Abstract
Varicella-Zoster virus (VZV) is a herpesvirus that becomes latent in sensory neurons after primary infection (chickenpox) and subsequently may reactivate to cause zoster. The mechanism by which this virus maintains latency, and the factors involved, are poorly understood. Here we demonstrate, by immunohistochemical analysis of ganglia obtained at autopsy from seropositive patients without clinical symptoms of VZV infection that viral regulatory proteins are present in latently infected neurons. These proteins, which localize to the nucleus of cells during lytic infection, predominantly are detected in the cytoplasm of latently infected neurons. The restriction of regulatory proteins from the nucleus of latently infected neurons might interrupt the cascade of virus gene expression that leads to a productive infection. Our findings raise the possibility that VZV has developed a novel mechanism for maintenance of latency that contrasts with the transcriptional repression that is associated with latency of herpes simplex virus, the prototypic alpha herpesvirus.
Latency has been defined as the reversible nonproductive infection of a cell by a replication-competent virus (1). Several viruses have developed strategies to establish latency in the infected host to prevent their elimination by the host immune response. Varicella-Zoster virus (VZV) is an alpha herpesvirus that becomes latent in dorsal root ganglia (DRG) after primary infection and subsequently may reactivate to cause zoster. It is essential to understand the molecular mechanisms governing VZV latency and reactivation, as approximately 15% of the human population will develop zoster (2, 3) and possibly experience postherpetic neuralgia, a debilitating pain syndrome associated with zoster (4).
A controversy regarding the localization of latent VZV (5–8) was resolved by the demonstration that VZV DNA is present both in neurons and satellite cells (9). The percentage of cells within an affected ganglion that are latently infected with VZV has been reported to range from 0.01% to 30% (5, 7, 9–11).
Despite a wealth of data indicating that the virus immediate early (IE) proteins IE62, IE63, IE4, and the putative IE gene product ORF61p, are involved in the regulation of VZV gene expression during productive infection (12–17), little is known about the behavior of the virus during latency and the conditions that cause its reactivation. Others have shown that transcription of some VZV genes occurs in human ganglia harboring latent virus as evidenced by the presence of virus specific transcripts for ORFs 21, 29, 62, and 63 (7, 8, 18–20). Although there is some uncertainty whether VZV latency-associated transcription takes place in nonneuronal satellite cells or in neurons, it is clear that both IE and putative early (E) VZV genes are transcribed during latency (5–8). One of the IE gene transcripts, that for ORF63, is translated during latency, and the IE63 protein has been detected in the cytoplasm of latently infected human neurons (21) and in the cytoplasm and nucleus of neurons from latently infected rats (22, 23). However, no late gene transcripts have been detected in latently infected DRG. These data raise two important questions. Is it the failure to express some of the virus regulatory genes, i.e., ORF4 and ORF61, or is it the inability of the IE and E latency-associated transcripts to be translated that is responsible for the lack of late VZV gene expression and maintenance of latency. To address the latter possibility we asked whether the products of the latency-associated VZV transcripts could be detected in DRG harboring latent VZV. Our approach involved immunohistochemical detection of VZV-encoded proteins in ganglia obtained at autopsy.
Our results indicate that the IE and E VZV gene transcripts are translated in neurons during latency, but their protein products display aberrant intracellular localization.
MATERIALS AND METHODS
Tissue Specimens.
DRG from three seropositive patients without clinical evidence of zoster and from one fetus without maternal history of varicella were obtained at autopsy. One ganglion from an 85-year-old man who had a zosteriform rash in the distribution of the right T11 sensory nerve at the time of death also was obtained. At autopsy he had a vesicular eruption in the right T11 distribution. All ganglia were removed and fixed in less than 24 hr after death.
Antibody Production and Purification.
Portions of the DNA regions from eight VZV (strain Ellen) ORFs (ORF4, 10, 14, 21, 29, 62, 63, and 67) were amplified by PCR using Vent (New England Biolabs). These regions encode amino acids 1–190 of the ORF4 product (IE4), 1–142 of the ORF10 product (ORF10p), 179–282 of the ORF14 product (gC), 878-1038 of the ORF21 product (ORF21p), 1086–1201 of the ORF29 product (ORF29p), 765–868 of the ORF62 product (IE62), 1–265 of the ORF63 product (IE63), and 592–666 of the ORF67 product (gI). The amplimers were cloned in the bacterial expression vector pALEX (24), which places glutathione S-transferase (GST) at the amino terminus and a six-histidine (6HIS) moiety at the carboxy terminus of the VZV peptide. The fusion proteins were expressed in Escherichia coli, strain BL21(DE3), and purified to apparent homogeneity by affinity chromatography on glutathione-Sepharose or nickel-agarose columns (24). The purity of the proteins was determined by SDS/PAGE (25), and these proteins were used to immunize rabbits.
