Summary
Cell-cell communication is essential for growth and development of multicellular organisms. In higher plants, the shoot organs are derived from three clonally distinct cell layers present in the meristem. The role of the outermost L1 cell-layer and its derived epidermis in coordinating growth of the inner cell-layers has long been debated. This question has been revisited recently using molecular tools to manipulate cell cycle progression or cell expansion, specifically in the epidermis. These studies conclude that cells in the epidermis both promote and restrict growth of the entire shoot by sending growth signals—either physical or chemical— to the inner layers.
Introduction
Multicellular organisms grow and develop as an integrated unit, relying on coordinated proliferation and expansion processes of individual cells. In higher plants, the shoot meristem gives rise to all above-ground organs, and has an organized pattern: the outermost layer is the L1, the cells immediately below comprise the L2, and the inner tissues define the L3 (Figure 1a) [1]. The L1 and L2 divide in a stereotypical manner, predominantly anticlinal to the plane, whereas the cells in the L3 undergo both anticlinal and periclinal divisions. Simply put, L1-derived cells form the epidermis and define the shoot/environment interface, the L2 gives rise to the photosynthesizing cells of the sub-epidermis, and L3-derived-cells comprise the ground tissues [2]. The extent to which each of these layers contribute to organ size and whether there is communication between these distinct cell layers has been a matter of interest for over a century; yet only recently have the molecular toolkits become available to address these questions in an intact, growing organism [3,4]. A number of techniques have been used to examine the autonomous or non-autonomous behavior of a specific layer during different developmental processes, including organ initiation and morphogenesis, regulation of leaf size, and elongation of stems. Though the details vary, these experiments demonstrate that inter-cell-layer-communication occurs to a large extent.
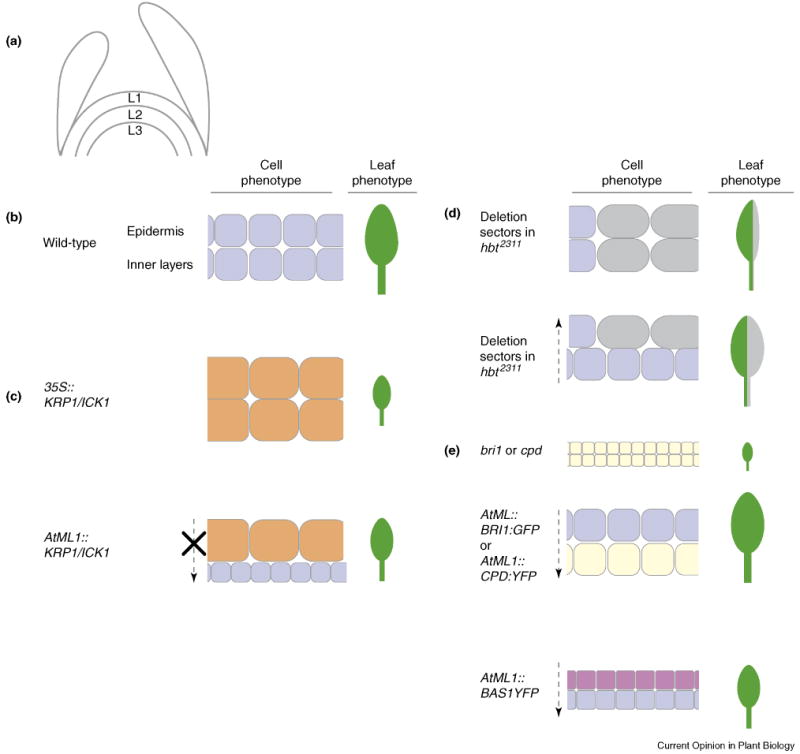
Inter cell-later communication during shoot growth. (a) Scheme of the shoot apical meristem and its cell-layers. L1 and L2 are single layers of cells which divide anticlinal to the plane and L3 is the remaining cells which divide both anticlinal and periclinal. L1 will form the epidermis, L2- the subepidermal mesophyll and L3 the inner mesophyll and the ground tissue. (b-e) Summary of autonomous and non-autonomous signals generated and perceived by the epidermis. A schematic organization of the cells in the leaf and the leaf phenotype are shown. (b) Wild-type. (c) Expression of the cell-cycle inhibitor KRP1/ICK1 under the ubiquitous 35S promoter (35S::KRP1/ICK1, upper panel) and the L1 specific promoter AtML1 (AtML1:: KRP1/ICK1, lower panel). Cells with KRP1/ICK1 expression are marked in red. Note that when KRP1/ICK1 is confined to the epidermis, cells in the inner layers have the same total cell number as in wild-type. (d) Deletion sectors of HBT in hbt2311. Sectors are marked in grey. Note the morphology of the leaf, which is more similar to wild type when sectors are derived solely from the epidermis. In this case, cell cycle, but not cell morphology is restored. (e) BR effects on cell expansion. BR deficient of insensitive mutant (cpd and bri1 respectively) cells are marked in yellow (upper panel). CPD and BRI1 fused to the yellow or green fluorescence protein are expressed under AtML1 promoter (AtML1::CPD:YFP and AtML1::BRI1:GFP respectively, middle panel, blue cells). Note the full rescue of the cell size in inner layers. Lowe panel, BR biosynthetic enzyme BAS1 fused to YFP expression (purple cells) in wild-type background. Cell morphology is as predicted, but has not been confirmed experimentally.
