Abstract
Both early-life stress and immune system activation in adulthood have been linked independently to depression in a number of studies. However, the relationship between early-life infection, which may be considered a “stressor”, and later-life depression has not been explored. We have reported that neonatal bacterial infection in rats leads to exaggerated brain cytokine production, as well as memory impairments, to a subsequent peripheral immune challenge in adulthood, and therefore predicted that stressor-induced depressive-like symptoms would be more severe in these rats as well. Rats treated on postnatal day 4 with PBS or E. coli were as adults exposed to inescapable tailshock stress (IS), and then tested for sucrose preference, social exploration with a juvenile, and overall activity, 1, 3, 5, and 7 days following the stressor. Serum corticosterone and extracellular 5-HT within the basolateral amygdala were measured in a second group of rats in response to the IS. IS resulted in profound depressive-like behaviors in adult rats, but, surprisingly, rats that suffered a bacterial infection early in life had blunted corticosterone responses to the stressor and were remarkably protected from the depressive symptoms compared to controls. These data suggest that early-life infection should be considered within a cost/benefit perspective, in which outcomes in adulthood may be differentially protected or impaired. These data also suggest that the immune system likely plays a previously unsuspected role in “homeostatic” HPA programming and brain development, which may ultimately lend insight into the often-contradictory literature on cytokines, inflammation, and depression.
Keywords: postnatal, cytokines, depression, sucrose preference, social exploration, corticosterone
Introduction
Research in human and non-human animals has suggested that events occurring early in life, such as stress, may predispose individuals to the development of mood disorders such as depression in adulthood (Kessler and Magee, 1993; Brewin et al., 2000; Ladd et al., 2000; Heim and Nemeroff, 2001). Glucocorticoids, the primary stress hormone, are critical for many aspects of normal development and thus are good candidates for so-called “programming” of brain and behavior during prenatal or early postnatal life (Owen et al., 2005). In support of this hypothesis a number of studies in rodents and humans have demonstrated a link between early-life stress, altered hypothalamic-pituitary-adrenal (HPA) activity, and increased depressive-like symptoms later in life (Heim and Nemeroff, 2001; Aisa et al., 2007; Lee et al., 2007; Lippmann et al., 2007).
An important and completely unexplored question is whether immune system activation early in life, such as occurs during infection, may influence the development of depression in adulthood. A significant relationship is not unexpected, as there is considerable crosstalk between stressors and immune system activation. For instance, administration of the pro-inflammatory cytokine interleukin [IL]-1 in neonatal mice leads to increased HPA reactivity in adulthood (del Rey et al., 1996). Similarly, tail shock stress in adult rats increases levels of IL-1 within the brain (Nguyen et al., 1998). Furthermore, prior stressor exposure can potentiate cytokine responses to a subsequent peripheral immune challenge (Johnson et al., 2002). Cytokines and immune-related pathology have also been linked to depression in a large number of studies (Dantzer et al., 1999; Pollak and Yirmiya, 2002; Hayley et al., 2005; Schiepers et al., 2005; Raison et al., 2006; Irwin and Miller, 2007), although not always (Whooley et al., 2007), and this is an area that remains controversial (see Glassman and Miller, 2007). Notably, elevation in brain IL-1 levels was recently shown to mediate the depressive-like symptoms induced by chronic mild stress, an established model of depression in mice (Goshen et al., 2007). Whereas the mechanisms underlying cytokine-associated depression remain unclear, the available data do suggest that stress, immune, and affective systems may all interact via influences on common neuromodulatory systems, such as serotonergic pathways (Leonard, 2005; Lee et al., 2007; Lowry et al., 2007).
It is estimated that up to 1/3 of pregnancies suffer complications involving infections or trauma of the uterus or newborn (Newton, 1993; Garnier et al., 2003), and an increasing body of evidence indicates that perinatal events involving the immune system may contribute to the development of behavioral or neuropsychiatric disorders (Rantakallio et al., 1997; Hornig et al., 1999; Shi et al., 2003; Zuckerman and Weiner, 2003). We have reported that bacterial infection on postnatal day 4 in rats, a developmental stage relatively comparable to the third trimester in humans during which significant brain growth occurs (Dobbing and Sands, 1979; Rodier, 1980), increases both peripheral and brain cytokines, as well as corticosterone for several hours (>48) following the infection (Bilbo et al., 2005a). Furthermore, as adults, these rats exhibit exaggerated cytokine production (IL-1) within the brain, as well as profound memory impairments, to a subsequent peripheral immune challenge in adulthood (Bilbo et al., 2005a; Bilbo et al., 2006; Bilbo et al., 2007). Importantly, preventing the synthesis of brain IL-1 prior to the immune challenge completely prevents the memory impairment, indicating a causal role for this cytokine in the impairment (Bilbo et al., 2005a). Collectively, these data led to the hypothesis that stressor-induced depressive-like behavior would be more pronounced in these rats compared to controls. Here we report that inescapable stress (IS) resulted in profound depressive-like behaviors in adult rats, but that, surprisingly, rats that suffered a bacterial infection early in life had blunted corticosterone responses to the stressor and were remarkably protected from depressive-like symptoms compared to controls.
Materials and Methods
Animals
Adult male and female Sprague-Dawley rats (70 days) were obtained from Harlan (Indianapolis, IN) and housed in same sex pairs in polypropylene cages with ad libitum access to food and water. Male juvenile Sprague-Dawley rats (21 days) to be used in social exploration tests were obtained from Harlan (Indianapolis, IN) and housed in groups of 4 in the colony room. The colony was maintained at 22°C on a 12:12-h light:dark cycle (lights on at 0600 MST), unless otherwise noted. Following acclimation to experimental conditions, males and females were paired into breeders. Sentinel animals were housed in the colony room and screened periodically for the presence of common rodent diseases (all screens were negative). All experiments were conducted with protocols approved by the University of Colorado Animal Care and Use Committee.
Neonatal Manipulations
Female breeders were visually examined daily for confirmation of pregnancy, and male breeders were removed from cages prior to the birth of pups (=postnatal day [P] 0). Litters were culled on P4 to a maximum of 10 pups/litter, retaining two female and as many male pups as possible. All studies were limited to males.
