SUMMARY
The folding of group II intron ribozymes has been studied extensively under optimal conditions for self-splicing in vitro (42 °C and high magnesium ion concentrations). In these cases, the ribozymes fold directly to the native state by an apparent two-state mechanism involving the formation of an obligate intermediate within intron Domain 1. We have now characterized the folding pathway under near-physiological conditions. We observe that compaction of the RNA proceeds slowly to completion, even at low magnesium (3 mM). Kinetic analysis shows that this compact species is a “near-native” intermediate state that is readily chased into the native state by the addition of high salt. Structural probing reveals that the “near-native” state represents a compact Domain 1 scaffold that is not yet docked with the catalytic domains (D3 and D5). Interestingly, native ribozyme reverts to the “near-native” state upon reduction in magnesium concentration. Therefore, while the intron can sustain the intermediate state under physiological conditions, the native structure is not maintained and is likely to require stabilization by protein cofactors in vivo.
Keywords: ribozyme, RNA folding, kinetics, mechanism, RNA structure
INTRODUCTION
Recent studies have demonstrated that RNA molecules follow a variety of folding pathways on their way to the native conformation 1. Some large RNAs like the Tetrahymena ribozyme or the B. Subtilis RNase P RNA proceed along a “rugged” folding pathway, forming stable, misfolded intermediates 1; 2; 3. These RNAs often fold slowly, with a rate-limiting step that is dominated by escape from a kinetic trap 2. Other ribozymes follow a smooth folding pathway that is devoid of kinetic traps. These molecules tend to fold rapidly and follow an apparent two-state folding pathway 1. Ribozymes derived from group II introns do not behave like molecules in either of these categories: They fold slowly, via an apparent two-state mechanism that involves formation of obligate, transient, intermediates 4; 5. At the temperature optimum for self-splicing activity in vitro (42°C), molecular collapse (i.e. formation of the compact state) and native state formation by group II ribozymes requires unusually high magnesium ion concentrations (100 mM) 5. Because of the unique folding paradigm that they provide, group II ribozymes represent an important model system for studying RNA folding.
Folding of group II introns has been extensively studied using a ribozyme derivative of intron ai5γ from S. cerevisiae mitochondria 5; 6; 7. This construct (D135) contains all the intronic domains that are important for reactivity (i.e. D1, D3 and D5 8; 9; 10). It is a highly active ribozyme that efficiently cleaves RNA oligonucleotides with multiple turnover 6; 7 and it was designed to catalyze only one reaction, which reduces the possibility of conformational rearrangements in the molecule. Indeed, ~ 90% of D135 molecules fold homogeneously to a uniform, native state that is catalytically active 6.
Folding studies on D135 have typically been carried out under conditions that were optimized for maximum catalytic activity in vitro: i.e. elevated temperature (42 °C) and high concentrations of monovalent and divalent cations (typically 500 mM monovalent and 100 mM MgCl2). While these studies have provided important insights into the ai5γ folding pathway, little is actually known about intron folding under conditions that are approximately physiological. The ai5γ intron is found within an essential cytochrome oxidase subunit gene, and given this context, it must undergo efficient self-splicing at the temperature of yeast growth (30 °C) in physiological concentrations of monovalent ions and magnesium in order to ensure survival of the organism.
It has been established that in vivo splicing of the ai5γ intron is assisted by various protein co-factors 11; 12. However, it is unclear how these factors contribute to splicing efficiency. It has been reported that the Neurospora CYT-19 protein facilitates ai5γ splicing in vitro under near-physiological conditions 11. Since CYT-19 is a DEAD-box protein that requires ATP hydrolysis for function, it has been hypothesized that it stimulates splicing by acting as an RNA helicase that relieves kinetic traps in the folding pathway 11. This would suggest that the intron folding pathway under physiological conditions is rugged and that it differs from the smooth pathway that was characterized at elevated temperatures and high salt concentrations, and for which kinetic traps are not operative. Given the lack of information on ai5γ folding under physiological conditions, it is difficult to evaluate this hypothesis.
An analysis of the ai5γ folding pathway under physiological conditions would accomplish several objectives: it would broaden the useful paradigm that is provided by group II intron folding, it would reveal features of the mechanism that are unique to intron folding at low temperature, it would reveal aspects of the mechanism that could be influenced by protein cofactors, and it would provide an opportunity to compare RNA tertiary folding pathways under drastically different reaction conditions.
We have therefore characterized the folding pathway of an intron-derived ribozyme at the temperature of yeast growth, under a range of ionic conditions that approximate physiological. Using a combination of native gel electrophoresis, kinetic analysis and DMS structural probing, we have found that the ribozyme collapses very slowly to a stable, compact, on-pathway intermediate that is readily chased to the native state upon addition of high concentrations of magnesium ions. Through DMS structural probing, we have defined the basic structural features of the intermediate and we observe important differences from the native state. We show that native ribozyme partially unfolds to the intermediate state upon reduction in magnesium ion concentrations, indicating that ai5γ cannot maintain the native structure at physiological magnesium and is likely to require stabilization by protein co-factors in vivo. Finally, our results suggest that formation of the compact intermediate is under kinetic control and that a large energy barrier separates this obligate intermediate from the unfolded state of the intron.
RESULTS
Experimental Design
Because a major objective of this study is to characterize folding of a group IIb intron under near-physiological conditions, it was desirable to utilize an intron construct that was relatively intact. This study therefore investigated the folding pathway of construct D1356, which contains all domains that are implicated in function of the ai5γ group II intron (Figure 1). The only difference between this construct and the D135 ribozyme, which was previously used for folding studies 5; 6; 7, is the presence of Domain 6 (D6). The inclusion of this domain is particularly important in light of the recent discovery of the D6 docking site in the coordination loop of D1 13. Ultimately, the presence of D6 was not observed to influence the folding behavior of D1356 relative to D135 (compare Figure 2 with data from 5).
Figure 1.
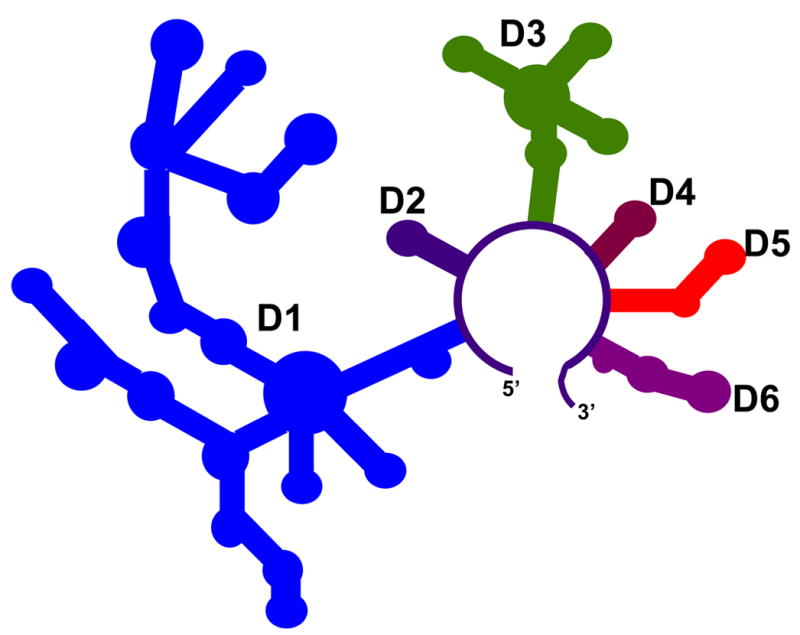
A schematic of the D1356 ribozyme construct derived from the ai5γ group II intron.
Figure 2.

Comparison of global compaction of the D1356 ribozyme at different magnesium concentrations after 20 minute incubation at 42 and 30 °C. ME, sample incubated in 10 mM MOPS, pH 6.0, 1 mM EDTA at 95 °C for 1 min and used as a control for the mobility of unfolded species.
Thermodynamic and biophysical characteristics of folding intermediates and of the native state were monitored using native gel electrophoresis, as described previously. Structural attributes of intermediate and native states were examined using DMS footprinting followed by reverse-transcription, as described in Methods, and their catalytic activity was assessed by kinetic analysis under multiple-turnover conditions.
