Abstract
Phosphatidylcholine-specific phospholipase C (PC-PLC) is a necessary intermediate in transducing apoptotic signals for tumor necrosis factor and Fas/Apo-1 ligands in nonneuronal cells. The data presented here show that PC-PLC also is required in oxidative glutamate-induced programmed cell death of both immature cortical neurons and a hippocampal nerve cell line, HT22. In oxidative glutamate toxicity, which is distinct from excitotoxicity, glutamate interferes with cystine uptake by blocking the cystine/glutamate antiporter, indirectly causing a depletion of intracellular glutathione. A PC-PLC inhibitor blocks oxidative glutamate toxicity, and exogenous PC-PLC potentiates glutamate toxicity. The inhibition of PC-PLC uncouples the cystine uptake from glutamate inhibition, allowing the maintenance of glutathione synthesis and cell viability. These data suggest that PC-PLC modulates neuronal cell death through a mechanism that is distinct from that involved in nonneuronal apoptosis.
Various neuropathological conditions appear to be associated with the activation of phospholipases, which release lipid metabolites that either are directly toxic to neurons or act as second messengers. The activities of phospholipases A2 (PLA2) and D are elevated in Alzheimer’s disease brains (1, 2), and that of PLA2 is increased during ischemia (3). Although phosphatidylcholine constitutes the majority of phospholipid in brain tissues, the role of phosphatidylcholine-specific phospholipase C (PC-PLC) in modulating neuronal survival or death is not known. In several other cell types, however, PC-PLC is critical in transducing apoptotic signals. For example, the inhibition of PC-PLC prevents tumor necrosis factor (TNF) or Fas/Apo-1-induced cell death in vitro and blocks TNF toxicity in vivo (4, 5).
Many models of neuronal programmed cell death have been established to address the mechanisms underlying neuronal degeneration (see, for example, refs. 6–9), including the glutamate-induced programmed cell death of immature cortical neurons (10) and of a hippocampal nerve cell line (11). In these cells, elevated levels of extracellular glutamate interfere with cystine uptake through the cystine/glutamate antiporter, which normally carries cystine into cells at the expense of the outflow of glutamate (11–13). The decreased cystine uptake leads to the depletion of intracellular glutathione (GSH), a cysteine-containing tripeptide vital for cell survival because of its ability to regulate cellular metabolism and to act as an antioxidant. This form of cell death, called “oxidative glutamate toxicity,” is distinct from excitotoxicity but is important to the understanding of neuronal degeneration in at least two situations. Many neurons contain no glutamate receptors yet are killed by excess glutamate, and neurons depleted of GSH by unknown mechanisms are specifically associated with Parkinson’s disease (14–16).
Several experiments suggest that PC-PLC might be involved in glutamate-induced programmed cell death. (i) 12-lipoxygenase (12-LOX) is required for cell death caused by oxidative glutamate toxicity (17), but the source of the 12-LOX substrate, arachidonic acid (AA), has not been determined. AA can be produced from phospholipids by the actions of PLA2, PLC, or PLD. Cleavage of phosphatidylcholine by PC-PLC yields diacylglycerol, which can give further rise to AA through the action of diacylglycerol lipase. (ii) Diacylglycerol also can act as a signal for the activation of protein kinase C (PKC), and PKC modulates glutamate toxicity (11). (iii) The PC-PLC pathway, which has been described in nonneuronal apoptosis (4, 5), may be involved in neuronal apoptosis. The experiments presented below show that PC-PLC is indeed involved in glutamate-induced programmed cell death and that PC-PLC acts through the direct regulation of the glutamate/cystine antiporter.
MATERIALS AND METHODS
Cells and Reagents.
The mouse hippocampal cell line HT22 (18) was grown and maintained in DMEM containing 10% fetal bovine serum. Primary cortical neurons were prepared from embryonic rats on day 17 of gestation as described (19) and were grown in MEM supplemented with 30 mM glucose, 2 mM glutamine, 1 mM pyruvate, and 10% fetal bovine serum. Experiments were performed 1 day after the initial plating of the neurons. Neither HT22 cells (18) nor the recently dissociated primary cortical neurons (20) contain active ionotropic glutamate receptors. Thus, they are killed by glutamate via the oxidative pathway, not by excitotoxicity.
Cell Culture Reagents.
AA and type IV PC-PLC from B. cereus were from Sigma. Tricyclodecan-9-yl xanthogenate (D609) was from Alexis (San Diego, CA). Baicalein was from Biomol (Plymouth Meeting, PA). 35S-Cystine was from NEN.