Bacterial Culture
E. coli culture (ATCC 15746; American Type Culture Collection, Manassas, VA) vial contents were hydrated and grown overnight in 30 ml of brain-heart infusion (BHI) (Difco Labs, Detroit, MI) at 37°C. Cultures were aliquoted into 1 ml stock vials supplemented with 10% glycerol and frozen at -20°C. One day before injections, a stock culture was thawed and incubated overnight in 40 ml of BHI at 37°C. The number of bacteria in cultures was read using a microplate reader (Bio-Tek Instruments, Inc., Winooski, VT) and quantified by extrapolating from previously determined growth curves. Cultures were centrifuged for 15 min at 4000 rpm, the supernatants were discarded, and the bacteria were re-suspended in the dose-appropriate volume of sterile Dulbecco’s PBS (Invitrogen Corp., Carlsbad, CA).
Bacterial Injections
Male pups were injected subcutaneously (30G needle) on P4 with either 0.1 × 106 colony forming units (CFU) of live bacterial E. coli/g suspended in 0.1 ml PBS, or 0.1 ml PBS alone. All pups were removed from the mother at the same time and placed into a clean cage with bedding, injected individually, and returned to the mother as a group. Elapsed time away from the mother was less than 5 min. All pups from a single litter received the same treatment due to concerns over possible cross-contamination from E. coli. All injections were given between 1530 and 1600 hr. The infection results in acute weight loss (Bilbo et al., 2005b) and a localized inflammatory response within the skin (redness, swelling, heat) that is resolved within several days (unpublished data). All pups are monitored visually daily. Any pups that present with significant tissue damage (e.g., open sore or lesion) or obvious weight loss or weakness (e.g., difficulty nursing or walking, lethargy) are removed from the study and euthenized if necessary. Such cases are rare and did not occur in the current experiment. Notably, the infection does not significantly alter maternal care (Bilbo et al., 2007). Pups were weaned on P21 into sibling pairs and remained undisturbed until adulthood. To control for possible litter effects, a maximum of two pups/litter were assigned to a single experimental group.
Experiment 1
Adult rats (2 mo) from each neonatal group were assigned randomly to stress or control groups (n=10/grp). Pair-housed rats from a single cage were assigned to the same group. Rats were transferred into a colony room with a reverse light:dark cycle (lights off at 0800 MST) and maintained on this cycle throughout the study in order to measure sucrose intake and social exploration during the active phase of their cycles. Rats were allowed 2 weeks to acclimate to the altered light cycle prior to subsequent procedures.
Sucrose Preference
Rats were habituated to a 2% sucrose solution for 3 days prior to the stressor in order to establish a baseline of sucrose preference. Each rat was transferred into an individual cage at 0900 MST and was given access to rat chow and one standard drinking bottle containing tap water, and a second identical bottle containing tap water with 2% sucrose. Rats were allowed to eat and drink undisturbed for 4 hours. Bottles and food were weighed before and after in order to measure consumption of each. The position of water versus sucrose bottles in the cage was rotated daily in order to prevent a place preference. This procedure was repeated for 3 consecutive days prior to the stressor, using the same individual cages for each rat throughout the experiment. Baseline sucrose preference did not differ between neonatal groups.
Inescapable Stress
All rats were weighed to establish a baseline. Each rat in the stress group was placed into a Plexiglas box (14 × 11 × 17 cm) with a Plexiglas rod protruding from the rear. The rat’s tail was secured to the rod with tape and affixed with copper electrodes. Rats received a single session of 100 tail shocks progressing from low to high intensity (1/3 at 1.0 mA, 1/3 at 1.3 mA, and 1/3 at 1.6 mA) with an average 60 s intertrial interval. Rats were returned to their home cages after the stressor. Non-shocked home cage (HC) rats remained undisturbed in the colony.
Social Exploration
On days 1, 3, 5, and 7 post-stressor, rats were transferred into their individual cages, and sucrose, water, and food intake were measured as described previously. After 4 hours, individual cages were taken individually into an adjacent room for social exploration testing, in which a male juvenile (P21-P28) conspecific was placed into the cage for 3 min. During this time, an observer blinded to the experimental conditions of the study recorded the total time in contact with the juvenile, but only contact that was directly initiated by the experimental rat, and defined as “social exploration” (e.g., sniffing, licking, or grooming, play behavior, etc.). Thus, leaning against or incidental side-by-side touching was not counted. Rats were also rated visually by the observer for overall activity. For this, a 4-point scale was used as follows: 4= very active/exploratory, 3= somewhat active/exploratory, 2= somewhat lethargic, and 1= very lethargic/unresponsive. Tail shock stress is a relatively severe stressor, and rats often exhibit symptoms of sickness behavior (Hart, 1988) in the days following (e.g., reduced grooming, piloerection, lethargy) (personal observations). Rats were weighed and returned to their home cages at the end of each test. Rats received a different juvenile stimulus rat for each test day, and juveniles were used no more than 4 times in a single day.
Experiment 2
Based on the results of Exp. 1, the goal of this experiment was to explore two potential mechanisms involved in behavioral responses to the stressor: 1) 5-HT in the basolateral amygdala (BLA), which increases following IS and mediates many of its behavioral consequences (Amat et al., 1998), and 2) circulating corticosterone.
Surgery
Adult rats from each neonatal group (n=9) were anesthetized with a Ketamine (100 mg/kg)/Xylazine (20 mg/kg)/Acepromazine (10 mg/kg) mixture via subcutaneous injection, and implanted with a CMA 12 microdialysis cannula guide (CMA microdialysis, Acton, MA, USA), ending just above the BLA: 3 mm caudal to bregma, 4.8 mm from midline and 6.2 mm from the dura matter. All coordinates followed the atlas of Paxinos and Watson (1998). The cannula guides were secured to the skull with four screws. A screw cap of a 15 ml conical centrifuge tube, whose central lid portion was removed, was also affixed to the skull so that its threads were exposed and it encircled the probe guide. This was done so that the skull assembly could later be protected during microdialysis. Following surgery, the rats were placed in individual plastic cages for a week for recovery.
Dialysis
The evening before the experiment, animals were transferred to the dialysis room and a 2 mm probe was inserted into the BLA (CMA 12, 0.5 mm in diameter with molecular weight cutoff of 20,000 daltons, from CMA/Microdialysis). Each animal was placed individually in a Plexiglas bowl (BAS, Westlafayette, IN, USA). Perfusion with isotonic Ringer’s solution (Baxter, Deerfield, IL, USA) began at a rate of 0.2 μl/min overnight. At about 0900h the following morning, the flow rate was increased to 1.5 μl/min and a 90 min stabilization period was allowed. Samples were collected at 20 min intervals throughout the dialysis session. Four baseline samples were collected before stressor onset. Following baseline sampling, the animals were removed from the Plexiglas bowls and placed into Plexiglas boxes, without interruption of the flow of the Ringer’s solution. The rats then received a single session of 100 tail shocks progressing from low to high intensity (1/3 at 1.0 mA, 1/3 at 1.3 mA, and 1/3 at 1.6 mA) with an average 60 s intertrial interval, as in Exp. 1. Five microdialysis samples were collected continuously during the roughly 2 h session. After the session was completed, the animals were returned to the Plexiglas bowls, without interrupting flow, and microdialysate samples were collected for a further 60 min.