Formation of the compact state: Different behaviors at 30°C and 42°C
Given that ai5γ is a yeast intron, it was important to monitor the folding of D1356 at the optimum temperature of yeast growth (30°C) and to compare it with the folding behavior at 42°C, which is the condition for previous folding studies and for optimal activity in vitro. Under the typical high-salt conditions used for previous studies (i.e. 100 mM Mg2+, 80 mM MOPS-potassium buffer, 20 minute incubation prior to loading), D1356 readily compacts and then folds to the native state at 42°C (Figure 2). Under the same conditions at 30°C, however, only half of the population has compacted.
The temperature dependence of compaction becomes particularly significant when one performs the experiments over a broad range of Mg2+ concentrations (Figure 2). Paradoxically, at 30°C, a small fraction of compact molecules is readily detectable at magnesium concentrations as low as 2 mM. Under this same condition at 42 °C, the sample is completely unfolded. This indicates that folding can occur at physiological temperatures, particularly at Mg2+ concentrations that are also physiological. At higher temperatures, where the molecule is presumably breathing more, additional Mg2+ is required to facilitate compaction.
The relatively small amount of compaction that is observed at 30°C could be due to several factors: A. The majority of the ribozyme population might be trapped irreversibly in a misfolded conformation. B. Ribozyme compaction at 30 °C does not reach equilibrium after 20 minutes of incubation. To differentiate these models, we monitored the magnesium-dependent compaction of D1356 as a function of time at 30 °C. Remarkably, ribozyme compaction ultimately proceeds to completion even at magnesium concentrations as low as 3 mM (Figure 3 (a), (b)). However, this process is 400 times slower than D1356 compaction at 42 °C and 100 mM MgCl2 5; 7 (0.0025 min−1, Table 1). This indicates that the rate constant for compaction has simply become extremely slow at lower temperature.
Figure 3.
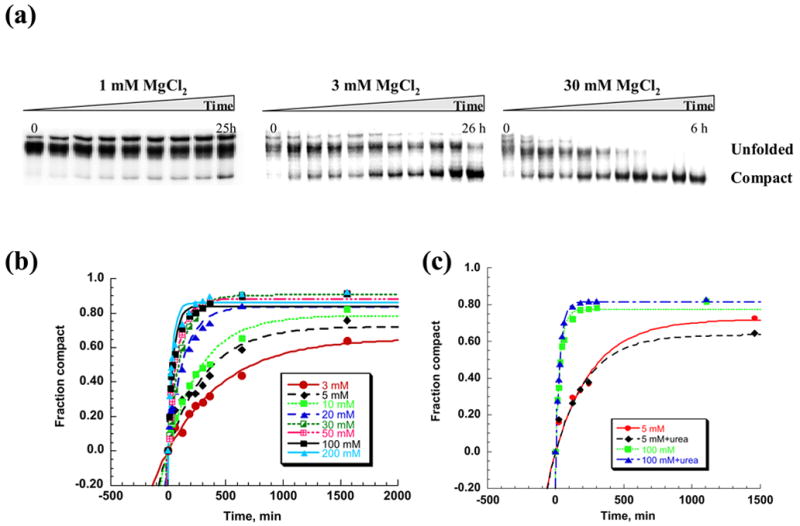
A time course of the D1356 ribozyme compaction at 30 °C. Native gel electrophoresis was used to monitor compaction over time (a). The fraction of compact population was plotted vs time and fit to a first order kinetic equation to calculate rate constants for compaction at different magnesium concentrations (b). (c) Comparison of the compaction time courses in the presence and in the absence of subdenaturing concentrations of urea at 5 mM and 100 mM MgCl2. The rate constants for compaction at 5 mM MgCl2 were 0.004±0.001 min−1 and 0.005±0.002 min−1 in the absence and presence of 0.5 M urea, respectively. The rate constants for compaction at 100 mM MgCl2 were 0.033±0.002 min−1 and 0.032±0.001 min−1 in the absence and presence of 1 M urea, respectively.
Table 1.
Compaction rates for the D1356 ribozyme at different magnesium concentrations. The error in determining the rate of compaction did not exceed 20%.
| [Mg2+], mM | 3 | 5 | 10 | 20 | 30 | 50 | 100 | 200 |
| (k*102), min−1 | 0.25 | 0.36 | 0.38 | 0.73 | 0.88 | 1.2 | 3.3 | 3.7 |
As magnesium concentration is increased at 30°C, the rate constant for global compaction of D1356 increases dramatically (Figure 3 (a), (b)), Table 1). This behavior is very different from that observed at 42 °C, where full compaction cannot be achieved at such low magnesium concentrations, at any timescale.
To examine whether the slow rate constant for D1356 collapse under physiological conditions is attributable to a kinetic trap (i.e. escape from a stable, misfolded intermediate), experiments were repeated in the presence of subdenaturing concentrations of urea. Urea had no effects on the rate constant for collapse at both high and low concentrations of Mg2+ (Figure 3 (c)), suggesting that the rate constant for compaction is not limited by a kinetic trap and that stable alternative structures do not obstruct the folding pathway. As hypothesized previously for D135 folding at higher temperatures, these results are consistent with a model in which D1356 folds via a direct pathway to the compact state under conditions that approximate physiological (i.e. 30°C and low Mg2+). However, given the slow rate constant for intron collapse, the results also underscore the need for protein co-factors to facilitate collapse in vivo. These proteins would act by catalyzing formation of intermediate structures rather than by unwinding misfolded conformations.
Catalytic activity of the collapsed species
We have established that one of the major features of global collapse of D1356 at 30°C is that the molecule compacts slowly, to completion, at magnesium concentrations more than 30 times lower than required for the native state formation. Perhaps more fundamental, the data demonstrate that D1356 forms a collapsed state at 30°C that is as compact as that previously observed for the native-state 5. However, the reaction conditions employed here are completely novel for the study of ai5γ ribozymes and we know little about the compact state that is formed under these conditions. The compact species could represent a native-like molecule, or it could be completely unreactive, irrespective of the folding mechanism.
To address whether the collapsed species has some form of catalytic activity, we measured rate constants for RNA cleavage by compacted D1356 molecules at 30°C. D1356 was allowed to completely collapse at 3, 5, 10, 20 or 100 mM MgCl2 (at 30°C) and then reacted with the substrate under multiple turnover conditions (i.e. conditions highly sensitive to the state of the enzyme). In order to ensure that chemical catalysis was fast and folding was rate limiting, reactions were performed at pH 8.0. Remarkably high levels of activity were observed, even at 5 mM MgCl2. In that case, the rate constant for reaction was only 15-fold lower than that for native ribozymes at 30 °C and 100 mM MgCl2. Activity increases as a function of Mg2+ such that, at 20 mM MgCl2, the rate constant was 3 times lower than that at 100 mM MgCl2 (Figure 4). These data clearly show that collapsed species formed at low Mg2+ and temperatures has the potential for reactivity and they suggest that the compact state represents some form of intermediate.
Figure 4.
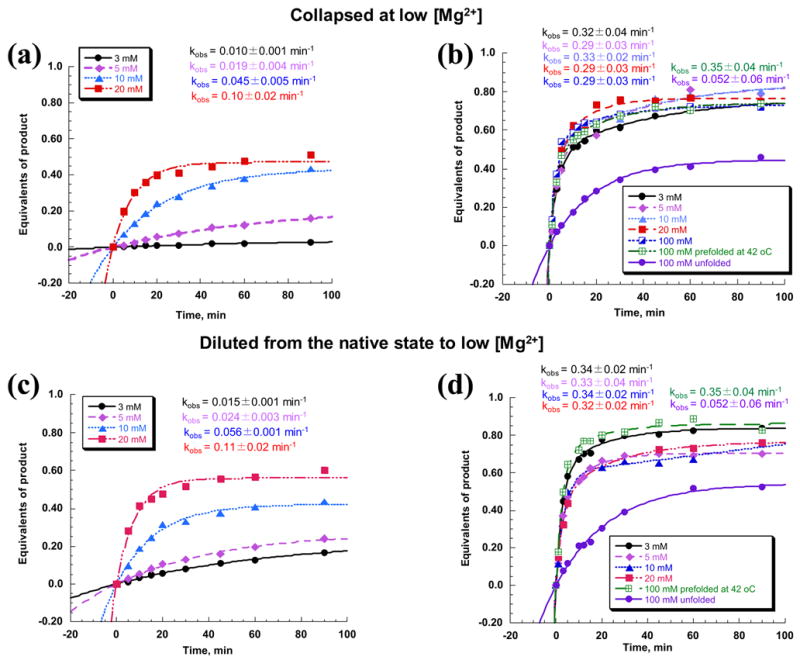
Multiple turnover kinetic analysis of RNA oligonucleotide substrate cleavage by the D1356 ribozyme. The ribozyme was either preincubated to full compaction at 30 °C and 3, 5, 10 or 20 mM MgCl2 ((a), (b)) or prefolded to a native state at 100 mM MgCl2 and 42 °C and then diluted to the same magnesium concentrations as above ((c), (d)). All reactions were carried out at 30 °C and either 3, 5, 10 or 20 mM MgCl2, respectively, ((a), (c)) or at 100 mM MgCl2 ((b), (d)). Unfolded and native ribozyme samples were used as controls for comparison of rate constants ((b), (d)).