Cell Viability Assays.
The viability of cells after various treatments was determined in 96-well plates by using the method of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide reduction and was confirmed by visual inspection. The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide assay in this system is a valid reflection of viable cell numbers as determined by colony forming assays (11). To insure maximum sensitivity to glutamate, HT22 cells were seeded at 2.5 × 103 cells/well, and primary cortical neurons were seeded at 2.5 × 104 cells/well. Cells were treated according to individual experimental designs, and viability was determined, usually 20 h later. For glutamate toxicity studies, cells were grown in 5% dialyzed fetal calf serum. For buthionine sulfoximine toxicity studies, cells were grown in 5% horse serum (17), and buthionine sulfoximine (BSO) was prepared just before use. For cystine-deprivation studies, cells initially were seeded in the normal DMEM medium and replaced with medium containing various amounts of cystine before other treatments.
Glutathione Measurement.
Glutathione content was determined by the enzymatic assay (21) as described (17). In brief, cells were lysed by 3.33% sulfosalicylic acid and centrifuged. The supernatant was neutralized with 50% triethanolamine and assayed for glutathione by using glutathione reductase and NADPH. The reduction of 5,5-dithiobis(2-nitrobenzoic acid) was assayed, and the content of glutathione was determined by comparing the rate of reaction of the samples to that of a standard. The sulfosalicylic acid-insoluble pellet was dissolved in 0.1 N NaOH, and the protein content was determined by using a commercial kit (Pierce, Rockford, IL). GSH content is stated as nanomol of glutathione per milligram of protein and is expressed relative to the controls.
Cystine Uptake.
The uptake of radioactive cystine was determined by the method of Ratan et al. (22) with some modifications. In brief, 5 × 104 HT22 cells were seeded onto 35-mm tissue culture dishes and, 12 h later, were treated with 5 mM glutamate and/or 50 μM D609 for various times. The medium was removed, and cells were washed three times with Hanks’ balanced saline solution. The uptake of radioactive cystine was then initiated by adding 1 ml of labeling mix (5 mM glucose/1 μM nonradioactive cystine/60 μM 35S-cystine per ml with or without 5 mM glutamate/50 μM D609) to each dish followed by incubation for 20 min in a 37°C water bath. Cells then were washed three times with cold Hanks’ balanced saline solution and then lysed. One-half of the cell lysate was used for radioactivity determination, and the other half was used to assay protein with a commercial kit (Pierce). The rate of cystine uptake is defined as picomol of cystine per microgram of protein per 20 min and is presented as a percentage of the control. Cystine uptake was linear for at least 40 min. Nonspecific cystine uptake also was determined as described above except that excess nonradioactive cystine was used (1 mM instead of 1 μM), and this value was subtracted from the above calculation.
RESULTS
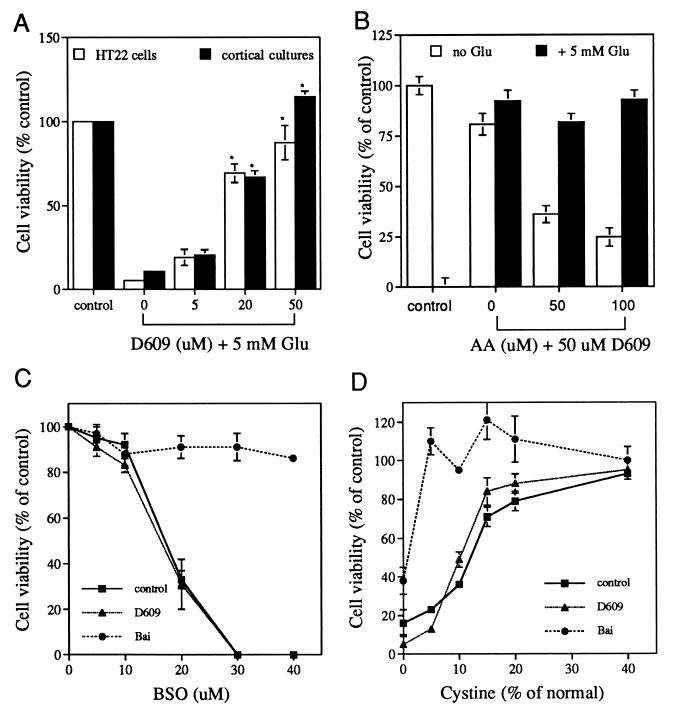
The hippocampal nerve cell line HT22 is sensitive to glutamate but does not possess active ionotropic glutamate receptors (18). These cells have been used extensively to study oxidative glutamate toxicity. Continuous exposure to 5 mM glutamate results in almost complete cell death after 20 h (refs. 11, 17, 18, and 23; Fig. 1A). Cell death requires the activity of 12-LOX (17), which uses AA as a substrate, so we asked which phospholipases are responsible for the generation of AA. A series of specific phospholipase inhibitors was used to define the role of each enzyme in glutamate toxicity. The inhibition of either phosphatidylinositol-specific PLC or PC-PLD with neomycine sulfate (up to 50 μM, the maximal nontoxic dose) did not have a protective effect, but the inhibition of PLA2 by 5 μM quancrine prevents glutamate-induced cell death by ≈35%. This protective effect is abolished when 50 μM exogenous AA is added to the growth medium. These data suggest that PLA2 contributes to at least the partial generation of AA required for downstream events in the cell death pathway. In contrast, the inhibition of PC-PLC completely blocks toxicity.