Blood Sampling
Blood samples were obtained immediately, 24 h, and 48 h after the last dialysis sample (=1 h post-stress) was collected. For each sample, the rat was wrapped gently in a towel, leaving the tail exposed. A small nick was made in the lateral tail vein using a scalpel and the tail was stroked until ∼100 μl of whole blood was collected into a microcentrifuge tube. The entire procedure lasted <2 min per rat. Between samples rats were returned to their home cages. Blood was centrifuged at 10,000 rpm at 4 °C for 10 min, and supernatant was collected and stored at -20°C until assayed for corticosterone.
Cannula verification
After the final blood sample an overdose of pentobarbital was administered and brains were removed and frozen. Forty micrometer sections were taken with a cryostat and stained with Cresyl Violet for cannula placement verifications using a light microscope. Only rats with at least 70% of the dialysis probe membrane within the BLA complex were included in the analysis.
5-HT analysis
5-HT concentration was measured in dialysates by HPLC with electrochemical detection. The system consisted of an ESA 5600A Coularray detector with an ESA 5014B analytical cell and an ESA 5020 guard cell (ESA, Chelmsford, MA, USA). The column was an ESA MD-150 (C-18, 150×3.2 mm) maintained at 37 °C, and the mobile phase was the ESA buffer MD-TM. The analytical cell potentials were kept at -100 mV and +200 mV and the guard cell at +220 mV. Twenty-five microliters of dialysate were injected with an ESA 542 autosampler which kept the dialysates at -6 °C. External standards (Sigma) were run each day to quantify 5-HT.
Exp. 2B
In order to examine corticosterone responses during the stressor, a second group of rats from each neonatal treatment group (n=9/grp) was cannulated and exposed to tail shock just as described above. Blood samples were collected from the tail vein 10 min into and immediately after the end of the tail shock session. Dialysates were not collected on these animals because blood sampling would have interfered with their interpretation; thus, surgery and overnight acclimation in the dialysis room were performed on these animals for consistency with the previous experiment.
Experiment 3
The goal of this experiment was to control for several variables in Exp. 2 that could alter the interpretation of any observed changes in corticosterone, including 1) the severity of the stressor, 2) surgery, and 3) single housing post-surgery, all of which likely alter corticosterone responses. A blood sample was obtained from the tail vein of a third group of pair-housed adult rats from each neonatal group (n=8/grp) in order to assess basal (non-stressed) corticosterone concentrations. One week later, rats were placed individually into well-ventilated Plexiglas restraint tubes (22 cm long and 9 cm in diameter) in a quiet experimental room for 30 min. Blood samples were collected immediately after and 2 h after the restraint session ended. Rats were returned to their home cages in the colony room between samples. Baseline and immediate post-stress samples were collected at the same time of day (1 week apart). Sucrose preference and social exploration tests were not conducted in this experiment because significant behavioral changes are not observed following this mild stressor (unpublished data).
Corticosterone Assessment
Total serum corticosterone concentrations were assessed from serum in 3 assays using a single colorimetric EIA kit from Assay Designs, Inc. (Ann Arbor, MI). The assays were run according to the manufacturer’s instructions except that serum (5 μl) was diluted 1:50 in 0.05% steroid displacement-modified assay buffer. The detection limit of the assay was 27 pg/ml, the intra-assay coefficients of variation for each assay were 9.25%, 10%, and 11%, and the inter-assay coefficient of variation was 4.5%.
Data Analysis and Statistics
All data were analyzed using analysis of variance (ANOVA) tests. Following significant F scores, post-hoc comparisons (Fisher’s protected least significant difference) were performed to further distinguish among groups, and all differences were considered statistically significant if p<0.05.
Results
Experiment 1
Neonatal infection attenuates the depressive-like behavioral changes observed following stress
Reduction in the preference for sweet solutions, anorexia, reduced body weight and diminished interest in the social environment have been considered as analogues of anhedonia and other depressive symptoms in rodents (Yirmiya, 1996; Willner, 2005). Thus, in the present experiment sucrose preference, food intake, and body mass were each subtracted from baseline for each rat and analyzed across days using separate repeated measures 2-way ANOVAs. For sucrose preference, a significant group × treatment effect (F1,108=4.8; p=0.03) revealed that stressed E. coli rats returned to baseline faster than did stressed PBS rats (p<0.05; Figure 1). For food intake, a significant group × treatment effect (F1,108=3.3; p<0.05) revealed that stressed E. coli rats returned to baseline faster than stressed PBS rats (p<0.05; Fig 1B). There were no significant differences in body weight (Fig 1C), and no neonatal group differences in HC (non-stressed) rats for any measure.
Figure 1.