However, it was still unclear whether this species represent a completely misfolded intermediate that must refold to become fully active, or whether it is a native-like obligate intermediate that forms en route to the native state. Given the experimental setup employed here (i.e. multiple-turnover at pH 8.0), if the compact species is an on-pathway intermediate, it will already contain some structural elements of the native state. If these molecules are subjected to a pulse of high Mg2+ concentration (100 mM), which enables the ribozyme to reach the native conformation, a partially-folded obligate intermediate is likely to have a structural advantage over a completely misfolded (non-native) or unfolded (denatured) ribozyme, and should react much faster with substrate. A completely misfolded intermediate might be expected to react more slowly, with a rate constant that reflects conformational change to the native state. Importantly, in the kinetic assay we employed here, the reaction amplitude for the first turnover of substrate reflects the population of active ribozymes. Therefore, the results will reflect whether the molecules migrating as the compact state represent a mixture of conformational populations. If this is the case, the amplitude of the first turnover will be lower than that of the native ribozyme species.
When D1356 was allowed to collapse to the compact state at 3, 5, 10 or 20 mM MgCl2, and then reacted with a 10-fold excess of substrate at 100 mM MgCl2 and pH 8.0, all of the D1356 samples reacted with the same rate constant, burst magnitude and amplitude as the native species (Figure 4 (b)). These data suggest that the compact species being characterized in this study represents a homogeneous, on-pathway intermediate along the folding pathway. In addition, since the reaction rate constants were identical to those of the native ribozyme and not lower, the transition from the intermediate to the native state is shown to occur very quickly, as previously proposed for folding at 42°C.
Thermodynamic stability of the compact intermediate
In order to better understand the thermodynamic stability of the compact intermediate state and to compare it with the native state, we employed urea titrations and determined the free energy for unfolding at 30°C. D1356 was allowed to fully collapse at various magnesium concentrations and these samples were then incubated with increasing concentrations of urea. Global unfolding as a function of urea was monitored by native gel electrophoresis (Figure 5 (a), (b)). The free energy of unfolding for samples collapsed at 3, 5 and 10 mM MgCl2 was 1.7–1.9 kcal/mol, whereas at 20 mM MgCl2 it increased to 2.8 kcal/mol. Notably, this latter value is within range of free energy values for the formation of different regions of the native state at 42 °C (2.4–3.5 kcal/mol) 4.
Figure 5.
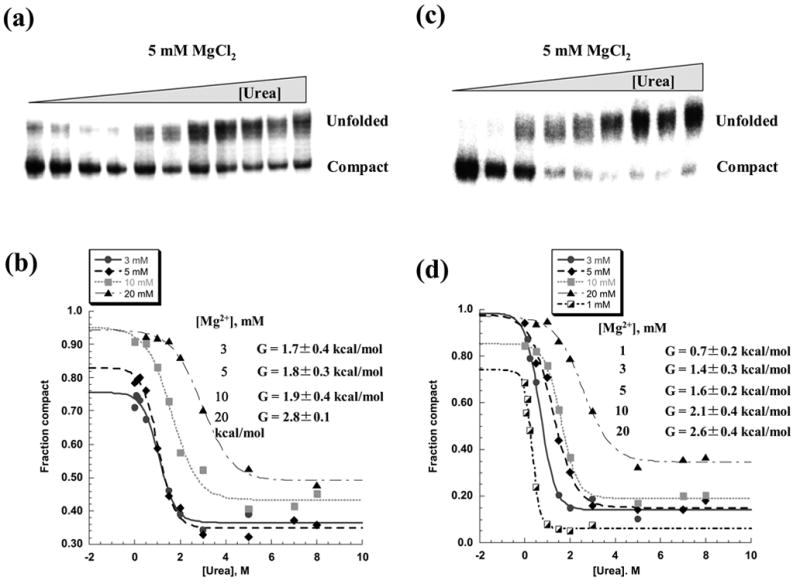
Unfolding of the compact intermediate in the presence of increasing concentrations of urea. Representative native gels showing urea-induced unfolding of the intermediate, which was either fully compacted at 30 °C and 5 mM MgCl2 (a) or diluted to the same concentration of magnesium from the native state (c). The fraction of compact population was plotted vs urea concentration and analyzed as previously described 4 in order to determine free energy of unfolding at different magnesium concentrations for compact species formed either after slow compaction at 30 °C (b) or after dilution from the native state (d).
The similarity in free energy of the intermediate and native states suggests that the structure of the collapsed state is reasonably close to that of the native state. After all, the highest Δ G° for the intermediate (i.e. 2.8 kcal/mol) is the lower limit of that reported for unfolding of individual intronic regions from the native state 4. Differences in thermodynamic stability of the intermediate and native states are probably due to the presence of additional stabilizing tertiary interactions in native state.
Structural probing of the folding intermediate
The thermodynamic analysis indicates that the compact intermediate does not contain all the structural elements that are present in the native state of D1356. The fact that we can isolate and study both the intermediate and the native state provides us with a unique opportunity to compare their structural features. To this end, we examined the structure of the compact species by analyzing accessibility of adenosine N1 residues to DMS at different magnesium concentrations. In this assay, adenosines that are involved in W-C base-pairs or in certain types of tertiary interactions will be protected from DMS modification.
DMS probing of the compact state was conducted in comparison with unfolded and native states. Analysis of the DMS protection pattern at increasing magnesium concentrations revealed a striking hierarchy of interactions that depend on the folded state of the intron. In the “unfolded state”, which contains potassium (80 mM from the buffer) but no magnesium, a large set of adenosine residues are protected, indicating that certain regions of secondary structure are stabilized under this condition at 30°C. This group of structures includes, for example, elements of the i stem and d-d”’ in D1 (Figure 6 (e), gray shading).
Figure 6.