Antibodies that crossreacted with E. coli proteins, GST and mammalian cell (Vero, HeLa, and 293) proteins, were removed by adsorption on columns containing these proteins. These columns were prepared by crosslinking extracts from E. coli BL21(DE3)/pALEX (induced for 4 hr with 0.5 mM isopropyl β-d-thiogalactoside after reaching A600 = 0.8) or from a mixture of Vero, HeLa, and 293 cells (approximately 2 × 1010 cells for each cell line) with cyanogen bromide-activated Sepharose 4B (Pharmacia Biotech), following the manufacturer’s instructions. Six milliliters from each antiserum were diluted 5-fold with PBS and applied sequentially to each column. The column flow-throughs were applied onto fresh columns, and the absence of Ig binding to the second set of columns served as an indication of the efficiency of the negative purification. Igs subsequently were partially purified by ammonium sulfate precipitation (50% saturation).
Western blot analyses were performed as described (26, 27), and protein concentrations were determined by using the Bradford method (28).
Immunohistochemistry.
Tissue sections were deparaffinized with xylenes, rinsed twice with ethyl alcohol, and treated with Serotec target unmasking fluid (Harlem Bioproducts for Science, Indianapolis, IN) according to the manufacturer’s recommendations. Cells and tissue sections then were blocked with 1% goat serum in PBS for 20 min and incubated with a 1:100 dilution of purified polyclonal rabbit anti-VZV and anti-GST antibodies or with ascites fluid from mouse containing an anti-VZV ORF62 mAb. After washing, the specimens analyzed with the rabbit polyclonal antibodies were incubated for 30 min with an alkaline phosphatase (AP)-labeled goat anti-rabbit antibody (Kirkegaard & Perry Laboratories) diluted 1:150 in PBS containing 1% goat serum. The specimens analyzed with the mouse mAb first were incubated for 30 min with a fluorescein-labeled anti-mouse secondary antibody (Boehringer Mannheim), and then incubated for another 30 min with an AP-conjugated goat anti-fluorescein antibody (Boehringer Mannheim). The slides then were washed in AP buffer (100 mM Tris, pH 9.5/100 mM NaCl/50 mM MgCl2), and the signal was visualized by light microscopy after developing for 20 min in development buffer [9 μl of nitroblue tetrazolium chloride (Boehringer Mannheim) and 3.5 μl of 5-bromo-4-chloro-3-indolyl phosphate (Boehringer Mannheim) in 2 ml of AP buffer].
DNA detection was performed as previously described (9).
RESULTS
The aim of this study was to ask whether the IE and E VZV gene transcripts, which have been detected previously in latently infected neuronal cells, are translated. In situ immunohistochemistry was performed to determine whether the protein products from these transcripts are present in latently infected ganglionic cells. At first a battery of polyclonal antibodies that recognize the products of ORFs 62, 63, 21, and 29 was generated. The genes encoding these proteins are known to be transcribed during latency (7, 8, 18–20). Antibodies to the products of ORFs 4, 10, 14, and 67 also were raised. IEs 62 and 63, the products of ORFs 62 and 63, are tegument proteins with transregulatory activities (29, 30), whereas the products of ORFs 21 and 29 are putative early proteins based on their homology to known herpesvirus DNA replication proteins (31, 32). The product of the ORF4 gene is an IE protein with transregulatory activity (16, 17, 33), whereas ORFs 10, 14 and 67 encode a virion-packaged transactivator (29, 34, 35) and two glycoproteins, which are all late proteins.