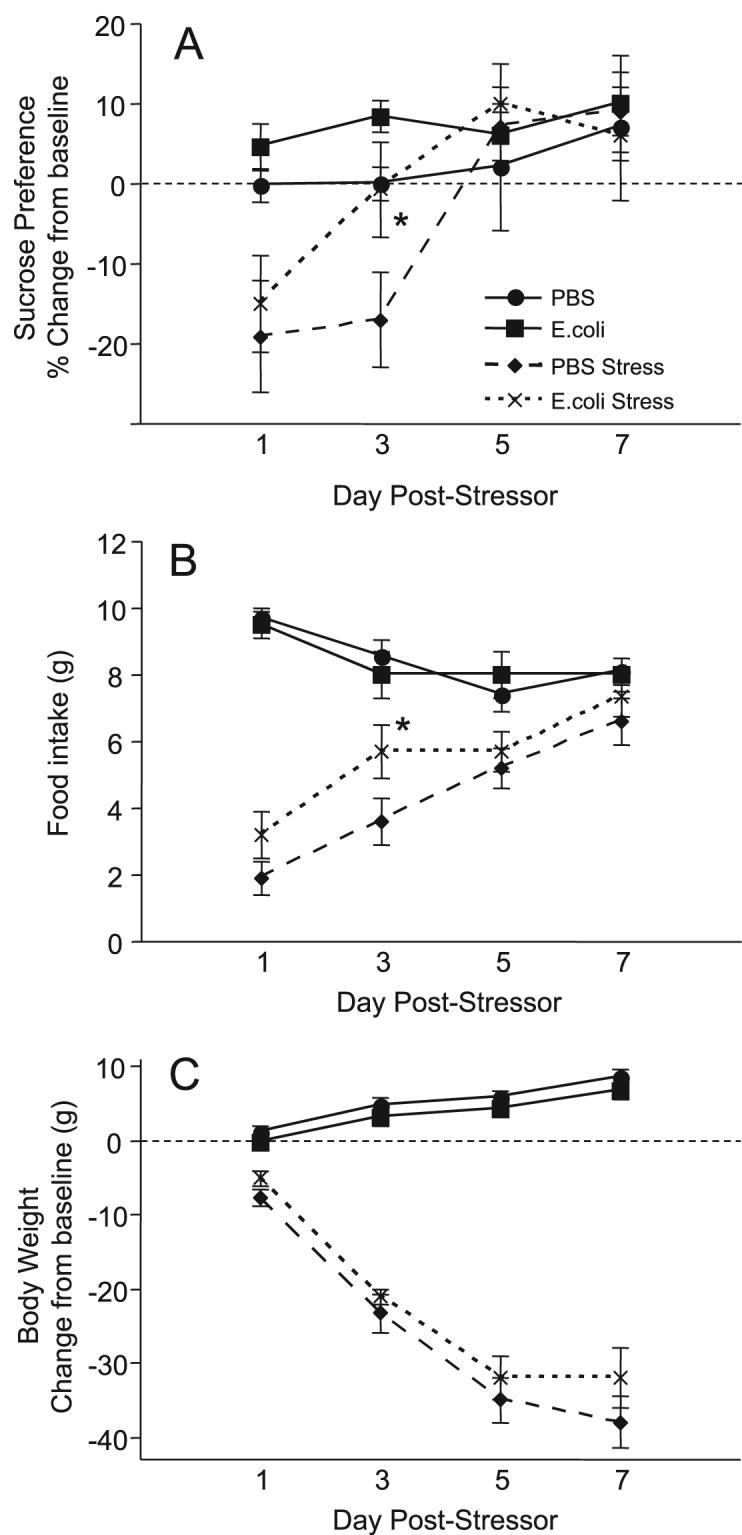
Neonatal infection attenuated the stressor-induced decrease in sucrose preference and food intake in adulthood (Experiment 1). Data are presented as mean±SEM (n=10/group). (A) Preference for a 2% sucrose solution over water (percent change from pre-stress baseline). (B) Food intake (g) during daily 4 h observation period. (C) Change in body weight from pre-stress baseline. *Significantly different from PBS Stress, p<0.05.
Social exploration was also analyzed across days using a repeated measures 2-way ANOVA. A significant day × group interaction (F3,108=3.1; p<0.03) revealed that E. coli rats were significantly less exploratory than PBS rats on the first day of testing (p<0.05), due to reduced exploration in E. coli HC rats. A significant 3-way interaction (F3,108=4.8; p=0.003) revealed that stressed E. coli rats returned to control levels of exploration by day 5, whereas PBS rats did not recover until day 7 (p<0.05; Figure 2). Ranked activity data were analyzed between stressed groups using a Mann-Whitney U test; because activity for all non-stressed HC groups did not vary from the highest ranking (=4), these were excluded from the analysis. Fig 2B illustrates that activity was significantly higher in stressed E. coli compared to stressed PBS rats (p=0.01).
Figure 2.
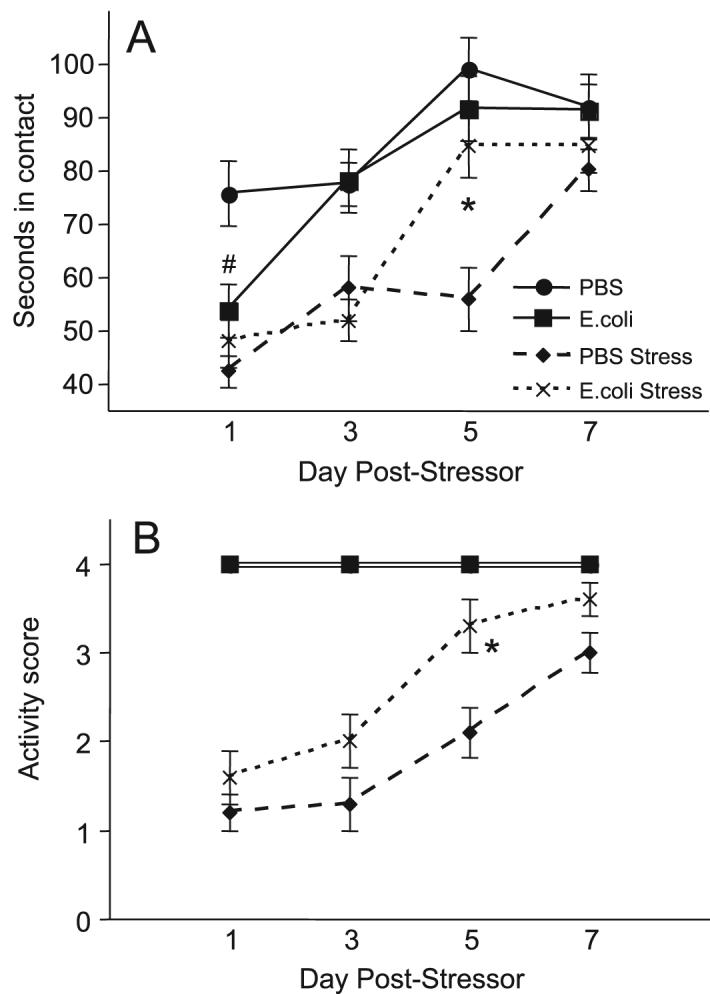
Neonatal infection attenuated the stressor-induced decreases in social exploration and activity in adulthood (Experiment 1). Data are presented as mean±SEM (n=10/group). (A) Total seconds in exploratory contact with juvenile (initiated by experimental animal) during 3 min test. (B) Activity score during social exploration test (1=lethargic/unresponsive, 4=very active/exploratory). #Significantly different from PBS, *Significantly different from PBS Stress, p<0.05.
Neonatal infection does not alter 5-HT in the basolateral amygdala during stress
Extracellular levels of 5-HT in the BLA were analyzed across sample time points using a repeated measures 2-way ANOVA. The last recovery sample was excluded in order to avoid artifacts (e.g., experimenter in the room). Three PBS-treated and one E. coli-treated rat were excluded from analyses because of improper cannulae placements. There were no significant differences in baseline, stress, or post-stress levels (Figure 3).
Figure 3.