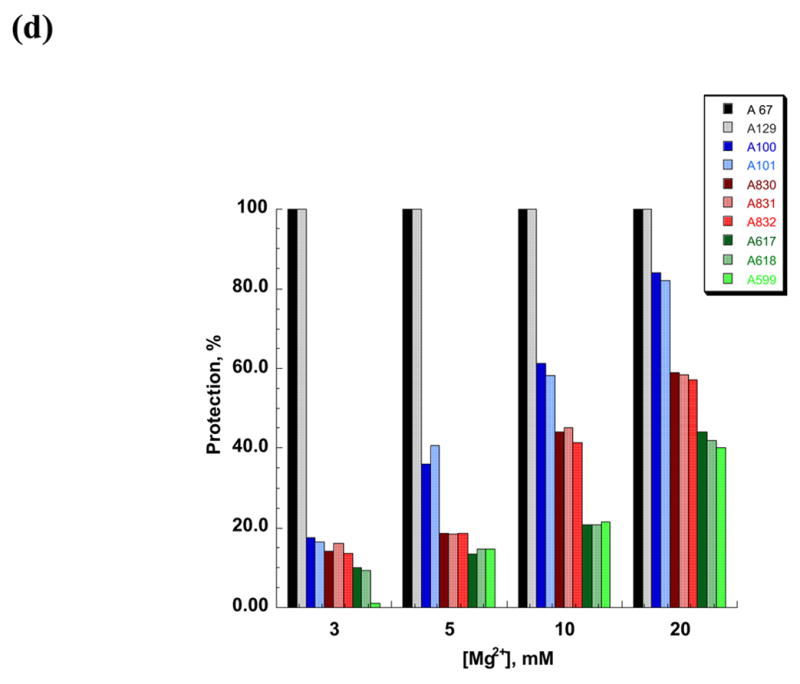
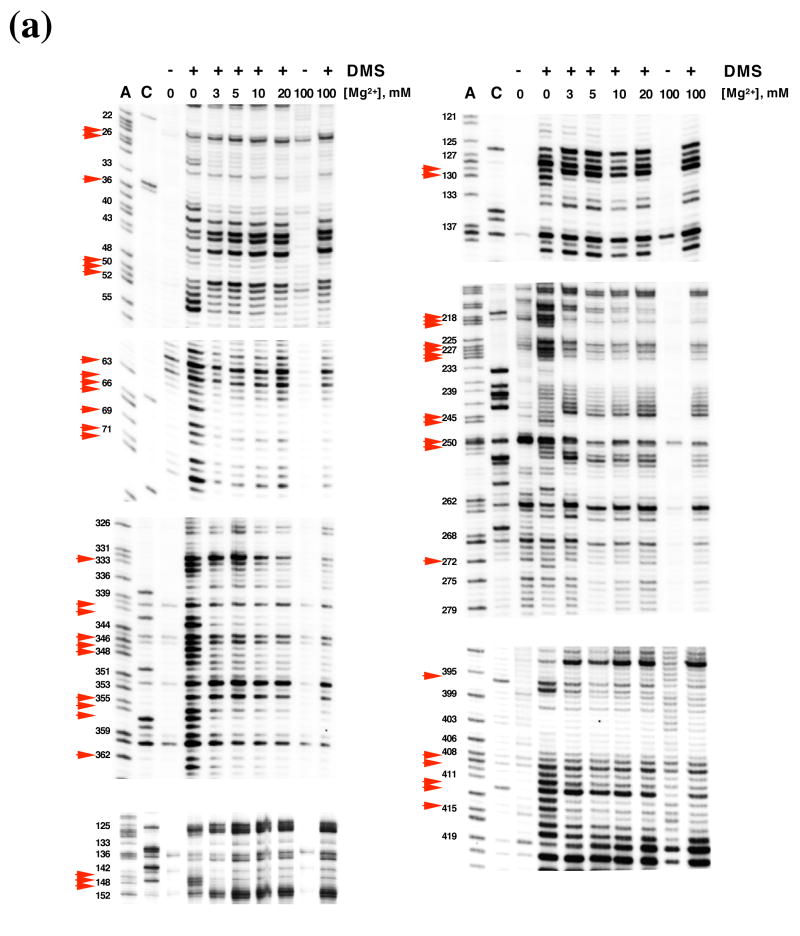
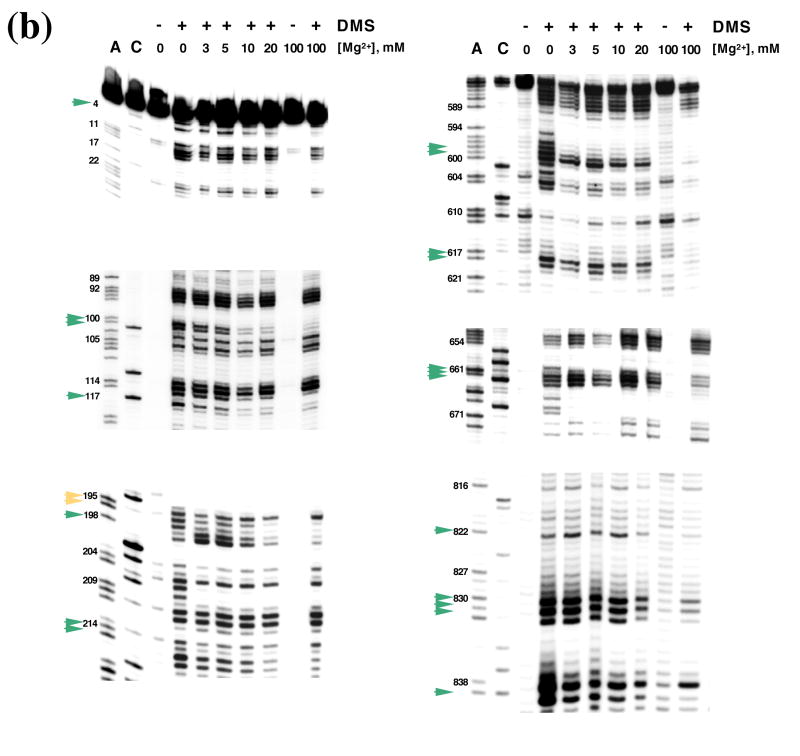
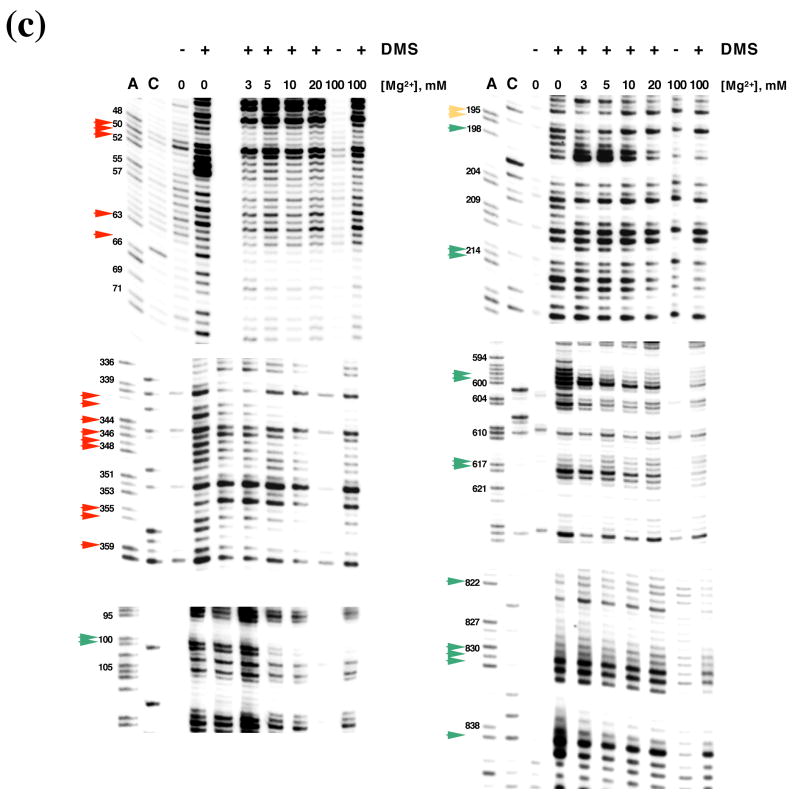
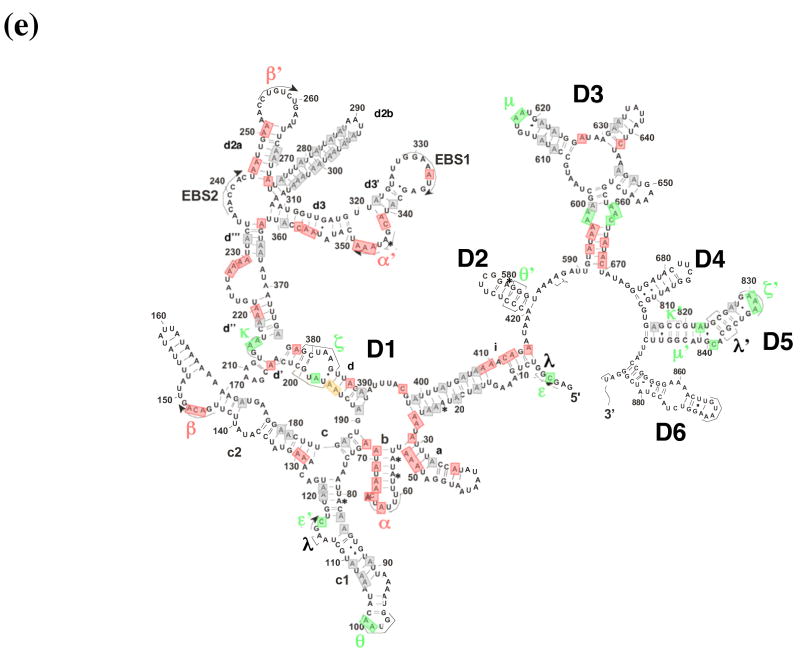
DMS structural probing of the compact folding intermediate. (a) Representative sequencing gels showing A and C positions protected against DMS modification already at 3 mM MgCl2 (red arrows). Prior to DMS treatment, samples were incubated at indicated magnesium concentrations at 30 °C until fully compact. (b) Representative sequencing gels showing A and C residues that gradually become protected against DMS at magnesium concentrations higher than 3 mM (green arrows). Yellow arrows indicate residues that are partially protected at 3 mM MgCl2 and gradually become fully protected at higher magnesium concentrations. Samples were treated as above (see legend to Figure 6 (a)). (c) The structure of the intermediate formed after dilution from the native state to indicated magnesium concentrations is identical to that of the intermediate formed by slow collapse at the same magnesium concentrations (compare to Figure 6 (a), (b)). Representative sequencing gels show A and C positions protected from DMS already at 3 mM MgCl2 (red arrows), gradually becoming protected with increasing magnesium (green arrows) or partially protected at 3 mM MgCl2 and gradually becoming fully protected at higher concentrations of magnesium (yellow arrows). (d) A bar graph showing extent of protection (%) at different magnesium concentration relative to the native state (100 %) for representative A and C positions that are fully protected already at 3 mM MgCl2 (shown in black and grey) and regions in D1 (blue), D5 (red) and D3 (green) that gradually become protected upon increasing magnesium. (e) The secondary structure map of the D1356 ribozyme showing regions corresponding to the compact intermediate (near-native state) (red) and regions gradually forming during the transition from the near-native to native state (green). Regions that are partially protected in the near-native state and gradually become fully protected in the native state are shown in yellow.