Figure 1.
PC-PLC inhibitor D609 prevents glutamate but not BSO or cystine deprivation-induced cell death via an AA-independent pathway. (A) Dose effect of D609. HT22 cells or primary cortical cultures were untreated (control) or treated overnight with 5 mM glutamate in the absence or presence of D609 at the indicated concentrations and cell viability determined. Results are presented relative to controls. ∗, P < 0.01, significantly different from glutamate treatment. (B) Effect of exogenous AA on the protective effect of D609. D609 is not toxic at 50 μM, and AA is not toxic at 50 μM but is slightly toxic at 100 μM. The experiment was done with HT22 cells and was repeated three times with similar results. (C) Effect of D609 and baicalein on BSO toxicity in HT22 cells. D609, 50 μM. BSO, 50 μM. Higher or lower doses of D609 were not protective either (data not shown). (D) Effect of D609 and baicalein on cell death induced by cystine deprivation in HT22 cells. D609, 50 μM. BSO, 50 μM. Higher or lower doses of D609 were not protective either (data not shown).
The Inhibition of PC-PLC Blocks Glutamate-Induced Neuronal Cell Death via an AA-Independent Pathway.
The gene encoding eukaryotic PC-PLC has not been cloned, but a highly specific and selective inhibitor, D609, is available and has been tested on a variety of cell types (4, 24–27). It has no effect on PLA2, PLD, or phosphatidylinositol-specific PLC (25). D609 inhibits glutamate-induced cell death in a dose-dependent manner in both HT22 cells and primary cortical neurons (Fig. 1A). Complete protection is achieved with 50 μM D609, a concentration similar to that used to inhibit PC-PLC in other cells (4, 24–27). With the concentrations of D609 that were used, glutamate-treated HT22 cells not only survive but also proliferate, showing that D609 does not have a significant effect on cell growth. This protective effect of D609 could not be overcome by exogenous AA (Fig. 1B), suggesting that the PC-PLC product diacylglycerol is not metabolized further to AA. In addition, RHC-80267, an inhibitor of diacylglycerol lipase that participates in the generation of AA from diacylglycerol (28), does not have a protective effect at any doses tested (data not shown), further supporting the above conclusion.
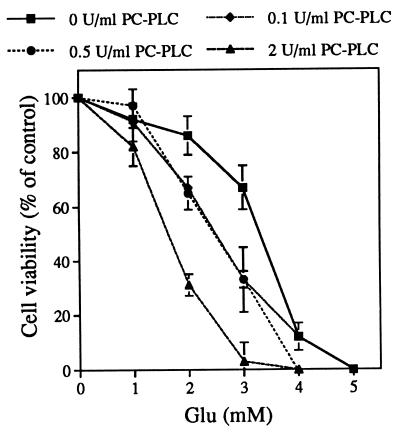
If D609 prevents cell death by inhibiting PC-PLC and, therefore, the generation of the downstream products, then the addition of exogenous PC-PLC should generate more of these products and thereby enhance cell death. Cells were treated with PC-PLC isolated from B. cereus and assayed for their sensitivity to marginally toxic levels of glutamate. Incubation of cells with 0.5–2.0 units/ml PC-PLC alone does not have any effect on cell viability (118 ± 8% and 100 ± 5%, respectively, relative to controls, n = 3) but greatly potentiates cell death induced by glutamate (Fig. 2). For example, compared with the 70% survival rate when cells are treated with 3 mM glutamate alone, the survival rate drops to ≈30% and 2% when cells are treated with 0.5 and 2.0 units/ml PC-PLC, respectively, together with 3 mM glutamate. Diacylglycerol does not potentiate toxicity, nor does the diacylglycerol lipase inhibitor block PC-PLC potentiation, ruling out the possible involvement of diacylglycerol in this experiment. These data suggest that PC-PLC is involved in glutamate-induced cell death via an AA-independent pathway.