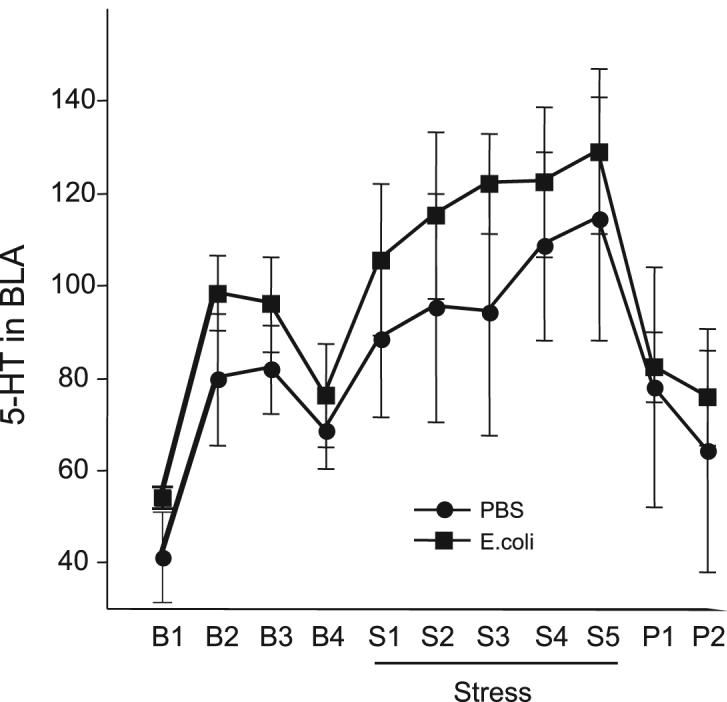
Extracellular levels of 5-HT in the basolateral amygdala expressed as a percentage of baseline for samples taken before, during, and after tail shock stress (Experiment 2). Data are presented as mean±SEM (n=9/group). There were no significant differences between groups.
Neonatal infection attenuates the corticosterone response to stress
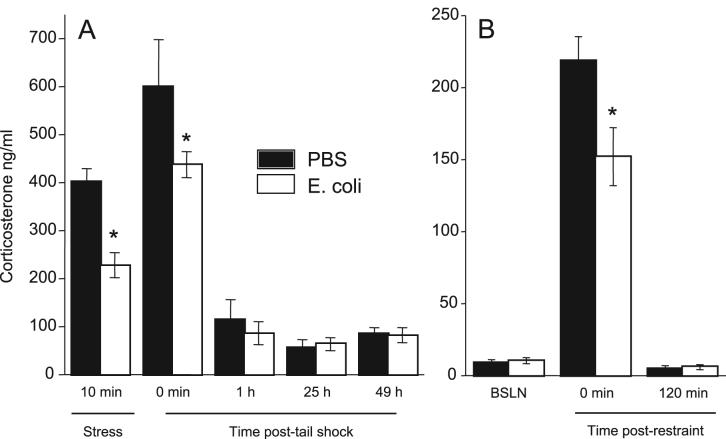
Corticosterone was analyzed separately for each experiment using 2-way repeated measures ANOVAs. In Exp. 2, there were no differences between neonatal groups in the concentrations of corticosterone at 1 h, 25 h, or 49 h post-stress (Figure 4). However, in Exp. 2B the increases in corticosterone during the tail shock stressor and immediately after were significantly attenuated in E. coli rats compared to PBS (F1,11=6.2; p<0.03). In Exp. 3, a significant time × group interaction (F2,28=4.9; p<0.01) revealed that the increase in corticosterone in response to restraint was also significantly attenuated in E. coli rats compared to PBS (p<0.05; Fig 4B), whereas basal and post-stress concentrations did not differ.
Figure 4.
Neonatal infection attenuated stressor-induced increases in corticosterone in adulthood. Data are presented as mean±SEM. (A) Serum corticosterone concentrations (ng/ml) during and after tail shock stress (Experiment 2; n=9/group). (B) Serum corticosterone concentrations before (BSLN) and after 30 min of restraint stress (Experiment 3; n=8/group). *Significantly different from PBS, p<0.05.
Discussion
Neonatal infection with E. coli results in sustained peripheral and brain cytokines, as well as corticosterone for several hours (>48) following the infection (Bilbo et al., 2005a). Because both early-life stress and exaggerated cytokine production is linked to depression, we predicted that depressive-like symptoms following a stressful experience would be more severe in neonatally-infected rats. However, bacterial infection early in life conferred a robust protection against stressor-induced depressive-like behavior in adulthood. This protection was associated with blunted corticosterone responses to the stressor in E. coli rats, whereas 5-HT levels within the BLA did not differ between groups.
The blunted corticosterone responses to the stressor in adult rats infected with E. coli is in contrast to our previous findings that corticosterone responses to a lipopolysaccharide (LPS) challenge in adulthood do not differ as a result of early infection (Bilbo et al., 2005a; Bilbo et al., 2007). LPS is the cell wall component of gram-negative bacteria that simulates infection and induces a robust immune response (Exton, 1997). Because corticosterone increased so dramatically in response to tail-shock in Exp. 2, one interpretation of decreased concentrations in E. coli rats is that these animals had reached a ceiling and were simply incapable of producing more. However, this possibility seems unlikely given that concentrations were also lower in E. coli rats in response to a mild restraint stressor (Exp. 3), in which peak concentrations were lower overall and were clearly capable of climbing much higher (see Fig 4). Rather, the data suggest the interesting possibility that LPS versus tail-shock stress activate the HPA axis in fundamentally different ways, along with opposing consequences for behavior, and that early-life “programming” within the immune system plays an important modulatory role in these responses.
The current data are also in contrast to several reports that neonatal LPS challenge (on P3 and P5) results in increased corticosterone responses to stress in adulthood (Shanks et al., 2000; Hodgson et al., 2001; Nilsson et al., 2002), although this is not always the case (Granger et al., 1996; Breivik et al., 2002). Beyond the obvious difference that LPS is not a replicating pathogen, one striking difference between the LPS model and E. coli infection is the pattern of corticosterone production following the challenge: LPS results in a dramatic increase in corticosterone (4 fold or more) the first day of challenge (P3), but virtually complete resistance to subsequent challenge on P5 (Walker et al., 2004a; 2004b). Following E. coli infection, concentrations increase 2-3 fold and remain elevated for at least 48 h (Bilbo et al., 2005a). These data suggest that caution should be used when generalizing the influence of immune system “activation” on physiological systems, but also suggest that the differences in HPA reactivity between the two models could be a valuable comparison. Unfortunately, there are no data, to our knowledge, on depressive-like behavior in adulthood following neonatal LPS. Interestingly, there are reports that P3/P5 LPS increases anxiety-like behavior in adulthood (Breivik et al., 2002; Walker et al., 2004a), which is often associated with depression. In contrast, Spencer et al. (2006) report no significant change in adult anxiety following LPS on P7.
