Google Scholar]
- 57.Rowland I, Faughnan M, Hoey L, Wahala K, Williamson G, Cassidy A. Bioavailability of phyto-oestrogens. Br J Nutr. 2003;89(Suppl 1):S45–58. doi: 10.1079/BJN2002796. [DOI] [PubMed] [Google Scholar]
- 58.Roy BN, Reid RL, Van Vugt DA. The effects of estrogen and progesterone on corticotropin-releasing hormone and arginine vasopressin messenger ribonucleic acid levels in the paraventricular nucleus and supraoptic nucleus of the rhesus monkey. Endocrinology. 1999;140(5):2191–2198. doi: 10.1210/endo.140.5.6684. [DOI] [PubMed] [Google Scholar]
- 59.Safe S. Transcriptional activation of genes by 17 beta-estradiol through estrogen receptor-SP1 interactions. Vitam.Horm. 2001;62:231–252. doi: 10.1016/s0083-6729(01)62006-5. [DOI] [PubMed] [Google Scholar]
- 60.Sanchez R, Nguyen D, Rocha W, White JH, Mader S. Diversity in the mechanisms of gene regulation by estrogen receptors. Bioessays. 2002;24(3):244–254. doi: 10.1002/bies.10066. [DOI] [PubMed] [Google Scholar]
- 61.Scott CJ, Tilbrook AJ, Simmons DM, Rawson JA, Chu S, Fuller PJ, Ing NH, Clark IJ. The distribution of cells containing estrogen receptor-alpha (ERalpha) and ERbeta messenger ribonucleic acid in the preoptic area and hypothalamus of the sheep: comparison of males and females. Endocrinology. 2000;141:2951–2962. doi: 10.1210/endo.141.8.7622. [DOI] [PubMed] [Google Scholar]
- 62.Shapiro RA, Xu C, Dorsa DM. Differential transcriptional regulation of rat vasopressin gene expression by estrogen receptor β and β. Endocrinology. 2000;141:4056–4064. doi: 10.1210/endo.141.11.7796. [DOI] [PubMed] [Google Scholar]
- 63.Share L, Wang Y-X, Crofton JT. Gender differences in the actions of vasopressin. In: Saito T, Kurokawa K, Yoshida S, editors. Neurohypophysis: Recent Progress in Vasopressin and Oxytocin Research. Elsevier Science B.V.; Amsterdam: 1995. pp. 3–13. [Google Scholar]
- 64.Shughrue PJ, Dellovade TL, Merchenthaler I. Estrogen modulates oxytocin gene expression in regions of the rat supraoptic and paraventricular nuclei that contain estrogen receptor-beta. Prog.Brain Res. 2002;139:15–20. doi: 10.1016/s0079-6123(02)39004-6. [DOI] [PubMed] [Google Scholar]
- 65.Shughrue PJ, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptor-a and -b mRNA in the rat central nervous system. J Comp Neurology. 1997;388:507–525. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 66.Shughrue PJ, Scrimo P, Lane M, Askew R, Merchenthaler I. The distribution of estrogen receptor-β mRNA in forebrain regions of the estrogen receptor-α knockout mouse. Endocrinology. 1997;138:5649–5652. doi: 10.1210/endo.138.12.5712. [DOI] [PubMed] [Google Scholar]
- 67.Simonian SX, Herbison AE. Differential expression of estrogen receptor and neuropeptide Y by brainstem A1 and A2 noradrenaline neurons. Neuroscience. 1997;76(2):517–529. doi: 10.1016/s0306-4522(96)00406-x. [DOI] [PubMed] [Google Scholar]
- 68.Skowsky WR, Swan L, Smith P. Effects of sex steroid hormones on arginine vasopressin in intact and castrated male and female rats. Endocrinology. 1979;104:105–108. doi: 10.1210/endo-104-1-105. [DOI] [PubMed] [Google Scholar]
- 69.Sladek CD, Badre SE, Morsette DJ, Sidorowicz HE. Role of non-NMDA receptors in osmotic and glutamate stimulation of vasopressin release: Effect of rapid receptor desensitization. J Neuroendocrin. 1998;10:897–903. doi: 10.1046/j.1365-2826.1998.00276.x. [DOI] [PubMed] [Google Scholar]