Figure 2.
Exogenous PC-PLC potentiates glutamate-induced cell death. HT22 cells were treated with glutamate and/or PC-PLC at various concentrations for 20 h, and cell viability was determined by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide assay. The experiment was repeated three times with similar results.
The Inhibition of PC-PLC Does Not Protect Cells from BSO Toxicity or Cystine Deprivation-Induced Cell Death.
PC-PLC acts independently of AA with respect to cell death, so it was important to determine where the enzyme functions in the cascade of events leading to glutamate-induced nerve cell death. Because glutamate induces cell death through GSH depletion via the blockade of cystine uptake (12), it was asked whether the inhibition of PC-PLC also blocks cell death induced by GSH depletion caused by a mechanism independent of cystine uptake. γ-Glutamylcysteine synthetase is the rate-limiting enzyme in GSH synthesis and is inhibited specifically by BSO, which kills HT22 cells in a dose-dependent manner (ref. 17; Fig. 1C). Fig. 1C shows that D609 is unable to prevent cell death induced by BSO. In contrast, baicalein, an inhibitor of 12-LOX that is activated by GSH depletion and is required for GSH depletion-induced cell death (17), protects cells from BSO toxicity (ref. 17; Fig. 1C).
Another way to reduce intracellular GSH is by eliminating exogenous cystine. Therefore, it was asked whether D609 blocks cystine deprivation-induced cell death. Fig. 1D shows that D609 cannot prevent cell death caused by cystine deprivation, but baicalein provides some protection (Fig. 1D). These results suggest that, unlike 12-LOX, which acts downstream of GSH depletion, PC-PLC is acting upstream of the GSH depletion step in the oxidative glutamate toxicity pathway.
PC-PLC Regulates the GSH Depletion Caused by Glutamate but Not by BSO.
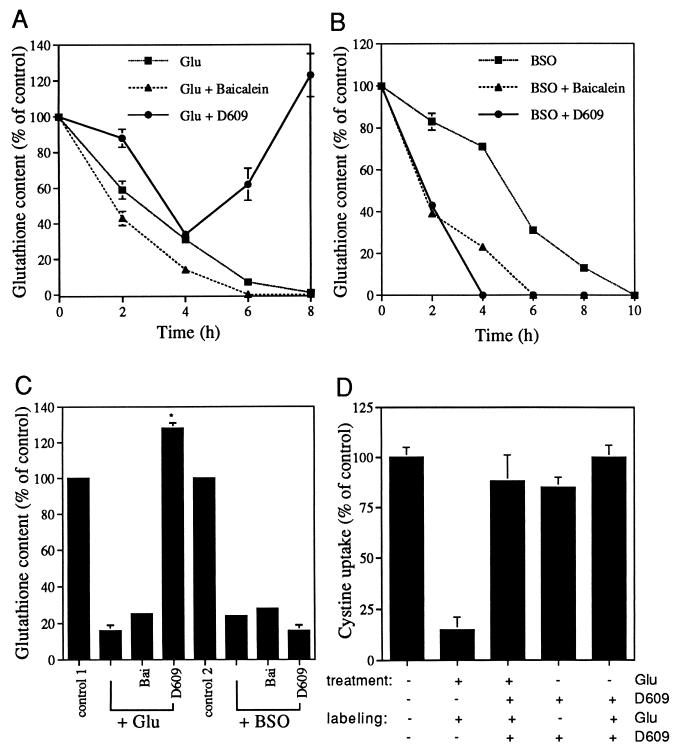
The above data suggest that PC-PLC is acting upstream of GSH depletion, so we asked whether PC-PLC activity directly regulates the intracellular GSH level. A time course study was done to examine intracellular GSH levels after treatment with glutamate or BSO in the presence or absence of D609. Treatment with glutamate or BSO alone results in a decrease in the level of GSH in a time-dependent fashion. By 6–10 h after treatment with glutamate or BSO, intracellular GSH drops to a level undetectable by the standard chemical assay (Fig. 3 A and B). In the presence of 50 μM D609, which is fully protective (Fig. 1A), glutamate initially still induces a decrease in GSH content (Fig. 3A). However, after a drop to ≈40% of the untreated control at 4 h, GSH levels start to increase (Fig. 3A). In contrast, D609 does not prevent BSO-induced GSH depletion; it even accelerates the decline in GSH content (Fig. 3B). As a control, the 12-LOX inhibitor baicalein does not block GSH depletion caused by either glutamate or BSO (Fig. 3 A and B). Similar results also were obtained with primary cortical neurons (Fig. 3C). These data suggest that D609 blocks glutamate-induced cell death by preventing the depletion of intracellular GSH at a step upstream of the enzyme γ-glutamylcysteine synthetase and, therefore, upstream of de novo GSH synthesis.