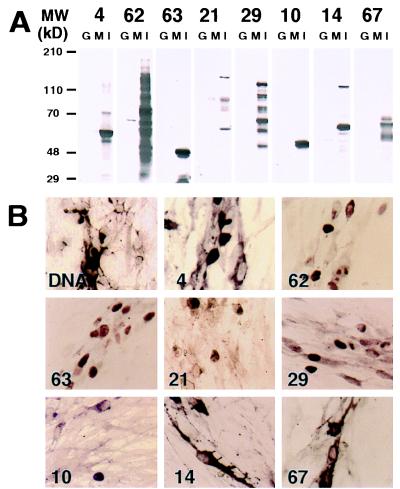
To ensure both the specificity of the antibodies and the lack of crossreactivity with host-cell proteins, the antibodies were purified as described in Materials and Methods. Unlike single positive affinity purification of antibodies with immobilized protein antigens this approach was expected to remove any antibodies recognizing epitopes common to virus and host cell proteins. The purified antibodies were tested for specificity and lack of crossreactivity with host cell proteins by using Western blot analysis and in situ immunohistochemistry. Western blot analysis demonstrated that the purified antibodies recognized proteins with the predicted molecular weights in extracts from human embryonic lung fibroblast cells infected with VZV. They also recognized some smaller polypeptides resulting from degradation of ORFs 62, 29, and 21 protein products. However, the antibodies showed minimal crossreactivity with proteins from uninfected cells and no crossreactivity with purified GST-6His (Fig. 1A), indicating that the purification was successful. In situ immunohistochemistry provided additional evidence for the lack of crossreactivity between the purified antibodies and host cell proteins. None of the antibodies reacted with uninfected cells (data not shown), whereas in infected cells, the antibodies reacted predominantly with cells displaying cytopathic effects of VZV infection (Fig. 1B). Each antibody recognized a VZV-encoded protein that localized to the expected cellular compartment. The protein products of the IE and E genes, ORFs 62, 63, 21, and 29 were found mainly in the nucleus (Fig. 1B, panels 62, 63, 21, and 29). These results are consistent with the previously identified functions of the ORF62 and ORF63 gene products as transcriptional regulators (12, 13) and those of the ORF21 and ORF29 gene products as putative components of the VZV DNA replication machinery (31, 32). In agreement with others (17, 36), IE4 was shown to localize in the cytoplasm and/or the nucleus of infected cells (Fig. 1B, panel 4). Similarly, the ORF10 protein, a transactivator (35), was detected both in the cytoplasm and/or the nucleus of infected cells (Fig. 1B, panel 10). In contrast, the glycoproteins encoded by the late genes, ORFs 14 and 67, were found predominantly in the cytoplasm (Fig. 1B, panels 14 and 67).
Figure 1.
Specificity of the anti-VZV protein antibodies by Western blot analysis and in situ immunohistochemistry. (A) Purified bacterially expressed GST protein (0.5 μg, G), and VZV-infected (I) or uninfected (M) human embryonic lung fibroblast cell lysates were analyzed by SDS/PAGE on 7–12% gradient gels, and the proteins subsequently were transferred electrophoretically onto nitrocellulose membranes. The membranes were probed with the purified antibodies at a 1/1,000 dilution. The VZV ORFs whose products were analyzed are identified at the top. The molecular weights of prestained size markers (GIBCO/BRL, high molecular weight) are indicated on the left. (B) In situ hybridization and immunohistochemical detection of VZV DNA and proteins was performed in human embryonic lung fibroblasts infected with VZV strain Ellen. VZV DNA was detected by using a fluoresceine-labeled oligonucleotide probe and an AP-conjugated antifluorescein antibody. The products of ORFs 4, 62, 63, 21, 29, 10, 14, and 67 were detected by using purified anti-VZV proteins rabbit antibodies and AP-conjugated goat anti-rabbit Ig secondary antibody. The signal was visualized by developing with AP substrate. In situ DNA hybridization analysis is shown in the top left (DNA), and the ORFs whose products were analyzed are identified in the lower left corner of each panel.
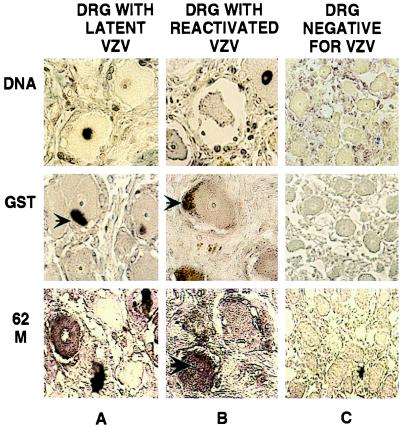
These purified antibodies were used to examine human ganglia obtained at autopsy from three VZV seropositive patients that were shown to harbor latent VZV DNA by DNA in situ hybridization (9) (Fig. 2, DNA row, column A). Sections from these ganglia were examined by in situ immunohistochemistry for the presence of VZV-encoded proteins. This analysis demonstrated the presence of all of the IE and E gene products tested, i.e., IE4, IE62, IE63, ORF21p, and ORF29p, in neuronal cells from latently infected ganglia (Figs. 3 and 4, columns A). To ascertain the specificity of these findings a number of control experiments were performed. Although the purified antisera failed to react with the GST-6His protein (Fig. 1A), it was important to prove that the signal did not represent staining of the neuronal cell GSTs. Thus, immunohistochemical analyses of the same sections were performed by using an affinity-purified anti-GST rabbit polyclonal antibody. This antibody did not react with neuronal proteins (Fig. 2, GST row).
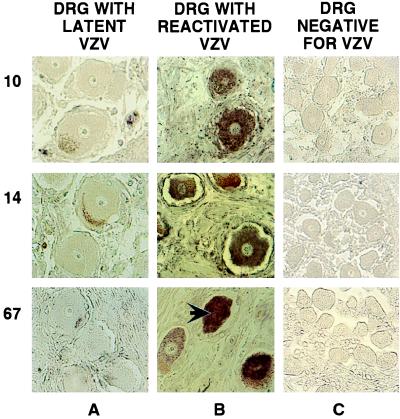
Figure 2.