In the compact intermediate state that has formed at 3 mM Mg2+, DMS footprinting highlights substructures that are required for specific collapse of D1356. Consistent with previous work implicating D1 as a folding scaffold, most of these substructures involve long-range interactions and elements that are critical for the shape of D1. (Figure 6 (a), (e)). For elements in this group, the DMS protection level was essentially the same in the intermediate state as in the final native state (Figure 6 (a), (e) (red shading)). Important substructures that are present in the compact intermediate include the long-range pairings α-α′ and β-β′ and their flanking helices (Figure 6 (a), (e)). In addition, several secondary structure elements that are disordered in an unfolded state become fully formed upon magnesium-induced compaction. These include stem d” (residues 219, 220), d3 (residues 355, 356, 357) and the 3′-part of the i stem (nucleotides 409, 410, 412, 413, 415). Interestingly, single-stranded regions such as the coordination loop (which later serves as the exon and branch-site docking site) 13 also become protected in the compact state (nucleotides 227–230) (Figure 6 (a), (e)). These data suggest that proper folding of some loop regions and/or their interaction with other structural elements may facilitate ribozyme collapse.
When magnesium is increased from 3 mM to100 mM Mg2+, one observes the sequential appearance of DMS footprints that reflect the formation of long-range interactions between domains that compose the catalytic core. (Figure 6 (b), (d), (e) (green shading)). The well-established tertiary interactions that dock D1 with D5 and D3 are fully formed only in the native state. For example, these additional footprints reflect the formation of ε-ε′, θ-θ′, ζ-ζ′, κ-κ′, and μ-μ′, which are elements that contribute to catalytic function. Since these structural elements form after initial compaction, they reflect the transition between the collapsed intermediate and the native conformation.
By comparing the results for the intermediate and native states, it is clear that D5 and D3 are not involved in ribozyme compaction, which is in excellent agreement with previously published observations 5. This underscores the basic structural similarity of intermediates formed at 30 and 42 °C.
Native ribozymes revert to the compact intermediate state at 30°C and low Mg2+
It remains unclear why maximal activity of D1356 requires high Mg2+ at 30°C, despite the fact that the molecule compacts at much lower [Mg2+]. One explanation is that the ribozyme requires high [Mg2+] to undergo a transition from intermediate to native states of the molecule. Alternatively, the native state may simply be unstable at low magnesium concentrations. If high concentration of magnesium is required only for reaching the native state, but not for stabilizing it, then the native structure will be preserved upon diluting out magnesium. If high concentration of magnesium ions is required for maintenance of the native state, the native structure will unfold or revert to the intermediate state upon dilution of magnesium.
To differentiate these models, we evaluated whether the native ribozyme (folded at 42 °C and 100 mM MgCl2) remains compact upon lowering the temperature to 30 °C and diluting magnesium to lower concentrations (0.5–20 mM). Indeed, native D1356 molecules remain strikingly compact upon lowering the temperature and reducing magnesium concentrations as low as 1 mM (Figure 7 (a)). This behavior contrasts sharply with that of control samples that were originally incubated at 42 °C at low magnesium concentrations (i.e. having never reached the native state). In the latter case, compact species were only detectable at 5 mM MgCl2. It is clear that 1 mM MgCl2 is the lowest concentration that still supports a collapsed state upon dilution at 30°C. When Mg2+ is diluted to lower concentrations (≤ 0.5 mM) pre-folded ribozymes revert to the completely unfolded state (Figure 7 (a)). The ability of D1356 to maintain a compact structure at magnesium concentrations that are 100-fold lower than optimal divalent ion requirements for the native state sets it apart from other large ribozymes. For example, pre-folded Tetrahymena group I introns revert to a mixture of unfolded/intermediate conformations when magnesium is diluted by only 10-fold (Figure 7 (b)).
Figure 7.
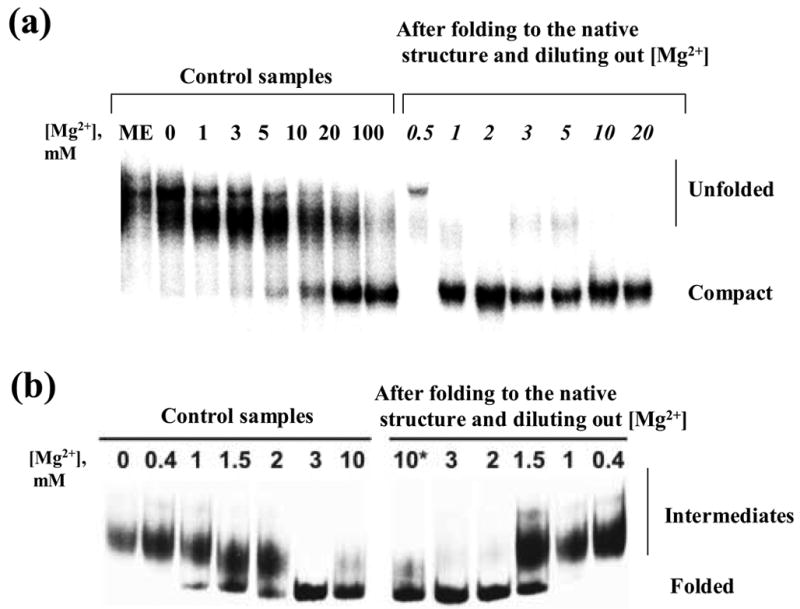
(a) Comparison of global compaction of the D1356 ribozyme folded at various magnesium concentrations at 42 °C (left) with that after folding to the native state at 42 °C and 100 mM MgCl2 followed by dilution to the same magnesium concentrations at 30 °C (right). (b) Comparison of global compaction of the Tetrahymena group I intron upon folding at various magnesium concentrations (left) with that after folding to the native state and dilution to the same magnesium concentrations (right).
To determine whether D1356 maintains the fully-reactive native state after diluting into low magnesium, ribozyme activity was evaluated after prefolding to the native state and diluting to low Mg2+ at 30°C. In this case, we observe that D1356 does does not preserve the native conformation at low [Mg2+]. Activity at 3–20 mM MgCl2 was lower than that of the native ribozyme (Figure 4 (c)), however it was identical to that observed previously for the compact intermediate (compare Figure 4 (a) and Figure 4 (c)). Furthermore, when the same diluted ribozyme samples were reacted with substrate at 100 mM MgCl2, reaction rates and amplitudes were equal to those of the native ribozyme (Figure 4 (d)), which again is identical to behavior of the slowly collapsed intermediate (See Figure 4 (b) for comparison). These results suggest that, upon dilution of magnesium, the native state destabilizes and reverts to the same intermediate structure that forms during slow collapse at low magnesium concentrations. This hypothesis is further supported by analysis of the relative thermodynamic stabilities of the ribozymes in the diluted and native states. Indeed, the free energy of unfolding for ribozyme samples that were prefolded and then diluted to lower magnesium was equal to that of slowly-collapsed ribozyme samples at the same magnesium concentrations (Figure 5). In addition, DMS structural probing of the pre-folded, diluted samples revealed that the protection pattern is identical to that of the collapsed species that formed directly (Figure 6 (c)). Taken together, the data indicate that high magnesium ion concentrations are indeed required to stabilize the native state in vitro, but that the compact intermediate state forms and is stabilized by low concentrations of Mg2+ at 30°C.