Figure 3.
D609 prevents glutamate but not BSO-induced GSH depletion and restores cystine uptake. (A and B) A time course analysis of intracellular GSH levels after the treatment of HT22 cells with glutamate (A) or BSO (B) in the presence or absence of the PC-PLC inhibitor, D609, and the 12-LOX inhibitor, baicalein. Results are presented relative to controls and are the mean ± SD of one typical experiment with three determinations. Error bars that are not visible are smaller than the width of the symbol. HT22 cells contain 12.4 ± 0.62 and 8.08 ± 0.08 nmol GSH/mg protein when grown in fetal calf serum (to assay for glutamate toxicity) and horse serum (to assay for BSO toxicity), respectively. Glu, 5 mM; BSO, 50 μM; D609, 50 μM; baicalein, 10 μM. (C) Effect of D609 on GSH levels in primary cortical neurons. Cells (5 × 106) were seeded in 100-mm dishes and were treated the next day with the indicated reagents for 12 h before the assay for GSH content. Results are presented relative to controls. Control 1 for glutamate treatment is 11.9 nmol GSH/mg protein. Control 2 for BSO treatment is 7.4 nmol GSH/mg protein. Glu, 5 mM; BSO, 50 μM; D609, 50 μM; baicalein, 1.5 μM. (D) Restoration of cystine uptake after treatment with D609. HT22 cells were pretreated with or without glutamate and D609 as indicated (treatment) for 8 h. The uptake of radioactive cystine then was monitored by incubating the cells with 35S-cystine for 20 min as described in Materials and Methods. In addition to 35S-cystine, the labeling mix also may contain glutamate and D609 as indicated (labeling) to distinguish cystine uptake via the cystine/glutamate antiporter from that via the other routes (see text for explanations). Glutamate, 5 mM; D609, 50 μM. Results are presented relative to controls without glutamate. The rate of cystine uptake for untreated controls is 22.7 pmol/μg protein/20 min.
The Inhibition of PC-PLC Restores Cystine Uptake.
The above observation that D609 restores GSH synthesis and blocks cell death caused by glutamate but not BSO or cystine deprivation suggests the possibility that cystine uptake is restored in the presence of D609. This possibility was examined by monitoring cystine uptake by HT22 cells after exposure to glutamate plus or minus D609. In the presence of 5 mM glutamate, the ability of cells to take up cystine is approximately one-fifth of that of the control (Fig. 3D). After 8 h of treatment with 50 μM D609, the ability of cells to take up cystine is almost equal to control values, even in the presence of 5 mM glutamate (Fig. 3D). However, treatment of cells with D609 for only 4 h was unable to restore the ability of cells to take up cystine in the presence of glutamate (relative cystine uptake of 51 ± 1% and 54 ± 3% for glutamate treatment and glutamate plus D609 treatment, respectively, compared with the nontreated control, n = 4). These data are consistent with Fig. 3A, which shows that intracellular GSH levels go up after 4 h of treatment.
The above data suggest that either the glutamate/cystine antiporter is uncoupled or another cystine channel is opened after the treatment of D609. To distinguish between these possibilities, cells were first treated with D609 for 8 h and cystine uptake was monitored in the presence or absence of 5 mM glutamate. If D609 treatment uncouples the glutamate/cystine antiporter, equal uptake of cystine would be expected in the presence or absence of glutamate. If D609 treatment activates another cystine channel, more uptake of cystine would be expected in the absence of glutamate than in the presence of glutamate. Fig. 3D shows that the cystine uptake is not significantly greater in the presence of D609. Therefore, D609 treatment leads to the deregulation of cystine uptake by glutamate.