Arrow represents unknown non-autonomous signal. Arrow with a cross represents autonomous behavior.
In this review, we limit our discussion to the fundamental question: what is the role of the L1 layer in plant growth and development? This seemingly simple question has led to different, often opposing, conclusions. We review classic studies and focus on novel insights gained from recent advances using the molecular toolkits now available. In particular we will address the autonomous and non-autonomous effects of the epidermis on different aspects of cell division and cell expansion and discuss the various proposals that have been put forward to explain the non-cell-autonomous role of the L1 in promoting and restricting shoot growth.
Genetic mosaics reveal inter-cell-layer communication
Our knowledge of cell lineage organization and inter-cell-layer communication has been gained largely from studies using chimeric plants or genetic mosaics [2,4,5]. These studies rely on the ability to easily identify two groups of genetically distinct cells. Early support that the three layers of the meristem are maintained as separate entities and that cells derived from all layers are all found in the shoot came from treatments of diploid seeds with colchicine. Colchicine induces polyploid periclinal chimeras (in periclinal chimeras, at least one entire apical layer is genetically distinct from the others) [1]. Polyploid cells can be distinguished from unaffected cells based on their large nuclei, thereby allowing a correlation of their meristematic position with their location in developing organs. Later studies used chlorophyll as a marker for distinct cells, which could be followed easily in white/green variegated sectors. As with the early studies, these experiments concluded that in dicots, the L1 layer forms the entire shoot epidermis, the L2 gives rise to the immediate underlying subepidermal tissue, and the L3 comprises the remaining inner tissues [4,5]. In summary, tissue organization could be studied by following cells that are visibly marked with a “neutral” autonomous trait.
Having established that distinct layers of the shoot arise from discrete zones of the meristem, an obvious and consequential question followed: Is there a developmental significance for the layer organization? A positive answer can be inferred if a specific cell-layer influences distinct developmental programs (i.e. initiation, morphology and size) of the entire organ. Indeed, the aforementioned early genetic mosaics uncovered several cases of “non-autonomous” traits. For example, in tomato, the L3 layer determines carpel number while the L1 and L2 do not [6]. Periclinal chimeras from grafting of tobacco species with different leaf morphologies revealed that both the L2 and L3, but not the L1, control leaf shape [5,7]. In contrast, the okra mutation in cotton, which modifies leaf shape, affects leaf morphology from both the L1 and L2 [8]. In addition, the L1 dictated flower development in Camellia [9]. Thus, while inter cell-layer-communication clearly exists, the layer in control varies between different experiments. This may be due to differences between species, the limitations of the experiment, or analysis of mutations that affect different traits. Regardless, these observations question the unique role of the L1 in controlling growth, as predicted by certain mechanical theories and as discussed next.
The epidermis as a barrier – a biophysical view
Theories of plant growth by mechanical concepts have been documented since the 17th century (for an interesting treatment of the different theories, see [10]). At the heart of these papers lies the observation that different isolated tissues exhibit different growth rates, and hence are under differential tension (Sachs, 1882 and 1875 in [11] and [10] respectively). Plant cells are immotile and glued together through their walls, necessitating that they grow together. As such, it was hypothesized that some tissues can grow passively owing to the driving force of the others. Indeed, various studies using sectioning methods have shown that when a growing stem is sectioned longitudinally and placed in water, the two halves will curl outward. This is because the inner layers (pith) are rapidly extended while the outer layers (epidermis and sometimes the attached underlying cortex) contract. This suggests that the compression of the former and the tension of the latter were relieved.