Finally, the current data are in stark contrast to a large body of data on rats subjected to a common model of early-life stress, maternal separation (MS), in which rats that are separated daily from their mothers for several hours (≥3) during the first two weeks of life exhibit HPA hyper-reactivity to stressors and increased depressive-like symptoms as adults (Diehl et al., 2007; Lippmann et al., 2007). We were originally interested in a comparison to this model because of the considerable crosstalk between stressors and immune system activation. As mentioned previously, E. coli infection on P4 results in a sustained increase in circulating corticosterone for 48+ hours following infection, a response that would be expected to reorganize or program the HPA axis and perhaps other physiological systems to some extent (Viau et al., 1996; Bakker et al., 2001). A close examination of the MS literature reveals surprisingly little information about corticosterone responses during the stressor, as opposed to later in life, making comparisons between the current findings and MS protocols difficult (but see Viau et al., 1996). Interestingly, Gareau et al (2007) recently demonstrated that probiotic, or “beneficial bacteria”, administration to rat pups successfully reversed MS-induced gut function abnormalities, and that this was achieved at least in part via normalization of HPA axis activity. Whereas the E. coli administered in the current study is not probiotic, these data do suggest that the immune system likely plays a previously unsuspected role in “homeostatic” HPA programming and brain development, with significant consequences for behavior throughout the remainder of the life span.
IS rats that were infected as neonates behaved strikingly like rats that have had “behavioral control” over the stressor. That is, rats that are allowed to turn a wheel during the session and thus terminate the shocks are protected from learned helplessness behavior (shuttle box escape) compared to yoked rats that do not have control (Maier and Watkins, 2005). Importantly, the two groups of rats receive the same amount of shock, so that the physical aspect of the stressor is identical. Recent experiments have shown that IS rats with control also recover more quickly in social exploration tests compared to IS rats without control (Dr. John Christianson, personal communication). Uncontrollable stress sensitizes 5-HT neurons with terminals in the BLA, which is important for its behavioral consequences (Amat et al., 1998; Maier and Watkins, 2005). Importantly, rats with control over the stressor do not exhibit the robust 5-HT increases. Based on these collective data, we measured 5-HT responses within the BLA, but found no differences between groups. These data are somewhat difficult to interpret, however, given the large variability, as well as, for unknown reasons, a relatively blunted 5-HT response to IS compared to previous studies (see Amat et al., 1998). Interestingly, Lowry et al (2007) recently reported that peripheral immune challenge with Mycobacterium activated only specific subsets of serotonergic neurons within the brains of mice, which notably resulted in reduced depressive-like behavior (immobility) in a forced swim test. These subsets were only detected using anatomical mapping of gene expression, suggesting that any differences as a result of early infection in the current study may have been undetectable using dialysis. In summary, this is an interesting area that deserves further investigation.
Taken together, bacterial infection early in life significantly influenced the expression of depressive-like behaviors following IS in adult rats, an effect that was related to decreased corticosterone responses to stress. These data, together with previous findings, suggest that early infection should be considered within a cost/benefit perspective, in which outcomes in adulthood may be differentially protected or impaired. It is both interesting and perplexing that the same neonatally-infected rats that exhibit exaggerated brain cytokine responses to an immune challenge in adulthood, which we have demonstrated several times, also exhibit decreased depressive-like behavior following stress, which seems to be in contrast to much of the literature (Dantzer et al., 1999; Pollak and Yirmiya, 2002; Hayley et al., 2005; Schiepers et al., 2005; Irwin and Miller, 2007). However, Goshen et al., (2007) have recently demonstrated that the depressive-like symptoms induced by stress-induced elevation of brain IL-1 are mediated by increases in corticosterone levels. Thus, reduced HPA responsiveness to IL-1 (and other cytokines) in the neonatally-infected rats may underlie the blunted depressive-like effects. Alternatively, it is possible that the brain cytokine response to stress (rather than LPS) in these animals is actually blunted, as is corticosterone. These possibilities need to be addressed in a future study, and future research should also determine any specific causal role of corticosterone in these responses, as well as the potential role of serotonin. These data may ultimately lend insight into the often-contradictory literature on cytokines, infection/inflammation, and depression.
Acknowledgements
The authors thank Alexis Northcutt for technical assistance. Supported in part by NIMH grant MH076320.
Role of Funding Source. Supported in part by NIMH grant MH076320; the NIMH had no further role in study design, in the collection, analysis and interpretation of data, in the writing of the report, or in the decision to submit the paper for publication to this journal.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aisa B, Tordera R, Lasheras B, Del Rio J, Ramirez MJ. Cognitive impairment associated to HPA axis hyperactivity after maternal separation in rats. Psychoneuroendocrinology. 2007;32:256–266. doi: 10.1016/j.psyneuen.2006.12.013. [DOI] [PubMed] [Google Scholar]
- Amat J, Matus-Amat P, Watkins LR, Maier SF. Escapable and inescapable stress differentially alter extracellular levels of 5-HT in the basolateral amygdala of the rat. Brain Res. 1998;812:113–120. doi: 10.1016/s0006-8993(98)00960-3. [DOI] [PubMed] [Google Scholar]