DISCUSSION
Although the molecular structure of eukaryotic PC-PLC is lacking, signaling through PC-PLC has been implicated in several cellular processes including mitogenesis (see, for example, refs. 29 and 30) and programmed cell death initiated via TNF and Fas/Apo-1 (4, 5). The above data show that PC-PLC also mediates glutamate-induced programmed cell death of immature cortical neurons and of the hippocampal cell line HT22. Although PC-PLC is involved in several models of programmed cell death, PC-PLC regulates glutamate-induced nerve cell death through a different mechanism. With TNF or Fas/Apo-1-induced apoptosis, PC-PLC functions to generate diacylglycerol, which activates an acidic sphingomyelinase with the resultant production of ceramide, which then activates downstream apoptotic machinery. In the case of glutamate toxicity, however, PC-PLC regulates the glutamate/cystine antiporter. The inhibition of PC-PLC activity uncouples the uptake of cystine from glutamate inhibition (Fig. 3D) and therefore restores the ability of cells to synthesize GSH, even in the presence of high concentrations of glutamate (Fig. 3 A and C). Cell death caused by GSH depletion via other mechanisms, such as BSO administration or cystine deprivation, is not prevented by the inhibition of PC-PLC (Fig. 1 C and D).
The specific PC-PLC inhibitor D609 (4, 24–27) was used to inhibit PC-PLC. Although D609 might directly modify the antiporter, two experiments suggest that such a direct modification is unlikely. First, treatment of cells with D609 for 4 h has no effect on the ability of glutamate to block cystine uptake. Consistent with this observation, glutamate still causes a drop in the level of GSH up to 4 h after the treatment of cells with D609 (Fig. 3A). A requirement for such a long duration to affect the antiporter likely excludes its direct interaction with the antiporter and suggests modifications of targets other than the antiporter. Second, exogenous PC-PLC potentiates glutamate toxicity (Fig. 2), suggesting that a PC-PLC product is involved in the toxicity pathway. It is therefore likely that PC-PLC plays a role in glutamate-induced nerve cell death by regulating the glutamate/cystine antiporter. It is unknown at this time whether PC-PLC is constitutively expressed or is up-regulated in the presence of glutamate.
The possible route from PC-PLC to the regulation of glutamate/cystine antiporter is yet to be determined. The signals induced by PC-PLC may be transduced via various molecules including protein kinases such as PKC (31) and mitogen-activated protein kinase (32), and transcriptional factors such as NF-κB (25, 33). The transduction of PC-PLC signals through PKC is appealing. The activation of PKC frequently is associated with the translocation of the protein to the plasma membrane, and membrane-associated proteins such as antiporters (e.g., the Na+/H+ exchanger) can serve as PKC substrates (34). However, the activation of PKC by phorbol ester prevents glutamate-induced nerve cell death in HT22 cells (11), so if PKC is involved, it must be a phorbol ester-insensitive form. Indeed, it has been shown recently that the transduction of a PC-PLC signal depends on an atypical PKC in Rat-1 cells (31). Regardless of the precise mechanism, it is clear that the action of PC-PLC does not involve an AA-dependent pathway because exogenous AA is unable to override the protective effect of D609 (Fig. 1B).
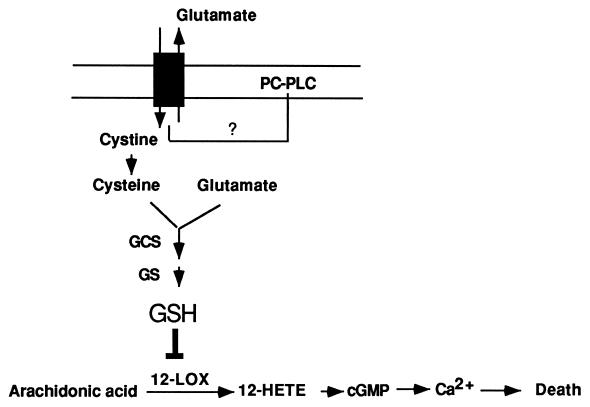
Combining the present data with previous studies suggests a model for oxidative glutamate-induced nerve cell death, which is depicted in Fig. 4. Cystine is taken up primarily via the cystine/glutamate antiporter, which is coupled to the export of glutamate (35). Once inside the cell, cystine is reduced to cysteine, which then is used as a substrate for GSH synthesis via γ-glutamylcysteine synthetase and glutathione synthetase. High levels of extracellular glutamate inhibit the cellular uptake of cystine, thereby indirectly causing the depletion of GSH (12). Low concentrations of GSH then activate 12-LOX (17), the products of which in turn activate soluble guanylate cyclase, leading to an elevation of intracellular cGMP. cGMP then activates a calcium channel, leading to the accumulation of intracellular calcium (23). The inhibition of the cystine/glutamate antiporter by glutamate is central to oxidative glutamate toxicity. The inhibition of PC-PLC abolishes the ability of glutamate to interfere with cystine uptake via the cystine/glutamate antiporter. Thus, targeting of the PC-PLC pathway may be an effective method to control oxidative glutamate-induced cell death in the brain (11–13, 36–39) and might be beneficial to people suffering from some neurodegenerative diseases associated with glutamate toxicity. Because such diseases also may be complicated by inflammation caused by factors such as TNF, targeting of this shared PC-PLC pathway may be particularly effective.