DISCUSSION
Slow collapse of a large RNA: An unusual folding paradigm
Extensive studies on the folding of large RNAs have suggested that, during early stages of folding, RNA rapidly collapses to a globular intermediate state that consists of specific or nonspecific structures 14. While this early process requires charge screening by cations, it does not necessarily require magnesium 14. In subsequent steps along the folding pathway, many RNA molecules engage in a slow conformational search for the native state through processes that require magnesium 1; 14. Our studies of D1356 under near-physiological conditions have revealed some similarities with the folding pathways of other ribozymes that do not form kinetic traps: in the presence of low magnesium concentrations, D1356 collapses to a specific, on-pathway intermediate, which is then transformed to the native state at higher magnesium concentrations (Figure 8 (a)). A similar pathway was observed for the Azoarcus ribozyme, which forms a specific compact intermediate at low magnesium and then adopts the native state at higher magnesium concentrations 15.
Figure 8.
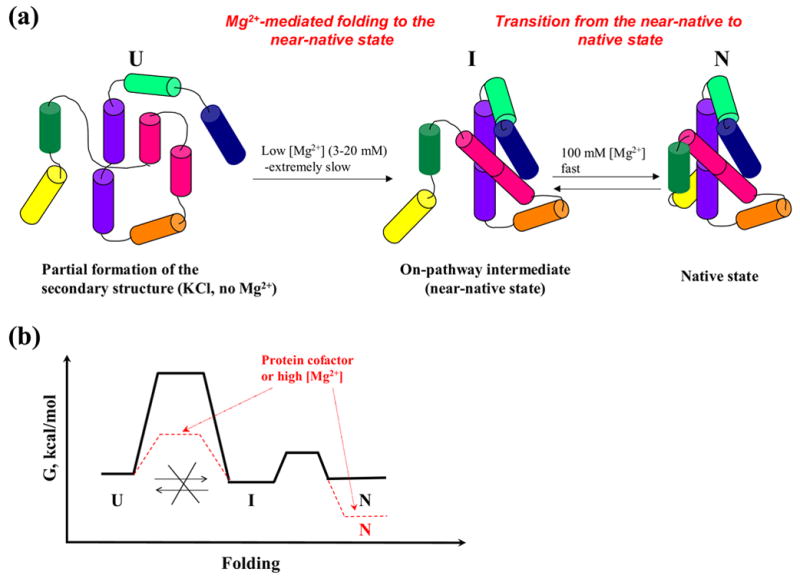
(a) A scheme illustrating folding pathway of the ai5γ-derived ribozyme at 30 °C. (b) A scheme of a hypothetical free energy diagram for the ribozyme folding to the near-native state and native states at low magnesium (solid black lines). Hypothetical changes in free energy landscape upon adding high concentrations of magnesium or protein co-factors are shown in red.
What sets D1356 apart from examples such as Azoarcus is the time scale over which each step occurs: At 3 mM magnesium, complete compaction of D1356 takes 26 hours (Figure 3, 8 (a)). However, subsequent steps to the native structure occur very rapidly when magnesium is increased (Figure 4, 8 (a)). This slow compaction followed by extremely rapid native state formation is the exact opposite of generalized folding pathways that have been proposed for other RNAs 14. Subdenaturing urea concentrations do not increase the rate constant for compaction, suggesting that this slow critical step likely proceeds without formation of kinetic traps. However, the compaction rate constant increases with increasing magnesium concentrations, suggesting that D1356 collapse may involve structural assembly around magnesium ion binding sites. Alternatively, increasing the concentration of delocalized magnesium ions might lower the electrostatic barrier to ribozyme compaction, which would also increase the rate constant for ribozyme collapse.
Although our data strongly support a folding pathway that involves a direct transition from the unfolded state to the collapsed intermediate state, at this stage we cannot completely exclude alternative folding models. For example, a misfolded structure might form and then refold without an increase in the exposed surface area of the transition state relative to the ground state. In such a case, the transition from the unfolded to the intermediate state might be insensitive to subdenaturing urea concentrations16.
The collapsed species represents a near-native state
The data provided herein suggests that the compact species formed at 3–20 mM MgCl2 represents an on-pathway intermediate, rather than the native state. This is based on numerous lines of evidence. First of all, apparent catalytic activity of the compact state is much lower than the native ribozyme that has been folded at 100 mM MgCl2 (Figure 4, (a)). Addition of the subdenaturing concentrations of urea does not accelerate the reaction rate. Rather, it renders the ribozyme inactive at 3 and 5 mM MgCl2 and drastically reduces activity at 10 and 20 mM MgCl2 (data not shown). These findings are inconsistent with formation of an off-pathway intermediate that behaves as a kinetic trap. Structural probing of the compact species at various magnesium concentrations revealed the same structural elements that are present in the native state, which is also inconsistent with a misfolded intermediate (Figure 6). Importantly, when D1356 is allowed to collapse at low magnesium and is then chased with high concentration of Mg2+ under multiple turnover conditions, the reaction rate constant and amplitude for the first turnover are practically indistinguishable from those of the native ribozyme. By contrast, unfolded ribozyme reacts very slowly, presumably because it must first undergo the transition to the compact intermediate (Figure 4 (b)). Taken together, all of these results are consistent with a folding paradigm in which the compact species represent a specific, homogeneous, on-pathway folding intermediate that is so readily converted to the native conformation that this transition does not affect the reaction rate constant. Based on these observations, we postulate that the collapsed intermediate that is formed at low magnesium concentrations represents the near-native state, similar to that previously observed during folding of the bI5 group I intron 17; 18.
Formation of the near-native state is under kinetic control
At 30°C and low magnesium concentrations, the compact state does not appear to be in equilibrium with the unfolded state, which is curious given that the thermodynamic stability of the compact state is relatively low (Figure 5). Insight into the free energy landscape is provided by the fact that formation of the compact state occurs extremely slowly. Furthermore, unfolding of the native state upon dilution of magnesium proceeds only to the near-native state, not to the unfolded state.
The implications of this become most obvious at very low concentrations of MgCl2. At 1 mM magnesium, only 10% of the population reaches the near-native state after 25 hours of incubation (Figure 3 (a)). If this ratio represented a process in equilibrium, one would expect that dilution of the native state from 100 mM to 1 mM magnesium would cause 10% of the population to adopt the near-native state and 90% of the population would completely unfold. Contrary to this expectation, dilution of the native state causes all of the molecules to adopt the compact, near-native state (Figure 7(a)). Importantly, none of the population undergoes complete unfolding despite the low thermodynamic stability of the compact intermediate (0.7 kcal/mol). These data indicate that a large energy barrier lies between the unfolded and near-native states. While this high barrier makes compaction extremely slow, it provides kinetic stability for the near-native state (Figure 8 (b)). These results suggest that D1356 adopts the near-native state through a process that is under kinetic control, much like that previously reported for bacterial α-lytic protease 19; 20; 21. Kinetic control often results in a folding pathway that can be precisely regulated and which is extremely specific for the proper structural state 19; 20; 21.
A hierarchy of events that occur during folding
Although an ai5γ folding intermediate has been implicated at 42 °C 5, its transient nature impeded direct structural analysis. In this study, we have lowered the temperature to 30 °C and experimented with low magnesium concentrations in order to “freeze” the ribozyme at distinct stages along the folding pathway. These species can then be subjected to structural analysis, such as chemical probing and footprinting. Elements that are formed at minimal magnesium concentrations (i.e 3 mM) are likely to reflect structural features of the near-native state (Figure 6 (a), (e)).
In this work, we conducted DMS footprinting experiments and compared the protection pattern of the near-native state (formed at 3 mM MgCl2 ) with that of the unfolded state (potassium only). These experiments showed that the structural transition from unfolded to near-native state results in completion of most intron secondary structures, including the formation of long-range D1 tertiary interactions α-α′ and β-β′. Near-native state formation also involves the collapse of loop regions such as the 5-way junction, and the κ–ζ region and coordination loop, which later serve as the docking sites for D5 and D6, respectively (Figure 6 (e)).
DMS footprinting reveals that many important intronic elements do not contribute to formation of the near-native state. However, these catalytically critical substructures begin to appear at higher magnesium concentrations (10–100 mM), thereby representing steps along the pathway from the compact intermediate to the native state. The DMS footprinting data indicate that ε-ε′ and θ-θ′ interactions form at slightly higher magnesium ion concentration (10 mM) than the near-native state (3 mM), suggesting that they represent an early stage in the native-state formation. At magnesium ion concentrations required for the native state formation (>20 mM) DMS footprints are consistent with docking of D5 (ζ-ζ′ and κ-κ′ interactions) and, finally, docking of D3 (Figure 6 (b), (d), (e)). Thus, the catalytically essential domains 5 and 3 likely bind last to a fully-formed D1 scaffold, thereby ensuring accurate formation of the ribozyme active site. A similar hierarchy in domain assembly is implicated by data at 42 °C 5, suggesting that the intermediates formed at 30 and 42 °C have similar structural organization and that the general folding pathway for D1356 at 42 °C resembles that under near-physiological conditions.