- Bakker JM, van Bel F, Heijnen CJ. Neonatal glucocorticoids and the developing brain: short-term treatment with life-long consequences? Trends Neurosci. 2001;24:649–653. doi: 10.1016/s0166-2236(00)01948-2. [DOI] [PubMed] [Google Scholar]
- Bilbo SD, Biedenkapp JC, Der-Avakian A, Watkins LR, Rudy JW, Maier SF. Neonatal infection-induced memory impairment after lipopolysaccharide in adulthood is prevented via caspase-1 inhibition. J Neurosci. 2005a;25:8000–8009. doi: 10.1523/JNEUROSCI.1748-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilbo SD, Levkoff LH, Mahoney JH, Watkins LR, Rudy JW, Maier SF. Neonatal infection induces memory impairments following an immune challenge in adulthood. Behav Neurosci. 2005b;119:293–301. doi: 10.1037/0735-7044.119.1.293. [DOI] [PubMed] [Google Scholar]
- Bilbo SD, Rudy JW, Watkins LR, Maier SF. A behavioral characterization of neonatal infection-facilitated cognitive impairment in adult rats. Beh Brain Res. 2006;169:39–47. doi: 10.1016/j.bbr.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Bilbo SD, Newsum NJ, Sprunger DB, Watkins LR, Rudy JW, Maier SF. Differential effects of neonatal handling on early life infection-induced alterations in cognition in adulthood. Brain Behav Immun. 2007;21:332–342. doi: 10.1016/j.bbi.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Breivik T, Stephan M, Brabant GE, Straub RH, Pabst R, von Horsten S. Postnatal lipopolysaccharide-induced illness predisposes to periodontal disease in adulthood. Brain Behav Immun. 2002;16:421–438. doi: 10.1006/brbi.2001.0642. [DOI] [PubMed] [Google Scholar]
- Brewin CR, Andrews B, Valentine JD. Meta-analysis of risk factors for posttraumatic stress disorder in trauma-exposed adults. J Consult Clin Psychol. 2000;68:748–766. doi: 10.1037//0022-006x.68.5.748. [DOI] [PubMed] [Google Scholar]
- Dantzer R, Wollman E, Vitkovic L, Yirmiya R. Cytokines and depression: fortuitous or causative association? Mol Psychiatry. 1999;4:328–332. doi: 10.1038/sj.mp.4000572. [DOI] [PubMed] [Google Scholar]
- Diehl LA, Silveira PP, Leite MC, Crema LM, Portella AK, Billodre MN, Nunes E, Henriques TP, Fidelix-da-Silva LB, Heis MD, Goncalves CA, Quillfeldt JA, Dalmaz C. Long lasting sex-specific effects upon behavior and S100b levels after maternal separation and exposure to a model of post-traumatic stress disorder in rats. Brain Res. 2007;1144:107–116. doi: 10.1016/j.brainres.2007.01.084. [DOI] [PubMed] [Google Scholar]
- del Rey A, Furukawa H, Monge-Arditi G, Kabiersch A, Voight KH, Besedovsky HO. Alterations in the pitutary-adrenal axis of adult mice following neonatal exposure to interleukin-1. Brain Behav Immun. 1996;10:235–248. doi: 10.1006/brbi.1996.0021. [DOI] [PubMed] [Google Scholar]
- Dobbing J, Sands J. Comparative aspects of the brain growth spurt. Early Hum Dev. 1979;3:79–83. doi: 10.1016/0378-3782(79)90022-7. [DOI] [PubMed] [Google Scholar]
- Exton MS. Infection-induced anorexia: active host defence strategy. Appetite. 1997;29:369–383. doi: 10.1006/appe.1997.0116. [DOI] [PubMed] [Google Scholar]
- Gareau MG, Jury J, Macqueen G, Sherman PM, Perdue MH. Probiotic treatment of rat pups normalizes corticosterone release and ameliorates colonic dysfunction induced by maternal separation. Gut. 2007 doi: 10.1136/gut.2006.117176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnier Y, Coumans AB, Jensen A, Hasaart TH, Berger R. Infection-related perinatal brain injury: the pathogenic role of impaired fetal cardiovascular control. J Soc Gynecol Investig. 2003;10:450–459. doi: 10.1016/s1071-5576(03)00150-3. [DOI] [PubMed] [Google Scholar]
- Glassman AH, Miller GE. Where There Is Depression, There Is InflammationSometimes! Biol Psychiatry. 2007;62:280–281. doi: 10.1016/j.biopsych.2007.05.032. [DOI] [PubMed] [Google Scholar]
- Goshen I, Kreisel T, Ben-Menachem-Zidon O, Licht T, Weidenfeld J, Ben-Hur T, Yirmiya R. Brain interleukin-1 mediates chronic stress-induced depression in mice via adrenocortical activation and hippocampal neurogenesis suppression. Mol Psychiatry. 2007 doi: 10.1038/sj.mp.4002055. [DOI] [PubMed] [Google Scholar]
- Granger DA, Hood KE, Ikeda SC, Reed CL, Block ML. Neonatal endotoxin exposure alters the development of social behavior and the hypothalamic-pituitary-adrenal axis in selectively bred mice. Brain Behav Immun. 1996;10:249–259. doi: 10.1006/brbi.1996.0022. [DOI] [PubMed] [Google Scholar]
- Hart BL. Biological basis of the behavior of sick animals. Neurosci Biobehav Rev. 1988;12:123–137. doi: 10.1016/s0149-7634(88)80004-6. [DOI] [PubMed] [Google Scholar]
- Hayley S, Poulter MO, Merali Z, Anisman H. The pathogenesis of clinical depression: stressor- and cytokine-induced alterations of neuroplasticity. Neuroscience. 2005;135:659–678. doi: 10.1016/j.neuroscience.2005.03.051. [DOI] [PubMed] [Google Scholar]
- Heim C, Nemeroff CB. The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biol Psychiatry. 2001;49:1023–1039. doi: 10.1016/s0006-3223(01)01157-x. [DOI] [PubMed] [Google Scholar]
- Hodgson DM, Knott B, Walker FR. Neonatal endotoxin exposure influences HPA responsivity and impairs tumor immunity in Fischer 344 rats in adulthood. Pediatr Res. 2001;50:750–755. doi: 10.1203/00006450-200112000-00020. [DOI] [PubMed] [Google Scholar]
- Hornig M, Weissenbock H, Horscroft N, Lipkin WI. An infection-based model of neurodevelopmental damage. Proc Natl Acad Sci U S A. 1999;96:12102–12107. doi: 10.1073/pnas.96.21.12102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin MR, Miller AH. Depressive disorders and immunity: 20 years of progress and discovery. Brain Behav Immun. 2007;21:374–383. doi: 10.1016/j.bbi.2007.01.010. [DOI] [PubMed] [Google Scholar]
- Johnson JD, O’Connor KA, Deak T, Stark M, Watkins LR, Maier SF. Prior stressor exposure sensitizes LPS-induced cytokine production. Brain Behav Immun. 2002;16:461–476. doi: 10.1006/brbi.2001.0638. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Magee WJ. Childhood adversities and adult depression: basic patterns of association in a US national survey. Psychol Med. 1993;23:679–690. doi: 10.1017/s0033291700025460. [DOI] [PubMed] [Google Scholar]