Figure 4.
A model for oxidative glutamate toxicity in nerve cells. The cystine/glutamate antiporter is designated by the gray box. Inhibition is designated by ⊥. See text for explanations. GCS, γ-glutamylcysteine synthetase; GS, glutathione synthetase.
Acknowledgments
We thank Drs. H. Kimura, Y. Liu, Y. Sagara, and S. Tan for critically reading the manuscript. This work was supported by National Institutes of Health grants to D.S. (R01 NS09658), P.M. (5PO-NS28121), and Y.L. (1 F32 AG 05769).
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: AA, arachidonic acid; BSO, buthionine sulfoximine; GSH, reduced glutathione; 12-LOX, 12-lipoxygenase; PLA2, phospholipase A2; PKC, protein kinase C; PLC, phospholipase C; PC-PLC, phosphatidylcholine-specific phospholipase C; TNF, tumor necrosis factor.
References
- 1.Stephenson D T, Lemere C A, Selkoe D J, Clemens J A. Neurobiol Dis. 1996;3:51–63. doi: 10.1006/nbdi.1996.0005. [DOI] [PubMed] [Google Scholar]
- 2.Kanfer J N, Singh I N, Pettegrew J W, McCartney D G, Sorrentino G. J Lipid Mediat. 1996;14:361–363. doi: 10.1016/0929-7855(96)00545-7. [DOI] [PubMed] [Google Scholar]
- 3.Rordorf G, Uemura Y, Bonventre J V. J Neurosci. 1991;11:1829–1836. doi: 10.1523/JNEUROSCI.11-06-01829.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cifone M G, Roncaioli P, Maria R D, Camarda G, Santoni A, Rubert G, Testi R. EMBO J. 1995;14:5859–5868. doi: 10.1002/j.1460-2075.1995.tb00274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Machleidt T, Kramer B, Adam D, Neumann B, Schutze S, Wiegmann K, Kronke M. J Exp Med. 1996;184:725–733. doi: 10.1084/jem.184.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Batistatou A, Greene L A. J Cell Biol. 1991;115:461–471. doi: 10.1083/jcb.115.2.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deckwerth T L, Johnson E M J. J Cell Biol. 1993;123:1207–1222. doi: 10.1083/jcb.123.5.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.D’Mello S R, Galli C, Ciotti T, Calissano P. Proc Natl Acad Sci USA. 1993;90:10989–10993. doi: 10.1073/pnas.90.23.10989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koh J-Y, Wie M B, Gwag B J, Sensi S L, Canzoniero L M T, Demaro J, Csernansky C, Choi D W. Exp Neurol. 1995;135:153–159. doi: 10.1006/exnr.1995.1074. [DOI] [PubMed] [Google Scholar]
- 10.Ratan R R, Murphy T M, Baraban J M. J Neurochem. 1994;62:376–379. doi: 10.1046/j.1471-4159.1994.62010376.x. [DOI] [PubMed] [Google Scholar]
- 11.Davis J B, Maher P. Brain Res. 1994;652:169–173. doi: 10.1016/0006-8993(94)90334-4. [DOI] [PubMed] [Google Scholar]
- 12.Murphy T H, Miyamoto M, Sastre A, Schnaar R L, Coyle J T. Neuron. 1989;2:1547–1558. doi: 10.1016/0896-6273(89)90043-3. [DOI] [PubMed] [Google Scholar]
- 13.Murphy T H, Schnaar R L, Coyle J T. FASEB J. 1990;4:1624–1633. [PubMed] [Google Scholar]
- 14.Perry T L, Godin D V, Hansen S. Neurosci Lett. 1982;33:305–310. doi: 10.1016/0304-3940(82)90390-1. [DOI] [PubMed] [Google Scholar]
- 15.Sofic E, Lange K W, Riederer P. Neurosci Lett. 1992;142:128–130. doi: 10.1016/0304-3940(92)90355-b. [DOI] [PubMed] [Google Scholar]
- 16.Sian J, Dexter D T, Lees A J, Daniel S, Agid Y, Javoy-Agid F, Jenner P, Marsden C D. Ann Neurol. 1994;36:348–355. doi: 10.1002/ana.410360305. [DOI] [PubMed] [Google Scholar]
- 17.Li Y, Maher P, Schubert D. Neuron. 1997;19:453–463. doi: 10.1016/s0896-6273(00)80953-8. [DOI] [PubMed] [Google Scholar]