- 70.Sladek CD, Johnson AK. The effect of anteroventral third ventricle lesions on vasopressin release by organ cultured hypothalamo-neurohypophyseal explants. Neuroendocrinology. 1983;37:78–84. doi: 10.1159/000123519. [DOI] [PubMed] [Google Scholar]
- 71.Sladek CD, Swenson KL, Kapoor R, Sidorowicz HE. The role of steroid hormones in the regulation of vasopressin and oxytocin release and mRNA expression in hypothalamo-neurohypophysial explants from the rat. Exp.Physiol. 2000;85S:171S–177S. doi: 10.1111/j.1469-445x.2000.tb00021.x. [DOI] [PubMed] [Google Scholar]
- 72.Smith DW, Sibbald JR, Khanna L, Day TA. Rat vasopressin cell responses to simulated hemorrhage: stimulus-dependent role for A1 noradrenergic neurons. Am J Physiology Regul Inegr Comp Physiol. 1995;268:R1336–R1342. doi: 10.1152/ajpregu.1995.268.5.R1336. [DOI] [PubMed] [Google Scholar]
- 73.Somponpun S, Sladek CD. Role of estrogen receptor β in regulation of vasopressin and oxytocin release in vitro. Endocrinology. 2002;143:2899–2904. doi: 10.1210/endo.143.8.8946. [DOI] [PubMed] [Google Scholar]
- 74.Somponpun SJ. Neuroendocrine regulation of fluid and electrolyte balance by ovarian steroids: contributions from central oestrogen receptors. J. Neuroendocrinology. doi: 10.1111/j.1365-2826.2007.01587.x. (in press) [DOI] [PubMed] [Google Scholar]
- 75.Somponpun SJ, Holmes MC, Seckl JR, Russell JA. Modulation of oestrogen receptor-beta mRNA expression in rat paraventricular and supraoptic nucleus neurones following adrenal steroid manipulation and hyperosmotic stimulation. J Neuroendocrinol. 2004;16(5):472–482. doi: 10.1111/j.1365-2826.2004.01190.x. [DOI] [PubMed] [Google Scholar]
- 76.Somponpun SJ, Johnson AK, Beltz T, Sladek CD. Estrogen receptor-alpha expression in osmosensitive elements of the lamina terminalis: regulation by hypertonicity. Am J Physiol Regul Integr Comp Physiol. 2004;287(3):R661–669. doi: 10.1152/ajpregu.00136.2004. [DOI] [PubMed] [Google Scholar]
- 77.Somponpun SJ, Johnson AK, Beltz T, Sladek CD. Osmotic regulation of estrogen receptor-β expression in magnocellular vasopressin neurons requires the lamina terminalis. Am.J.Physiol.Regul.Integr.Comp.Physiol. 2004;286:R465–R473. doi: 10.1152/ajpregu.00478.2003. [DOI] [PubMed] [Google Scholar]
- 78.Somponpun SJ, Sladek CD. Osmotic regulation of estrogen receptor-β in rat vasopressin and oxytocin neurons. J.Neurosci. 2003;23:4261–4269. doi: 10.1523/JNEUROSCI.23-10-04261.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Somponpun SJ, Sladek CD. Depletion of oestrogen receptor (ER)-b expression in magnocellular vasopressin neurones by hypovolaemia and dehydration. J Neuroendocrin. 2004;16(6):544–549. doi: 10.1111/j.1365-2826.2004.01200.x. [DOI] [PubMed] [Google Scholar]
- 80.Spruce BA, Baylis PH, Burd J, Watson MJ. Variation in osmoregulation of arginine vasopressin during the human menstrual cycle. Clin Endocrinology. 1985;11:37–42. doi: 10.1111/j.1365-2265.1985.tb01062.x. [DOI] [PubMed] [Google Scholar]
- 81.Stachenfeld NS, DiPietro L, Kokoszka CA, Silva C, Keefe DL, Nadel ER. Physiological variability of fluid-regulation hormones in young women. J Appl Physiol. 1999;86(3):1092–1096. doi: 10.1152/jappl.1999.86.3.1092. [DOI] [PubMed] [Google Scholar]
- 82.Steckelbroeck S, Jin Y, Gopishetty S, Oyesanmi B, Penning TM. Human cytosolic 3alpha-hydroxysteroid dehydrogenases of the aldo-keto reductase superfamily display significant 3beta-hydroxysteroid dehydrogenase activity: implications for steroid hormone metabolism and action. J Biol Chem. 2004;279(11):10784–10795. doi: 10.1074/jbc.M313308200. [DOI] [PubMed] [Google Scholar]