- Ladd CO, Huot RL, Thrivikraman KV, Nemeroff CB, Meaney MJ, Plotsky PM. Long-term behavioral and neuroendocrine adaptations to adverse early experience. Prog Brain Res. 2000;122:81–103. doi: 10.1016/s0079-6123(08)62132-9. [DOI] [PubMed] [Google Scholar]
- Lee JH, Kim HJ, Kim JG, Ryu V, Kim BT, Kang DW, Jahng JW. Depressive behaviors and decreased expression of serotonin reuptake transporter in rats that experienced neonatal maternal separation. Neurosci Res. 2007;58:32–39. doi: 10.1016/j.neures.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Leonard BE. The HPA and immune axes in stress: the involvement of the serotonergic system. Eur Psychiatry. 2005;20(Suppl 3):S302–306. doi: 10.1016/s0924-9338(05)80180-4. [DOI] [PubMed] [Google Scholar]
- Lippmann M, Bress A, Nemeroff CB, Plotsky PM, Monteggia LM. Long-term behavioural and molecular alterations associated with maternal separation in rats. Eur J Neurosci. 2007;25:3091–3098. doi: 10.1111/j.1460-9568.2007.05522.x. [DOI] [PubMed] [Google Scholar]
- Lowry CA, Hollis JH, de Vries A, Pan B, Brunet LR, Hunt JR, Paton JF, van Kampen E, Knight DM, Evans AK, Rook GA, Lightman SL. Identification of an immune-responsive mesolimbocortical serotonergic system: potential role in regulation of emotional behavior. Neuroscience. 2007;146:756–772. doi: 10.1016/j.neuroscience.2007.01.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier SF, Watkins LR. Stressor controllability and learned helplessness: the roles of the dorsal raphe nucleus, serotonin, and corticotropin-releasing factor. Neurosci Biobehav Rev. 2005;29:829–841. doi: 10.1016/j.neubiorev.2005.03.021. [DOI] [PubMed] [Google Scholar]
- Newton ER. Chorioamnionitis and intraamniotic infection. Clin Obstet Gynecol. 1993;36:795–808. doi: 10.1097/00003081-199312000-00004. [DOI] [PubMed] [Google Scholar]
- Nguyen KT, Deak T, Owens SM, Kohno T, Fleshner M, Watkins LR, Maier SF. Exposure to acute stress induces brain interleukin-1beta protein in the rat. J Neurosci. 1998;18:2239–2246. doi: 10.1523/JNEUROSCI.18-06-02239.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson C, Jennische E, Ho HP, Eriksson E, Bjorntorp P, Holmang A. Postnatal endotoxin exposure results in increased insulin sensitivity and altered activity of neuroendocrine axes in adult female rats. Eur J Endocrinol. 2002;146:251–260. doi: 10.1530/eje.0.1460251. [DOI] [PubMed] [Google Scholar]
- Owen D, Andrews MH, Matthews SG. Maternal adversity, glucocorticoids and programming of neuroendocrine function and behaviour. Neurosci Biobehav Rev. 2005;29:209–226. doi: 10.1016/j.neubiorev.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Pollak Y, Yirmiya R. Cytokine-induced changes in mood and behaviour: implications for ‘depression due to a general medical condition’, immunotherapy and antidepressive treatment. Int J Neuropsychopharmacol. 2002;5:389–399. doi: 10.1017/S1461145702003152. [DOI] [PubMed] [Google Scholar]
- Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol. 2006;27:24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rantakallio P, Jones P, Moring J, Von Wendt L. Association between central nervous system infections during childhood and adult onset schizophrenia and other psychoses: a 28-year follow-up. Int J Epidemiol. 1997;26:837–843. doi: 10.1093/ije/26.4.837. [DOI] [PubMed] [Google Scholar]
- Rodier PM. Chronology of neuron development: animal studies and their clinical implications. Dev Med Child Neurol. 1980;22:525–545. doi: 10.1111/j.1469-8749.1980.tb04363.x. [DOI] [PubMed] [Google Scholar]
- Schiepers OJ, Wichers MC, Maes M. Cytokines and major depression. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:201–217. doi: 10.1016/j.pnpbp.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Shanks N, Windle RJ, Perks PA, Harbuz MS, Jessop DS, Ingram CD, Lightman SL. Early-life exposure to endotoxin alters hypothalamic-pituitary-adrenal function and predisposition to inflammation. Proc Natl Acad Sci U S A. 2000;97:5645–5650. doi: 10.1073/pnas.090571897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L, Fatemi SH, Sidwell RW, Patterson PH. Maternal influenza infection causes marked behavioral and pharmacological changes in the offspring. J Neurosci. 2003;23:297–302. doi: 10.1523/JNEUROSCI.23-01-00297.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer SJ, Martin S, Mouihate A, Pittman QJ. Early-life immune challenge: defining a critical window for effects on adult responses to immune challenge. Neuropsychopharmacology. 2006;31:1910–1918. doi: 10.1038/sj.npp.1301004. [DOI] [PubMed] [Google Scholar]
- Viau V, Sharma S, Meaney MJ. Changes in plasma adrenocorticotropin, corticosterone, corticosteroid-binding globulin, and hippocampal glucocorticoid receptor occupancy/translocation in rat pups in response to stress. J Neuroendocrinol. 1996;8:1–8. doi: 10.1111/j.1365-2826.1996.tb00680.x. [DOI] [PubMed] [Google Scholar]
- Walker FR, Brogan A, Smith R, Hodgson DM. A profile of the immediate endocrine, metabolic and behavioural responses following a dual exposure to endotoxin in early life. Physiol Behav. 2004a;83:495–504. doi: 10.1016/j.physbeh.2004.08.030. [DOI] [PubMed] [Google Scholar]
- Walker FR, March J, Hodgson DM. Endotoxin exposure in early life alters the development of anxiety-like behaviour in the Fischer 344 rat. Behav Brain Res. 2004b;154:63–69. doi: 10.1016/j.bbr.2004.01.019. [DOI] [PubMed] [Google Scholar]
- Whooley MA, Caska CM, Hendrickson BE, Rourke MA, Ho J, Ali S. Depression and inflammation in patients with coronary heart disease: findings from the heart and soul study. Biol Psychiatry. 2007;62:314–320. doi: 10.1016/j.biopsych.2006.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willner P. Chronic mild stress (CMS) revisited: consistency and behavioural-neurobiological concordance in the effects of CMS. Neuropsychobiology. 2005;52:90–110. doi: 10.1159/000087097. [DOI] [PubMed] [Google Scholar]
- Yirmiya R. Endotoxin produces a depressive-like episode in rats. Brain Res. 1996;711:163–174. doi: 10.1016/0006-8993(95)01415-2. [DOI] [PubMed] [Google Scholar]
- Zuckerman L, Weiner I. Post-pubertal emergence of disrupted latent inhibition following prenatal immune activation. Psychopharmacology (Berl) 2003;169:308–313. doi: 10.1007/s00213-003-1461-7. [DOI] [PubMed] [Google Scholar]