- 18.Maher P, Davis J B. J Neurosci. 1996;16:6394–6401. doi: 10.1523/JNEUROSCI.16-20-06394.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abe K, Takayanagi M, Saito H. Jpn J Pharmacol. 1990;53:221–227. doi: 10.1254/jjp.53.221. [DOI] [PubMed] [Google Scholar]
- 20.Murphy T H, Baraban J M. Dev Brain Res. 1990;57:146–150. doi: 10.1016/0165-3806(90)90195-5. [DOI] [PubMed] [Google Scholar]
- 21.Tietze F. Anal Biochem. 1969;27:502–522. doi: 10.1016/0003-2697(69)90064-5. [DOI] [PubMed] [Google Scholar]
- 22.Ratan R, Murphy T, Baraban J M. J Neurosci. 1994;14:4385–4392. doi: 10.1523/JNEUROSCI.14-07-04385.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li Y, Maher P, Schubert D. J Cell Biol. 1997;139:1317–1324. doi: 10.1083/jcb.139.5.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muller-Decker K. Biochem Biophys Res Commun. 1989;162:198–205. doi: 10.1016/0006-291x(89)91981-5. [DOI] [PubMed] [Google Scholar]
- 25.Schutze S, Potthoff K, Machleidt T, Berkovic D, Wiegmann K, Kronke M. Cell. 1992;71:765–776. doi: 10.1016/0092-8674(92)90553-o. [DOI] [PubMed] [Google Scholar]
- 26.Cobb R R, Felts K A, Parry C N, Mackman N. Mol Pharmacol. 1996;49:998–1004. [PubMed] [Google Scholar]
- 27.Cowen D S, Sowers R S, Manning D R. J Biol Chem. 1996;271:22297–22300. doi: 10.1074/jbc.271.37.22297. [DOI] [PubMed] [Google Scholar]
- 28.Balsinde J, Diez E, Mollinedo F. J Biol Chem. 1991;266:15638–15643. [PubMed] [Google Scholar]
- 29.Cai H, Erhardt P, Troppmair J, Diaz-Meco M T, Sithanandam G, Rapp U R, Moscat J, Cooper G M. Mol Cell Biol. 1993;13:7645–7651. doi: 10.1128/mcb.13.12.7645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu X-X, Tessner T G, Rock C O, Jackowski S. Mol Cell Biol. 1993;13:1522–1533. doi: 10.1128/mcb.13.3.1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Dijk M C M, Muriana F J G, van der Hoeven P C J, van Widt J, Schaap D, Moolenaar W H, van Blitterswijk W J. Biochem J. 1997;323:693–699. doi: 10.1042/bj3230693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Dijk M C M, Muriana F J G, de Widt J, Hilkmann H, van Blitterswijk W J. J Biol Chem. 1997;272:11011–11016. doi: 10.1074/jbc.272.17.11011. [DOI] [PubMed] [Google Scholar]
- 33.Arenzana-Seisdedos F, Fernandez B, Dominguez I, Jacque J M, Thomas D, Diaz-Meco M T, Moscat J, Virelizier J L. J Virol. 1993;67:6596–6604. doi: 10.1128/jvi.67.11.6596-6604.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Exton J H. Biochim Biophys Acta. 1994;1212:26–42. doi: 10.1016/0005-2760(94)90186-4. [DOI] [PubMed] [Google Scholar]
- 35.Bannai S. Biochim Biophys Acta. 1984;779:289–306. doi: 10.1016/0304-4157(84)90014-5. [DOI] [PubMed] [Google Scholar]
- 36.Vornov J J, Coyle J T. J Neurochem. 1991;56:996–1006. doi: 10.1111/j.1471-4159.1991.tb02020.x. [DOI] [PubMed] [Google Scholar]
- 37.Kato S, Negishi K, Teranishi T, Ishita S. Neuroscience. 1992;48:903–914. doi: 10.1016/0306-4522(92)90278-a. [DOI] [PubMed] [Google Scholar]
- 38.Oka A, Belliveau M J, Rosenberg P A, Volpe J J. J Neurosci. 1993;13:1441–1453. doi: 10.1523/JNEUROSCI.13-04-01441.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.La Bella V, Alexianu M E, Colom L V, Ionescu A, Mohamed A H, Appel S H. Neuroscience. 1996;70:1039–1052. doi: 10.1016/0306-4522(95)00401-7. [DOI] [PubMed] [Google Scholar]