In situ detection of VZV DNA and immunohistochemical analyses of VZV and GST proteins in human DRG. DRG harboring latent (A) or reactivated (B) virus and a control fetal DRG (C) were analyzed. VZV DNA and GST proteins were detected as described in the legend to Fig. 1. The product of VZV ORF62 was detected by using primary mouse mAbs, fluoresceine-labeled goat-anti-mouse secondary antibodies, and AP-conjugated goat-antifluoresceine tertiary antibody. The signal was visualized by developing with AP substrate. The specimens used are shown at the top, and the individual products analyzed are shown on the left. The large arrow indicates a neuron with a positive nucleus, and the small arrows point to coloration of lipofuschin.
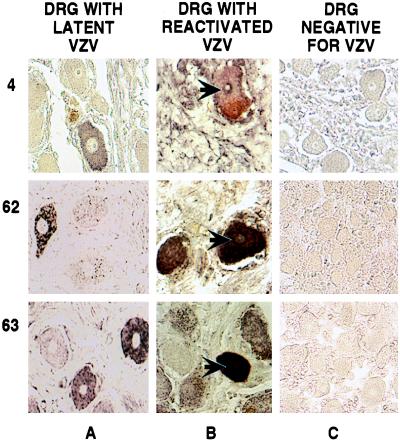
Figure 3.
Immunohistochemical detection of VZV IE proteins in human DRG. DRG harboring latent (A) or reactivated (B) virus and a control fetal DRG (C) were analyzed. The products of ORFs 4, 62, and 63 were detected as described in the legend to Fig. 1. The specimens used are shown at the top, and the individual genes whose products were analyzed are shown on the left. The arrows indicate neurons with positive nuclei. The staining seen near the plasma membrane of some neurons is from coloration of lipofuschin.
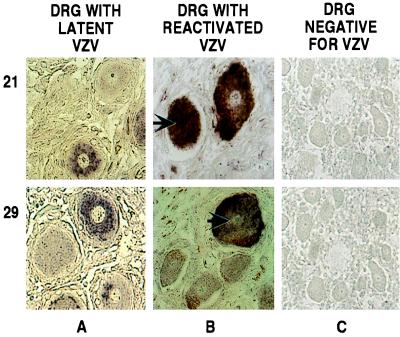
Figure 4.
Immunohistochemical detection of VZV E proteins in human DRG. DRG harboring latent (A) or reactivated (B) virus and a control fetal DRG (C) were analyzed. The products of ORFs 21 and 29 were detected as described in the legend to Fig. 1. The specimens used are shown at the top, and the individual genes whose products were analyzed are shown on the left. The arrows indicate neurons with positive nuclei. The staining seen near the plasma membrane of some neurons is from coloration of lipofuschin.
To further eliminate the possibility that the results represent nonspecific interactions between these antibodies and neuronal cell proteins, VZV DNA negative fetal ganglionic cells were examined for crossreactivity by in situ immunohistochemical analyses (Fig. 2, DNA row, column C). None of the antibodies react with proteins from these VZV-free fetal ganglionic cells (Figs. 3–5, columns C). Thus, the staining of VZV proteins in neuronal cells is specific.
Figure 5.
Immunohistochemical detection of VZV late proteins in human DRG. DRG harboring latent (A) or reactivated (B) virus and a control fetal DRG (C) were analyzed. The products of ORFs 10, 14, and 67 were detected as described in the legend to Fig. 1. The specimens used are shown at the top, and the individual genes whose products were analyzed are shown on the left. The arrow indicates a neuron with a positive nucleus. The staining seen near the plasma membrane of some neurons is from coloration of lipofuschin.
One obvious question was whether every cell harboring latent VZV contains IE and E viral proteins or whether these proteins are present only in a small fraction of the latently infected neurons. To answer this question, the number of neurons, in each ganglion, scoring positive by DNA hybridization was compared with the number of neurons positive for each of the IE and E VZV proteins. The number of neurons containing VZV DNA were comparable to the number of neurons containing each of the viral proteins tested (Table 1); however, the numbers of neurons scoring positive for the IE62 and IE63 proteins were lower than those scoring positive for VZV DNA, or for the IE4, ORF21p, and ORF29p proteins (Table 1). This variation may just represent differences in avidity among the antibodies, or it may have real biological significance. Collectively, these findings suggest that the presence of viral IE and E proteins in the latently infected neurons does not reflect spurious expression of these proteins in some neurons, nor virus in the process of reactivation. The fact that no late VZV proteins were detected, i.e., ORF10p, gC, and gI, in the same ganglia (Fig. 5, column A and Table 1) provides further evidence that the virus is not reactivating.