Given that cell expansion is not homogeneous within a plant, which tissue controls plant growth? The answer has been debated. Sachs proposed that the outer layers passively follow the actively growing inner layers [12] in [10]. Alternative biophysical theories favored a role for the epidermis in restricting and controlling the rate and direction of plant growth [13,14]. Green hypothesized that the epidermis can “restrain more stress than it generates” [13]. This argument seems reasonable since the turgor pressure generated and experienced by the inner cells is predicted to occur in all directions (because each cell wall is surrounded by walls of its neighboring cells). In contrast, the cells in the epidermal layer interface with the environment. Therefore, the wall properties of the epidermis and its ability to counteract internal forces are expected to influence the extent of growth. In agreement, the outer wall of the epidermis is thicker (sometimes 5-10 fold) than inner cell walls.
In addition to notable differences in cell wall thickness, the epidermis is highly responsive to auxin-induced wall extensibility and elongation [14], again emphasizing the importance of the epidermis for organogenesis and morphogenesis [15]. The genetic mosaic studies described in the previous section suggest that layers other than the L1 can often control different developmental aspects. However, as mentioned above, it is not clear where in the signaling pathway the analyzed gene products are acting to eventually modulate cell proliferation and expansion and hence where and when the involvement of cell-cell movement of different players takes place.
Recently, alternative ways to examine the role of a particular layer for growth and development were used. In one study, the cell wall loosening enzymes, expansins, were shown to induce turgor-driven cell expansion [16,17]. Local exogenous applications of expansins to the SAM could induce a primordia-like structure [18]. Later modification of the technique involved local and inducible expression of an endogenous expansin in the SAM, which led to the initiation and development of normal leaves [19]. The difference in leaf morphology between these two experiments was attributed to limited penetration and thus restricted accumulation of the expansin in the L1 in the former, while expansin was expressed in all layers in the latter [19]. In a different experiment, spatio-temporal examination of the L1 in the SAM by means of laser-based ablation techniques revealed that it was essential for organ formation [20], since leaf primordia were not formed at the ablated site. If L1-ablation alleviates the surface barrier, then this experiment does not support the mechanical prediction upon which cells in the inner layers are consequently “free” to grow. On the other hand, cell ablation does not interfere solely with biophysics, as it also disrupts overall cellular processes, including auxin transport, which is essential for organ formation [21,22]. In summary, biophysical manipulation of the plant surface was able to guide organ formation, in agreement with the L1 being a mechanical barrier for growth. However, organogenesis was better programmed if cell walls were modified in all layers.
Inter-layer coordination of cell proliferation
To manipulate the epidermis independently of the L2 and L3 layers, L1-specific promoters have been used to express genes of interest. One such a promoter in Arabidopsis, called AtML1, was used to examine the coordination between the L1 and the inner layers when cell division was inhibited in the former [23**]. To this end, plants with epidermal expression of two cyclin-dependent kinase (CDK) inhibitor genes, KRP1/ICK1 and KRP4, were analyzed (Figure 1c). Over-expression of KRP1/ICK1, as well as additional family members under the ubiquitous 35S promoter, had been previously shown to inhibit cell division. This was accompanied by a compensated increase in cell volume, decrease in the overall size of the plant and altered morphology [24,25]. Similar to their ubiquitous expression, over-expression of CDK inhibitors in the epidermis resulted in smaller plants. As predicted, cells in the epidermis were reduced in number with a compensating increased size as compared to control plants. However, cell numbers in the inner layers, such as cortex and mesophyll, were not different from the wild type. This intriguing result demonstrates clearly that cells in the inner layers act independently of cells in the L1. In other words, the L1 cannot send a “stop dividing” signal to the L2. This lack of inter-layer coordination was further reflected by the distorted orientation of cell division in the inner layers, likely a result of constrained space.
In what seems to oppose the CDK inhibitor studies, genetic mosaic analyses with the HOBBIT (HBT) gene suggest that there is inter layer communication [26*]. HBT is a CDC27 homolog, which encodes a subunit of the Anaphase Promoting Complex or Cyclosome (APC/C) [27]. Analysis of hbt plants suggests that HBT functions primarily post-embryonically to maintain normal cell division and expansion Figure 1d) [26*]. Deletion marked sectors (representing HBT genomic excision) in a hbt rescued mutant were generated using the Cre/lox system [26*]. Sectors encompassing L1, L2 and L3 layers contained fewer cells with elongated morphology as compared with the surrounding cells. However, sectors derived from the L1 of comparable size revealed that cell division was rescued by the presence of wild type HBT in cells from the underlying layers. In contrast, cell morphology was not. Thus, reduced cell proliferation attributed to a key player in cell division control could be overcome by a yet unknown mode of inter-cell-layer communication.