The likely role of protein co-factors in group II intron folding
These studies indicate that, in the absence of protein co-factors, folding of the ai5γ intron is hindered by a series of problems: A. Initial molecular collapse is extremely slow and it proceeds under kinetic control due to a large energy barrier between the unfolded and near-native states. It is therefore likely that co-factors are required to accelerate this process in vivo, possibly via selective binding of the transition state and lowering the energy barrier (Figure 8 (b)). B. Although the ribozyme can maintain the near-native state under near-physiological conditions, it is incapable of maintaining the native conformation at low magnesium concentrations. This suggests a potential need for protein co-factors to stabilize the native state in vivo (Figure 8(b)). It also suggests that, despite apparent self-splicing activity in vitro, the intron is unlikely to splice itself out in vivo during transcription of the pre-mRNA. Rather, it is likely to require the assistance of co-factors and these would play a role in regulation of cytochrome oxidase gene expression at the level of pre-mRNA splicing. In vitro, the requirement for co-factors that stabilize the native state has been circumvented by conducting experiments in high concentrations of magnesium and monovalent ions.
Finally, as in all previous studies on ai5γ folding, the results reported herein are inconsistent with a kinetically trapped intermediate along the pathway to the near-native or the native state. This suggests that, while RNA helicase enzymes are excellent RNA binding proteins that may play a role in ATP-regulated folding of the intron, they are unlikely to do so by unwinding intermediates along the folding pathway, unless precursor RNA molecules are impacted by exon misfolding.
MATERIALS AND METHODS
RNA preparation
The plasmid encoding D1356 was prepared from the full splicing construct by site-directed mutagenesis, as previously described 22 and linearized with the Bam HI restriction enzyme prior to transcription. The D1356 ribozyme was transcribed as described earlier 23. Synthesis, deprotection and purification of the 17/7 RNA oligo substrate was carried out as described 24. Internal labeling of the D1356 ribozyme and 5′-end labeling of the 17/7 RNA oligo was carried out as previously described 23.
Analysis of global compaction
To compare global compaction at 42 and 30 °C as a function of magnesium concentration, internally labeled D1356 ribozyme (5 nM) was denatured at 95 °C in 80 mM MOPS pH 6.0, then MgCl2 was added to final concentrations of 0, 1, 2, 3, 5, 10, 20, 50 and 100 mM and the samples were incubated at 42 or 30 °C in the final volume of 10 μl for 20 min, then the native gel loading buffer was added and the samples were analyzed on a 6 % native gel containing 3 mM MgCl2 in 34 mM Tris-66 mM HEPES buffer as previously described 25. Alternatively, after 20 min incubation samples were combined with pre-chilled loading buffer and stored at 4 °C overnight, then analyzed the next day, in order to determine whether sample storage at 4 °C would affect the results. The extent of ribozyme compaction at respective magnesium concentrations was the same regardless of whether the samples were analyzed immediately after incubation or stored at 4 °C prior to analysis.
In order to determine the rate of compaction at various magnesium concentrations, the ribozyme samples were prepared as above and incubated at 30 °C in the final volume of 100 μl. Final concentrations of MgCl2 in the ribozyme samples were 3, 5, 10, 20, 30, 50, 100 and 200 mM. Aliquots of 5 μl were taken at respective time points (see Figure 3) and combined with pre-chilled (4 °C) loading buffer to prevent further compaction, then stored at 4 °C prior to analysis on a native gel as described above.
The rate of compaction in the presence of subdenaturing concentrations of urea was measured as above except 0.5 or 1M urea (final concentrations) was added to the samples containing 5 or 100 mM MgCl2, respectively, prior to incubation at 30 °C.
Monitoring of global compaction after dilution of the native ribozyme
Internally labeled ribozyme samples were denatured in 80 mM MOPS pH 6.0 at 95 °C, then folded at 100 mM MgCl2 at 42 °C for 20 min and cooled to 30 °C. Then samples were diluted with 80 mM MOPS pH 6.0 to 0.5, 1, 3, 5, 10 and 20 mM MgCl2, incubated at 30 °C for 20 min and analyzed on a native gel as above. Ribozyme was at 5 nM after dilution.
Folding of the Tetrahymena ribozyme was carried out essentially as described 26. Dilution of the native structure to lower magnesium concentrations (Figure 7) was carried out at 22 or 10 °C.
Analysis of thermodynamic stability by urea titration
Internally labeled ribozyme samples (50 nM) were denatured at 95 °C in 80 mM MOPS pH 6.0, then MgCl2 was added to final concentrations of 3, 5, 10 and 20 mM and the samples were incubated at 30 °C in the final volume of 10 μl until full compaction was reached (26 h). Then urea solution in the same buffer (9 μl) was added to 1 μl of the compacted sample to reach final urea concentrations of 0.125, 0.25, 0.5, 1, 1.5, 2, 3, 5 and 7 M for the sample containing 3 mM MgCl2, and to 0.5, 1, 1.5, 2, 3, 5, 7 and 8 M for other samples. All samples were incubated at 30 °C for 30 min and then analyzed on a native gel as above. Longer incubation times with urea did not result in any difference in the fraction of unfolded species.
In order to analyze stability of the compact ribozyme samples obtained after dilution of the native state to 3, 5, 10 or 20 mM MgCl2, the samples were prefolded at 100 mM MgCl2 at 42 °C for 20 min as described above, then cooled to 30 °C, diluted with 80 mM MOPS pH 6.0 to respective concentrations of magnesium and then treated with urea and analyzed as above. The concentration of the ribozyme was adjusted so that after dilution and addition of urea solution it was the same for all the samples (5 nM).
Multiple turnover kinetic analysis
Ribozyme samples were denatured, then allowed to compact at 3, 5, 10, 20 or 100 mM MgCl2 for 26 h, or folded to the native state and then diluted to 3, 5, 10 or 20 mM MgCl2 as described above, except the reaction buffer was 80 mM HEPES pH 8.0. The substrate (17/7) was denatured in 80 mM HEPES, pH 8.0, then MgCl2 was added to 3, 5, 10 or 20 mM final concentration MgCl2 and incubated at 30 °C for 20 min. For analysis of the ribozyme activity at low magnesium, the ribozyme and substrate incubated at respective magnesium concentrations were mixed and incubated at 30 °C. Aliquots were taken at respective time points and analyzed as described 23; 27. For analysis of catalytic activity at 100 mM MgCl2 ribozyme and substrate incubated at respective magnesium concentrations were mixed with simultaneous addition of MgCl2 to final concentration of 100 mM in the ribozyme-substrate mixture, then the samples were incubated at 30 °C, aliquots were taken at respective time points and analyzed as described. The final concentration of the ribozyme in all reaction mixtures was 20 nM, substrate − 200 nM.
To analyze catalytic activity of the native ribozyme, the ribozyme and substrate were denatured, preincubated separately in 80 mM HEPES pH 8.0 at 42 °C and 100 mM MgCl2, then cooled to 30 °C, mixed together and incubated at 30 °C. Aliquots were collected at respective time points and analyzed as described 23; 27. Final concentrations of ribozyme and substrate were the same as above.
To analyze catalytic activity of the unfolded ribozyme, the ribozyme and substrate were denatured in 80 mM MOPS pH 8.0, mixed together with simultaneous addition of 100 mM MgCl2 (final concentration), and incubated at 30 °C. Aliquots at respective time points were collected and analyzed as described 23; 27.
Preincubation of the substrate sample in the presence of magnesium or adding magnesium to the substrate sample prior to the reaction did not result in any difference in the reaction rate.