Table 1.
VZV DNA and proteins in ganglia during latency
| Percent neurons positive for VZV DNA or protein
|
||||
|---|---|---|---|---|
| DNA/ORF | Patient 1 | Patient 2 | Patient 3 | Mean ± SD |
| DNA | 11 | 18 | 10 | 13 ± 4.4 |
| 4 | 10 | 21 | 20 | 17 ± 6.1 |
| 62 | 6 | 10 | 9 | 8 ± 2.1 |
| 63 | 3 | 9 | 6 | 6 ± 3 |
| 21 | 9 | 23 | 17 | 16 ± 7 |
| 29 | 18 | 22 | 24 | 21 ± 3.1 |
| 10 | 0 | 0 | 0 | 0 |
| 14 | 0 | 0 | 0 | 0 |
| 67 | 0 | 0 | 0 | 0 |
VZV DNA was detected as previously described (9), and the proteins were detected as described in Materials and Methods. The table shows results obtained with ganglia from three seropositive patients with no clinical signs of VZV infection (latency). The results are expressed as percentage of neurons with detectable nuclear VZV DNA or cytoplasmic VZV protein. Zero indicates the absence of detectable protein. None of our antibodies reacted with VZV proteins located in the nuclei of these neurons. A total of 200 neurons were scored for each ganglion examined. The SD is provided for the mean values.
A surprising finding is that the localization of the IE and E proteins found in the latently infected neurons is different than that observed in productively infected cells. All of these proteins, with the exception of IE4, which is found in the cytoplasm and the nucleus (17, 36), localize mainly in the nucleus of productively infected cells (Fig. 1B and refs. 23, 29, 31, and 37). In contrast, these proteins were found to localize in the cytoplasm rather than in the nucleus of latently infected neurons (Figs. 3 and 4). Although low levels of these proteins could be present in the nuclei, below the threshold of detection of this assay, these data demonstrate a clear difference in the localization of these proteins in latent versus productive infection.
To define whether the intracellular localization of these proteins in neurons is altered during the course of VZV reactivation, a similar analysis on a DRG obtained at autopsy from a patient with zoster was performed. This ganglion innervated the site of reactivation and contained VZV DNA in the majority of its neurons, as demonstrated by in situ hybridization (Fig. 2, DNA row, column B and Table 2). Immunohistochemical analysis of this ganglion demonstrated that the IE and E VZV proteins tested were not restricted to the cytoplasm, but were also present in the nuclei of neurons (Figs. 3, and 4, columns B). The majority of VZV DNA-positive neurons (more than 60%) also scored positive for nuclear IE and E protein localization (Table 2). As with the latently infected ganglia, the numbers of neurons containing IE and E proteins were found to be comparable to those positive for VZV DNA (Table 2). The presence of late VZV proteins (Fig. 5, column B) provides evidence that the virus has reactivated in these neurons. These data indicate that the presence of IE and E VZV proteins in the nuclei of neurons, containing reactivated virus, does not represent an aberration taking place in a limited number of cells. Rather, they suggest that it is a discrete relocalization associated with reactivation.
Table 2.
VZV proteins detected in ganglia during latency or reactivation
| ORF | Product name | Function | Gene class | Latency
|
Reactivation
|
||
|---|---|---|---|---|---|---|---|
| N | C | N | C | ||||
| 4 | IE4 | Transactivator | IE | 0 | 17 | 74 | 88 |
| 62 | IE62 | Transregulator | IE | 0 | 8 | 66 | 74 |
| 63 | IE63 | Transregulator | IE | 0 | 6 | 84 | 93 |
| 21 | ORF21p | DNA replication | E | 0 | 16 | 48 | 62 |
| 29 | ORF29p | DNA replication | E | 0 | 21 | 42 | 62 |
| 10 | ORF10p | Transactivator | L | 0 | 0 | 47 | 61 |
| 14 | gC | Glycoprotein | L | 0 | 0 | 55 | 83 |
| 67 | gI | Glycoprotein | L | 0 | 0 | 59 | 81 |
The immunocytochemical detection of IE, E and late (L) virus proteins was performed as described in Materials and Methods. The table compares results obtained with ganglia harboring latent VZV (Table 1, mean) with those obtained with a ganglion, from a patient with zoster, innervating the site of the VZV infection (reactivation). The results are expressed as percentages of neurons with detectable VZV protein within each ganglion. Zero indicates absence of detectable protein from the nucleus (N) or the cytoplasm (C). All identified neurons (80 to 120) within the ganglion harboring reactivated virus were scored for the presence of VZV DNA and proteins. Seventy eight percent of neurons scored positive for VZV DNA by in situ hybridization in this ganglion.