Can the observed differences in cell proliferation coordination be resolved? One possibility is related to the design of the two experiments. In the KRP1/ICK1, the perturbation in the L1 is of dominant nature due to overexpression of an inhibitor. In contrast, in the HBT experiment the perturbation occurs by a loss-of-function. In a scenario where inter-cell-layer communication involves cell-cell movement of cell-cycle components (from inner layers to L1), then loss of HBT can be easily rescued. However, such a scenario won't be able to rescue the high inhibitory activity of KRP1/ICK1. In this regard, it would be interesting to test the reciprocal situation in which ICK targets (CDKs) are depleted from the L1 (for example by deletion sectors of CDKA;1 [28,29]). In any case (it could be a signal, or it could be movement of wild type HBT or its targets), the rescue signal of cell division in the HBT experiment comes from the L2/L3 layers. An open question is whether L1 can similarly rescue cell division defects in the inner layers, since the corresponding sectors (and of equivalent size) for HBT have not been reported.
Another interesting aspect that emerges from L1-specific expression of KRP1/ICK1 is the reduction in cell size of the underlying cells. Thus, while the L1 cells are larger in size as compared to wild type (perhaps due to compensation, as mentioned above), cells in the inner layer (palisade mesophyll) are significantly smaller than their equivalent wild-type cells. Hence, as opposed to the autonomous behavior of cell proliferation in the L2, cell volume responds to the mechanical constraints generated by reduced cell number in the L1, in agreement with the L1 being a barrier for growth (see above). With a similar rationale, can inner cells expand in response to extension of the outer layer? This question was studied in early works using exogenous application of auxin and more recently through molecular manipulation of brassinosteroid signaling or levels, as elaborated next.
Epidermis control of cell expansion – the hormone aspect
Seventy years ago, Thimann proposed that differential tissue tension in cylindrical elongating organs is a result of differential sensitivity to auxin [11]. Using microsurgery and explants, he then showed that the inner layers were more sensitive to a given auxin concentration then the epidermis. In contrast, later experiments concluded that the epidermis (and occasionally the cortex) was more responsive to auxin than the inner layers, suggesting that the epidermis was the preferred target of auxin action [14]. These experiments were limited to surgical methods and exogenous application of hormones to plant segments, which may be the reason for the large disagreements between different laboratories [30].
In the past 10 years, hormone biosynthetic, catabolic, and response pathway mutants of Arabidopsis have been identified [31]. This has allowed scientists to revisit the old questions in an intact organism. One recent paper manipulated the levels and signaling pathway of the steroid growth hormones, brassinosteroids (BRs) [32**]. These experiments were possible because BR biosynthetic and catabolic mutants are known and their corresponding genes have been cloned [33]. The same is true for the BR receptor, BRI1, and most of the major signaling components [34]. Interpretation of the results was made easier by the fact that BRs are predominantly involved in cell expansion, and unlike auxin, do not appear to play an important role in organ formation and patterning. Thus, restricting biosynthesis and perception of BR to the epidermis can be used to examine whether inter-cell-layer communication occurs during the cell expansion phase of growth [32**].
The experiment was to use an L1-specific promoter AtML1 (see above) to drive the expression of the BR receptor BRI1 or the BR biosynthesis enzyme CPD, in their corresponding dwarf mutant backgrounds. In both cases, a remarkable rescue of plant growth occurred (Figure 1e). The cells of the epidermis were rescued to wild-type size, and remarkably, so were the cells in the L2. Therefore, not only the BR hormone itself, but also BR perception from the epidermis is sufficient to send growth signals to the inner layers. As opposed to expression from the L1, targeted expression from the L3 (using a vasculature specific promoter) had a very minor effect on growth in the case of CPD and almost no effect in the case of BRI1. These experiments demonstrate that growth, mainly a result of cell expansion, can be controlled by a signal(s) initiated at the epidermis. In agreement, BES1 (and its closest homolog BZR1), which are downstream transcription factors in the signaling pathway can also drive plant growth when are restricted to the L1 in bri1, although to a much lesser extent as compared with CPD or BRI1.
An important question then is how the ground tissue senses the growing outer layer? The answer is still unknown. One hypothesis predicts cell-cell movement of a BR signaling component from the L1 to inner layers. Cell-cell movement of transcription factors through plasmodesmata has explained various cases of inter-cell-layer communication [35]. Alternatively, cell expansion could be either restricted or enhanced by mechanical stimuli, as we have mentioned above. Finally, it is possible that BR mediated growth responses in plants are active predominantly in the outer layers. In this case, the inner layers of bri1 should maintain their growth capacity (which is being restricted by the lack of BR activity in the epidermis). Indeed, BR is required in the outer layer for normal growth. This was shown by expressing the BR catabolic gene BAS1 in the L1 of wild-type plants, resulting in their significant growth inhibition [32**].