- 83.Stricker EM, Hoffmann ML. Inhibition of vasopressin secretion when dehydrated rats drink water. Am J Physiol Regul Integr Comp Physiol. 2005;289(5):R1238–1243. doi: 10.1152/ajpregu.00182.2005. [DOI] [PubMed] [Google Scholar]
- 84.Sun J, Meyers M, Fink BE, Rajendran R, Katzenellenbogen JA, Katzenellenbogen BS. Novel ligands that function as selective estrogens or antiestrogens for estrogen receptor-alpha or estrogen receptor-beta. Endocrinology. 1999;140:800–804. doi: 10.1210/endo.140.2.6480. [DOI] [PubMed] [Google Scholar]
- 85.Sunn N, McKinley MJ, Oldfield BJ. Identification of efferent neural pathways from the lamina terminalis activated by blood-borne relaxin. Journal of Neuroendocrinology. 2001;13:432–437. doi: 10.1046/j.1365-2826.2001.00650.x. [DOI] [PubMed] [Google Scholar]
- 86.Suzuki S, Handa RJ. Regulation of estrogen receptor-beta expression in the female rat hypothalamus: differential effects of dexamethasone and estradiol. Endocrinology. 2004;145(8):3658–3670. doi: 10.1210/en.2003-1688. [DOI] [PubMed] [Google Scholar]
- 87.Swenson KL, Badre SE, Morsette DJ, Sladek CD. N-methyl-D-aspartic (NMDA) stimulation of vasopressin release: Role in osmotic regulation and modulation by gonadal steroids. Journal of Neuroendocrinology. 1998;10:679–685. doi: 10.1046/j.1365-2826.1998.00257.x. [DOI] [PubMed] [Google Scholar]
- 88.Swenson KL, Sladek CD. Gonadal steroid modulation of vasopressin secretion in response to osmotic stimulation. Endocrinology. 1997;138:2089–2097. doi: 10.1210/endo.138.5.5142. [DOI] [PubMed] [Google Scholar]
- 89.Thomas P, Pang Y, Filardo EJ, Dong J. Identity of an estrogen membrane receptor coupled to a G protein in human breast cancer cells. Endocrinology. 2005;146(2):624–632. doi: 10.1210/en.2004-1064. [DOI] [PubMed] [Google Scholar]
- 90.Torn S, Nokelainen P, Kurkela R, Pulkka A, Menjivar M, Ghosh S, Coca-Prados M, Peltodeto H, Isomaa V, Vihko P. Production, purification, and functional analysis of recombinant human and mouse 17beta-hydorxysteroid dehydrogenase type 7. Biochem. Biophys. Res. Commun. 2003;305:37–45. doi: 10.1016/s0006-291x(03)00694-6. [DOI] [PubMed] [Google Scholar]
- 91.Trigoso WF, Wesly JM, Meranda DL, Shenker Y. Vasopressin and atrial natriuretic hormone response to hypertonic saline during the follicular and luteal phases of the menstrual cycle. Hum Reprod. 1996;11(11):2392–2395. doi: 10.1093/oxfordjournals.humrep.a019121. [DOI] [PubMed] [Google Scholar]
- 92.Uht RM, McKelvy JF, Harrison RW, Bohn MC. Demonstration of Glucocorticoid Receptor-Like Immunoreactivity in Glucocorticoid-Sensitive Vasopressin and Corticotropin-Releasing Factor Neurons in the Hypothalamic Paraventricular Nucleus. Journal of Neuroscience Research. 1988;19:405–411. doi: 10.1002/jnr.490190404. [DOI] [PubMed] [Google Scholar]
- 93.Voisin DL, Simonian SX, Herbison AE. Identification of estrogen receptor-containing neurons projecting to the rat supraoptic nucleus. Neuroscience. 1997;78:215–228. doi: 10.1016/s0306-4522(96)00551-9. [DOI] [PubMed] [Google Scholar]
- 94.Vokes TJ, Weiss NM, Schreiber J, Gaskill MB, Robertson GL. Osmoregulation of thirst and vasopressin during normal menstrual cycle. American Journal of Physiology. 1988;254:R641–R647. doi: 10.1152/ajpregu.1988.254.4.R641. [DOI] [PubMed] [Google Scholar]