To corroborate these findings a mouse mAb against the IE62 protein was used. In situ immunohistochemistry on ganglia containing either latent or reactivated VZV yielded results that were similar to those obtained with the polyclonal antibodies, i.e., cytoplasmic localization in latency and nuclear and cytoplasmic in reactivation (Fig. 2, row 62M).
As previously shown (9), VZV DNA also was detected in the satellite cells of ganglia harboring both latent and reactivated virus (Fig. 2, DNA row, columns A and B). Although it is possible that VZV proteins are expressed in these satellite cells, limitations in the sensitivity of the assay preclude detection, much less localization of VZV proteins in these cells.
DISCUSSION
In the present study we demonstrate several findings that contribute to understanding the maintenance of VZV latency. First, we show that the virus is not entirely dormant during latency; all of the IE proteins tested and two E proteins were detected in latently infected neurons. Second, we find that the intracellular localization of these VZV proteins in neurons is different from that in productively infected cells. Specifically, these proteins, which are normally intranuclear, accumulate predominantly in the cytoplasm of latently infected neurons. Third, we show that the localization of these IE and E proteins changes during reactivation; they become detectable in the nuclei as well as the cytoplasm of neurons harboring reactivated virus.
A common property of herpesviruses is that, after primary infection, they establish latent or asymptomatic infections that persist for the lifetime of the host. Knowledge of the exact molecular mechanisms involved in establishment, maintenance, and exit from latency is sparse. In the case of herpes simplex virus (HSV), the prototypic alpha herpesvirus, latency is associated with almost complete transcriptional repression of the virus genome (38). The only HSV-encoded transcripts that accumulate during latency are the latency-associated transcripts, and they are restricted to the nucleus (38). The biological role of these transcripts in latency is unknown, and no protein products encoded by them have been identified in neurons latently infected with HSV. Reactivation is associated with activation of transcription of HSV genes, and the viral regulatory protein ICP0 can initiate this process (39, 40).
In contrast to HSV, VZV does not appear to be transcriptionally inactive in ganglionic cells during latency. Several VZV-specific transcripts, for genes belonging to the IE and E kinetic classes, have been detected in ganglia harboring latent virus (7, 8, 18–20). Although VZV has been found in both neurons and satellite cells during latency, the cellular origin of these RNAs is not clear because pooled ganglia were used in the experiments where these RNAs were detected. Nevertheless the presence of IE63 protein in the cytoplasm of neurons harboring latent VZV offers evidence that VZV transcription does take place in neurons (21). We extended this finding by using an in situ immunohistochemical method to show that not only IE63, but also IEs 62 and 4, as well as ORFs 21p and 29p, are present in latently infected neurons. Transcripts encoding all of these proteins, except IE4, have been detected in ganglia (7, 8, 18–20).
The possibility that VZV transcription and protein accumulation are postmortem events can not be excluded. The only certain way to rule out this possibility would be to examine surgically extirpated ganglia or to develop an animal model of VZV latency. VZV latency thus does not appear to be the result of a translational block in neuronal cells. These proteins appear to accumulate in, and to be restricted to, the cytoplasm of latently infected neurons. This distribution contrasts with their nuclear localization in productively infected cells. It is not clear why these proteins fail to be transported into the nucleus during latency. A lack of factors essential for the transport of viral proteins to the nucleus or the presence of neuronal cell-specific inhibitory factors could be invoked as possible mechanisms. We cannot exclude the possibility that VZV proteins are in the nuclei of the latently infected neurons at levels below the detection threshold of our method.
It is tempting to speculate that control of the nuclear import of VZV regulatory and other proteins is involved in maintenance and exit from latency. If this hypothesis is true, then one would expect these proteins to relocalize to the nuclei of neurons during reactivation. Indeed, examination of a ganglion that innervated an area of VZV reactivation revealed that the IE and E proteins tested were present in the nucleus and the cytoplasm of the infected neurons. As expected, late VZV proteins were present in neurons from this ganglion with reactivated virus. It would be desirable to repeat this experiment with several ganglia containing reactivated virus. Practical limitations, however, attributed to difficulty in obtaining these specimens from patients who die with active zoster, prevent us from including more samples in the present study. Nevertheless, our results indicate that the restriction imposed on the nuclear import of IE and E VZV proteins during latency is, at least partially, removed during reactivation.