DMS footprinting
Samples were denatured in 80 mM potassium cacodylate pH 7.0, then either allowed to compact at 0 (unfolded sample), 3, 5, 10 or 20 mM MgCl2 for 26 h or prefolded at 42 °C at 100 mM MgCl2 and then diluted to the same magnesium concentrations as described above (except 0). The native sample was denatured in the same buffer and then folded at 42 °C at 100 mM MgCl2. Then DMS solution in ethanol was added to a final dilution of 1:500, mixture was incubated at room temperature for 30 min, quenched with β-mercaptoethanol, ethanol precipitated and analyzed by primer extension as previously described 28. Band intensities were quantified using ImageQuant software (Molecular Dynamics). Non-specific breaks in the RNA as well as DMS modification sites in loop regions that remain unprotected throughout the whole range of magnesium concentrations (0–100 mM) were used as internal standards to normalize band intensities for loading differences. Degree of protection against DMS modification was calculated as a ratio between normalized band intensities for unfolded samples and samples incubated at a given magnesium concentration. The average value from at least three independent experiments was determined and only 2-fold or higher protection values were considered. In order to facilitate comparison between various regions with different degrees of DMS protection (Fig. 6(d)), protection values for intermediates were normalized to those for the native state (100 mM MgCl2), which represent the highest possible degree of protection against DMS modification (100 %).
Acknowledgments
We thank Dr. Yong Xiong for many helpful discussions and A. Solem for critically reading the manuscript. We also thank Dr.Inna Shcherbakova for the gift of Tetrahymena ribozyme RNA. OF is a Research Specialist and AMP is an Investigator of the Howard Hughes Medical Institute. This work was supported by a grant from the NIH (GM50313 to AMP) and from the Austrian Science Foundation (Schroedinger fellowship J2332 to CW).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Woodson S. Recent insights on RNA folding mechanisms from catalytic RNA. Cell Mol Life Sci. 2000;57:796–808. doi: 10.1007/s000180050042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Treiber D, Williamson JR. Exposing the kinetic traps in RNA folding. Curr Opin Struct Biol. 1999;9:339–345. doi: 10.1016/S0959-440X(99)80045-1. [DOI] [PubMed] [Google Scholar]
- 3.Treiber D, Rook M, Zarrinkar P, Williamson JR. Kinetic intermediates trapped by native interactions in RNA folding. Science. 1998;279:1943–1946. doi: 10.1126/science.279.5358.1943. [DOI] [PubMed] [Google Scholar]
- 4.Su L, Brenowitz M, Pyle AM. An alternative route for the folding of large RNAs: apparent two-state folding by a group II intron ribozyme. J Mol Biol. 2003;334:639–652. doi: 10.1016/j.jmb.2003.09.071. [DOI] [PubMed] [Google Scholar]
- 5.Su LJ, Waldsich C, Pyle AM. An obligate intermediate along the slow folding pathway of a group II intron ribozyme. Nucl Acids Res. 2005;33:6674–6687. doi: 10.1093/nar/gki973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Swisher J, Duarte C, Su L, Pyle A. Visualizing the solvent-inaccessible core of a group II intron ribozyme. EMBO J. 2001;20:2051–2061. doi: 10.1093/emboj/20.8.2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Swisher J, Su L, Brenowitz M, Anderson V, Pyle A. Productive Folding to the Native State by a Group II Intron Ribozyme. J Mol Biol. 2002;315:297–310. doi: 10.1006/jmbi.2001.5233. [DOI] [PubMed] [Google Scholar]
- 8.Lehmann K, Schmidt U. Group II introns: structure and catalytic versatility of large natural ribozymes. Crit Rev Biochem Mol Biol. 2003;38:249–303. doi: 10.1080/713609236. [DOI] [PubMed] [Google Scholar]
- 9.Pyle AM, Lambowitz AM. Group II introns: ribozymes that splice RNA and invade DNA. In: Gesteland RF, Cech TR, Atkins JF, editors. The RNA world. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, New York: 2006. pp. 469–506. [Google Scholar]
- 10.Qin PZ, Pyle AM. The architectural organization and mechanistic function of group II intron structural elements. Curr Opin Struct Biol. 1998;8:301–308. doi: 10.1016/s0959-440x(98)80062-6. [DOI] [PubMed] [Google Scholar]
- 11.Mohr S, Matsuura M, Perlman PS, Lambowitz AM. A DEAD-box protein alone promotes group II intron splicing and reverse splicing by acting as an RNA chaperone. Proc Natl Acad Sci U S A. 2006;103:3569–3574. doi: 10.1073/pnas.0600332103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang H, Rowe CE, Mohr S, Jiang Y, Lambowitz AM, Perlman PS. The splicing of yeast mitochondrial group I and group II introns requires a DEAD-box protein with RNA chaperone function. Proc Natl Acad Sci U S A. 2005;102:163–168. doi: 10.1073/pnas.0407896101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamill S, Pyle AM. The receptor for branch-site docking within a group II intron active site. Mol Cell. 2006 doi: 10.1016/j.molcel.2006.07.017. in press. [DOI] [PubMed] [Google Scholar]
- 14.Woodson S. Metal ions and RNA folding: a highly charged topic with a dynamic future. Curr Opin Chem Biol. 2005;9:104–109. doi: 10.1016/j.cbpa.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 15.Perez-Salas U, Rangan P, Krueger S, Briber RM, Thirumalai D, Woodson SA. Compaction of a bacterial group I ribozyme coincides with the assembly of core helices. J Mol Biol. 2003;329:229–238. doi: 10.1021/bi035642o. [DOI] [PubMed] [Google Scholar]
- 16.Shelton VM, Sosnick TR, Pan T. Applicability of urea in the thermodynamic analysis of secondary and tertiary RNA folding. Biochemistry. 1999;38:16831–16839. doi: 10.1021/bi991699s. [DOI] [PubMed] [Google Scholar]
- 17.Buchmueller KL, Webb AE, Richardson DA, Weeks KM. A collapsed non-native RNA folding state. Nat Struct Biol. 2000;7:362–366. doi: 10.1038/75125. [DOI] [PubMed] [Google Scholar]
- 18.Buchmueller K, Weeks KM. Near native structure in an RNA collapsed state. Biochemistry. 2003;42:13869–13878. doi: 10.1021/bi035476k. [DOI] [PubMed] [Google Scholar]
- 19.Sohl J, Jaswal SS, Agard DA. Unfolded conformations of alpha-lytic protease are more stable than its native state. Nature. 1998;395:817–819. doi: 10.1038/27470. [DOI] [PubMed] [Google Scholar]
- 20.Jaswal S, Sohl JL, Davis JH, Agard DA. Energetic landscape of alpha-lytic protease optimizes longevity through kinetic stability. Nature. 2002;415:343–346. doi: 10.1038/415343a. [DOI] [PubMed] [Google Scholar]
- 21.Cunningham E, Jaswal SS, Sohl JL, Agard DA. Kinetic stability as a mechanism for protease longevity. Proc Natl Acad Sci U S A. 1999;96:11008–11014. doi: 10.1073/pnas.96.20.11008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kunkel TA, Bebenek K, McClary J. Efficient site-directed mutagenesis using uracil-containing DNA. Methods Enzymol. 1991;204:125–139. doi: 10.1016/0076-6879(91)04008-c. [DOI] [PubMed] [Google Scholar]
- 23.Pyle AM, Green JB. Building a kinetic framework for group II intron ribozyme activity: quantitation of interdomain binding and reaction rate. Biochemistry. 1994;33:2716–2725. doi: 10.1021/bi00175a047. [DOI] [PubMed] [Google Scholar]
- 24.Wincott F, DiRenzo A, Shaffer C, Grimm S, Tracz D, Workman C, Sweedler D, Gonzalez C, Scaringe S, Usman N. Synthesis, deprotection, analysis and purification of RNA and ribozymes. Nucl Acids Res. 1995;23:2677–2684. doi: 10.1093/nar/23.14.2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rangan P, Masquida B, Westhof E, Woodson SA. Assembly of core helices and rapid tertiary folding of a small bacterial group I ribozyme. Proc Natl Acad Sci U S A. 2003;100:1574–1579. doi: 10.1073/pnas.0337743100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pan J, Deras M, Woodson S. Fast folding of a ribozyme by stabilizing core interactions: evidence for multiple folding pathways in RNA. J Mol Biol. 2000;296:133–44. doi: 10.1006/jmbi.1999.3439. [DOI] [PubMed] [Google Scholar]
- 27.Swisher J. Folding studies of the group II intron ai5γ. Columbia University; 2000. [Google Scholar]
- 28.Waldsich C, Masquida B, Westhof E, Schroeder R. Monitoring intermediate folding states of the td group I intron in vivo. EMBO J. 2002;21:2300–2312. doi: 10.1093/emboj/cdf504. [DOI] [PMC free article] [PubMed] [Google Scholar]