- 95.Wang H, Ward AR, Morris JF. Oestradiol acutely stimulates exocytosis of oxytocin and vasopressin from dendrites and somata of hypothalamic magnocellular neurons. Neuroscience. 1995;68:1179–1188. doi: 10.1016/0306-4522(95)00186-m. [DOI] [PubMed] [Google Scholar]
- 96.Wang Y-X, Edwards RM, Nambi P, Stack EJ, Pullen M, Share L, Crofton JT, Brooks DP. Sex difference in the antidiuretic activity of vasopressin in the rat. Am.J.Physiol.Regul.Integr.Comp.Physiol. 1993;265:R1284–R1290. doi: 10.1152/ajpregu.1993.265.6.R1284. [DOI] [PubMed] [Google Scholar]
- 97.Wang YX, Crofton JT, Liu H, Sato K, Brooks DP, Share L. Estradiol attenuates the antidiuretic action of vasopressin in ovariectomized rats. Am.J.Physiol.Regul.Integr.Comp.Physiol. 1995;268:R951–R957. doi: 10.1152/ajpregu.1995.268.4.R951. [DOI] [PubMed] [Google Scholar]
- 98.Warembourg M, Leroy D. Comparative distribution of estrogen receptor alpha and beta immunoreactivities in the forebrain and the midbrain of the female guinea pig. Brain Res. 2004;1002(1−2):55–66. doi: 10.1016/j.brainres.2003.12.014. [DOI] [PubMed] [Google Scholar]
- 99.Waters WW, Ziegler MG, Meck JV. Postspaceflight orthostatic hypotension occurs mostly in women and is predicted by low vascular resistance. J. Appl. Physiol. 2001;92:582–594. doi: 10.1152/japplphysiol.00544.2001. [DOI] [PubMed] [Google Scholar]
- 100.Wells CC, Riazi S, Mankhey RW, Bhatti F, Ecelbarger C, Maric C. Diabetic nephropathy is associated with decreased circulating estradiol levels and imbalance in the expression of renal estrogen receptors. Gend Med. 2005;2(4):227–237. doi: 10.1016/s1550-8579(05)80052-x. [DOI] [PubMed] [Google Scholar]
- 101.Williams TDM, Edwards A, Fairhall KM, Robinson ICAF, McGarrick GM, Lightman SL. Influence of endogenous and exogenous oestrogens on posterior pituitary secretion in women. Clin.Endocrinol.(Oxf.) 1985;22:589–596. doi: 10.1111/j.1365-2265.1985.tb02994.x. [DOI] [PubMed] [Google Scholar]
- 102.Xue B, Hay M. 17beta-estradiol inhibits excitatory amino acid-induced activity of neurons of the nucleus tractus solitarius. Brain Res. 2003;976(1):41–52. doi: 10.1016/s0006-8993(03)02629-5. [DOI] [PubMed] [Google Scholar]
- 103.Yagil C, Sladek CD. Effect of extended exposure to hypertonicity on vasopressin mRNA content in hypothalamo-neurohypophyseal explants. Endocrinology. 1990;127:1428–1435. doi: 10.1210/endo-127-3-1428. [DOI] [PubMed] [Google Scholar]
- 104.Yue C, Mutsuga N, Scordalakes EM, Gainer H. Studies of oxytocin and vasopressin gene expression in the rat hypothalamus using exon- and intron-specific probes. Am J Physiol Regul Integr Comp Physiol. 2006;290(5):R1233–1241. doi: 10.1152/ajpregu.00709.2005. [DOI] [PubMed] [Google Scholar]
- 105.Yue C, Mutsuga N, Verbalis J, Gainer H. Microarray analysis of gene expression in the supraoptic nucleus of normoosmotic and hypoosmotic rats. Cell Mol Neurobiol. 2006;26(4−6):957–976. doi: 10.1007/s10571-006-9017-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhou L, Blaustein JD, De Vries GJ. Distribution of androgen receptor immunoreactivity in vasopressin- and oxytocin-immunoreactive neurons in the male rat brain. Endocrinology. 1994;134:2622–2627. doi: 10.1210/endo.134.6.8194487. [DOI] [PubMed] [Google Scholar]
- 107.Zingg HH, Lefebvre D, Almazan G. Regulation of vasopressin gene expression in rat hypothalamic neurons. Response to osmotic stimulation. J.Biol.Chem. 1986;261:12956–12959. [PubMed] [Google Scholar]