The validity of these observations depends on the quality of the reagents. Precautions were taken to ensure that there were no artifacts caused by crossreactivity of the antibodies with host cell proteins. The specificity and lack of crossreactivity of these antibodies was verified by Western blot analyses and in situ immunohistochemistry. None of the antibodies reacted with neuronal proteins from fetal ganglia or uninfected human embryonic lung fibroblast cells. To further reduce the possibility of antibody-specific artifacts, the in situ immunohistochemistry was repeated with a mAb to IE62 with similar results. Furthermore, the correlation between the numbers of neurons containing VZV DNA and those found to contain IE and E proteins further argues for the validity of these findings. The correlation between these two numbers indicates that almost every neuron containing latent VZV also expresses IE and E proteins. This result also rules out the possibility that the presence of these VZV proteins in neurons results from spurious reactivation of the virus in a small percentage of latently infected neurons. Further evidence that the virus is not reactivating comes from our finding that no late VZV proteins are detected in these neurons. The only consistent source of background observed in our experiments was from nonspecific coloration of lipofuschin. This staining could be differentiated from specific staining because of its different color and the fact that it occurs only within a limited area of each affected cell. Our estimate of the percentage of VZV-infected neurons (6–21%) is significantly higher than those reported by others (5, 7, 10, 11). This difference might reflect the enhanced sensitivity of our method. Notably, the percentage of VZV positive neurons in this study is remarkably close to that reported in our previous study (9) where a different set of latently infected ganglia was used.
Our findings suggest that VZV latency results from the absence of nuclear import of virus proteins responsible for regulation of gene expression and/or DNA replication. The putative failure of import would prevent these proteins from encountering the virus genome, which is located in the nuclei of latently infected neurons, and thus performing their functions. It will be important to understand the molecular basis for exclusion of VZV IE and E proteins from the nucleus of latently infected neurons. Identification of the mechanism involved in this process will provide insight into the maintenance of VZV latency and provide a possible target for therapeutic intervention to prevent reactivation and the debilitating disease that is associated with recrudescence.
Acknowledgments
These studies were supported by a grant to A.A.G. and S.J.S. from the National Institutes of Health (AI 124021). O.L. was supported by a fellowship from the Varicella-Zoster Virus Research Foundation. P.W.A. is supported by Grant AI 01409 from the National Institutes of Health.
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: VZV, Varicella-Zoster virus; DRG, dorsal root ganglia; IE, immediate early; E, early; GST, glutathione S-transferase; AP, alkaline phosphatase; HSV, herpes simplex virus.
References
- 1.Garcia-Blanco M A, Cullen B R. Science. 1991;254:815–820. doi: 10.1126/science.1658933. [DOI] [PubMed] [Google Scholar]
- 2.Hope-Simpson R E. Proc R Soc Med. 1965;58:9–20. doi: 10.1177/003591576505800106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ragozzino M W, Melton L J, 3rd, Kurland L T, Chu C P, Perry H O. Medicine (Baltimore) 1982;51:310–316. doi: 10.1097/00005792-198209000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Straus S E. J Am Med Assoc. 1993;269:1836–1839. doi: 10.1001/jama.269.14.1836. [DOI] [PubMed] [Google Scholar]
- 5.Hyman R W, Ecker J R, Tenser R B. Lancet. 1983;2:814–816. doi: 10.1016/s0140-6736(83)90736-5. [DOI] [PubMed] [Google Scholar]
- 6.Gilden D H, Rozenman Y, Murray R, Devlin M, Vafai A. Ann Neurol. 1987;22:377–380. doi: 10.1002/ana.410220315. [DOI] [PubMed] [Google Scholar]
- 7.Croen K D, Ostrove J M, Dragovic L J, Straus S E. Proc Natl Acad Sci USA. 1988;85:9773–9777. doi: 10.1073/pnas.85.24.9773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meier J L, Holman R P, Croen K D, Smialek J E, Straus S E. Virology. 1993;193:193–200. doi: 10.1006/viro.1993.1115. [DOI] [PubMed] [Google Scholar]
- 9.Lungu O, Annunziato P W, Gershon A, Staugaitis S M, Josefson D, LaRussa P, Silverstein S J. Proc Natl Acad Sci USA. 1995;92:10980–10984. doi: 10.1073/pnas.92.24.10980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vafai A, Murray R S, Wellish M, Devlin M, Gilden D H. Proc Natl Acad Sci USA. 1988;85:2362–2366. doi: 10.1073/pnas.85.7.2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mahalingam R, Wellish M, Lederer D, Forghani B, Cohrs R, Gilden D H. J Virol. 1993;67:2381–2384. doi: 10.1128/jvi.67.4.2381-2384.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jackers P, Defechereux P, Baudoux L, Lambert C, Massaer M, Merville-Louis M P, Rentier B, Piette J. J Virol. 1992;66:3899–3903. doi: 10.1128/jvi.66.6.3899-3903.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perera L P, Mosca J D, Sadeghi-Zadeh M, Ruyechan W T, Hay J. Virology. 1992;191:346–354. doi: 10.1016/0042-6822(92)90197-w. [DOI] [PubMed] [Google Scholar]
- 14.Moriuchi H, Moriuchi M, Smith H A, Straus S E, Cohen J I. J Virol. 1992;66:7303–7308. doi: 10.1128/jvi.66.12.7303-7308.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moriuchi M, Moriuchi H, Straus S E, Cohen J I. Virology. 1994;200:297–300. doi: 10.1006/viro.1994.1190. [DOI] [PubMed] [Google Scholar]
- 16.Perera L P, Kaushal S, Kinchington P R, Mosca J D, Hayward G S, Straus S E. J Virol. 1994;68:2468–2477. doi: 10.1128/jvi.68.4.2468-2477.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Defechereux P, Debrus S, Baudoux L, Rentier B, Piette J. J Virol. 1997;71:7073–7079. doi: 10.1128/jvi.71.9.7073-7079.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cohrs R J, Srock K, Barbour M B, Owens G, Mahalingam R, Devlin M E, Wellish M, Gilden D H. J Virol. 1994;68:7900–7908. doi: 10.1128/jvi.68.12.7900-7908.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cohrs R J, Barbour M B, Mahalingam R, Wellish M, Gilden D H. J Virol. 1995;69:2674–2678. doi: 10.1128/jvi.69.4.2674-2678.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cohrs R J, Barbour M, Gilden D H. J Virol. 1996;70:2789–2796. doi: 10.1128/jvi.70.5.2789-2796.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mahalingam R, Wellish M, Cohrs R, Debrus S, Piette J, Rentier B, Gilden D H. Proc Natl Acad Sci USA. 1996;93:2122–2124. doi: 10.1073/pnas.93.5.2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sadzot-Delvaux C, Debrus S, Nikkels A, Piette J, Rentier B. Neurology. 1995;45:S18–S20. doi: 10.1212/wnl.45.12_suppl_8.s18. [DOI] [PubMed] [Google Scholar]
- 23.Debrus S, Sadzot-Delvaux C, Nikkels A, Piette J, Rentier B. J Virol. 1995;69:3240–3245. doi: 10.1128/jvi.69.5.3240-3245.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Panagiotidis C A, Silverstein S J. Gene. 1995;164:45–47. doi: 10.1016/0378-1119(95)00417-5. [DOI] [PubMed] [Google Scholar]
- 25.Laemmli U K. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 26.Panagiotidis C A, Huang S C, Canellakis E S. Int J Biochem Cell Biol. 1995;27:157–168. doi: 10.1016/1357-2725(94)00068-m. [DOI] [PubMed] [Google Scholar]
- 27.Towbin H, Staehelin T, Gordon J. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bradford M. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 29.Kinchington P R, Hougland J K, Arvin A M, Ruyechan W T, Hay J. J Virol. 1992;66:359–366. doi: 10.1128/jvi.66.1.359-366.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kinchington P R, Bookey D, Turse S E. J Virol. 1995;69:4274–4282. doi: 10.1128/jvi.69.7.4274-4282.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kinchington P R, Inchauspe G, Subak-Sharpe J H, Robey F, Hay J, Ruyechan W T. J Virol. 1988;62:802–809. doi: 10.1128/jvi.62.3.802-809.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meier J L, Straus S E. Neurology. 1995;45:S30–S32. doi: 10.1212/wnl.45.12_suppl_8.s30. [DOI] [PubMed] [Google Scholar]
- 33.Defechereux P, Melen L, Baudoux L, Merville-Louis M P, Rentier B, Piette J. J Virol. 1993;67:4379–4385. doi: 10.1128/jvi.67.7.4379-4385.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McKee T A, Disney G H, Everett R D, Preston C M. J Gen Virol. 1990;71:897–906. doi: 10.1099/0022-1317-71-4-897. [DOI] [PubMed] [Google Scholar]
- 35.Moriuchi H, Moriuchi M, Straus S E, Cohen J L. J Virol. 1993;67:2739–2746. doi: 10.1128/jvi.67.5.2739-2746.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Defechereux P, Debrus S, Baudoux L, Schoonbroodt S, Merville M P, Rentier B, Piette J. J Gen Virol. 1996;77:1505–1513. doi: 10.1099/0022-1317-77-7-1505. [DOI] [PubMed] [Google Scholar]
- 37.Stevenson D, Xue M, Hay J, Ruyechan W T. J Virol. 1996;70:658–662. doi: 10.1128/jvi.70.1.658-662.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roizman B, Sears A. In: Herpes Simplex Viruses and Their Replication. Fields B N, Knipe D M, Howley P M, editors. Vol. 2. Philadelphia: Lippincott; 1996. pp. 2231–2295. [Google Scholar]
- 39.Harris R A, Everett R D, Zhu X, Silverstein S, Preston C M. J Virol. 1989;63:3513–3515. doi: 10.1128/jvi.63.8.3513-3515.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhu X, Chen J, Young C S H, Silverstein S. J Virol. 1990;64:4489–4498. doi: 10.1128/jvi.64.9.4489-4498.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]