Different growth hormones may be active in preferential spatial domains. This theory fits the recent idea according to which plant hormones have very few common downstream targets, and in some cases regulate distinct members of a given gene family [36]. Hence, detailed expression patterns combined with functional mapping of other plant hormones should be tested. Interestingly, in the periclinal chimera of the grapevine Pinot Meunier, the L1 layer has the dominant mutated allele of the DELLA homolog of the Arabidopsis GAI [37]. This mutation confers gibberellin insensitivity. In this case, while trichome density is impaired, the size of Pinot Meunier is indistinguishable from – the non-chimeric variant. However, plants regenerated from the L1, but not from the L2, are dwarf. The fact that the DELLA mutation confined to the L1 has no effect on plant growth may be attributed to growth compensation signal from the underlying tissues. On the other hand, this idea is not in agreement with recent molecular data that supports the epidermis as a barrier for growth. Another possibility is that GAI is not important for growth in the L1, but rather in the inner layers. These scenarios await examination using the toolkits now available.
Conclusions and future perspectives
New molecular studies have provided data that the epidermis can restrict plant growth, as predicted over a century ago. This occurs through inhibition of cell expansion and may be attributed to mechanical constraints. However, if and how mechanotransduction functions during growth remains to be shown [38*]. In contrast, while the epidermis restricts cell expansion of underlying layers, it does not affect their cell proliferation program. On the other hand, in a different experimental context, cell proliferation in the L1 can receive signals from the underlying layers, suggesting also non-autonomous control of cell proliferation (for elaborated discussion, see above). It would be interesting to further study the extent of inter-layer-coordination and examine the consequence for plant growth when cell cycle is specifically perturbed in the inner layers during different developmental stages.
In addition to the epidermis' role as a barrier, it can also send growth signals initiated by BRs, to the inner layers. It is now important to determine what is the nature of the signal and how and where other hormones act to regulate growth. The use of tissue specific promoters is not a perfect experiment, as they can drive lower or higher expression levels than normally occur. However, it is a powerful method for generating specific spatio-temporal perturbation in various processes related to plant growth. Recent advances in the hormone field, including characterization of hormone receptors, signaling and biosynthesis proteins, as well as collection of corresponding mutants, provide the basis for follow-up studies. At the same time it will be important to develop other molecular strategies, for example, to develop sensors that monitor the activity of a given hormone signaling pathway in real time. Finally, mutants which are specifically impaired in cell proliferation in the L1 can be used to further address its role in inter-cell-layer communication [39].
Acknowledgments
We thank Dr. James Umen for critically reading of the manuscript and Dr. Xuelin Wu for helpful discussions. We apologize to those colleagues whose work we could not cite because of space limitations. Our research on brassinosteroid signaling is funded by The Howard Hughes Medical Institute and grants from the USDA and NSF to J.C. SS-G was supported by fellowships from US–Israel Bi-National Agricultural Research and Development Fund (BARD) and the Salk Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Satina S, Blakeslee AF, Avery AG. Demonstration of the three germ layers in the shoot apex of Datura by means of induced polyploidy in periclinal chimeras. Am J Bot. 1940;27:895–905. [Google Scholar]
- 2.Stewart RN, Burk LG. Independence of tissues derived from apical layers in ontogeny of the tobacco leaf and ovary. Am J Bot. 1970;S7:1010–1016. [Google Scholar]
- 3.Ingram GC. Between the sheets: inter-cell-layer communication in plant development. Philos Trans R Soc Lond B Biol Sci. 2004;359:891–906. doi: 10.1098/rstb.2003.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Szymkowiak EJ, Sussex IM. What Chimeras Can Tell Us About Plant Development. Annu Rev Plant Physiol Plant Mol Biol. 1996;47:351–376. doi: 10.1146/annurev.arplant.47.1.351. [DOI] [PubMed] [Google Scholar]
- 5.Marcotrigiano M. Genetic mosaics and the analysis of leaf development. International Journal of Plant Sciences. 2001;162:513–525. [Google Scholar]
- 6.Szymkowiak EJ, Sussex IM. The internal meristem layer (L3) determines floral meristem size and carpel number in tomato periclinal chimeras. Plant Cell. 1992;4:1089–1100. doi: 10.1105/tpc.4.9.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McHale NA, Marcotrigiano M. LAM1 is required for dorsoventrality and lateral growth of the leaf blade in Nicotiana. Development. 1998;125:4235–4243. doi: 10.1242/dev.125.21.4235. [DOI] [PubMed] [Google Scholar]
- 8.Dolan L, Poethig RS. The Okra leaf shape mutation in cotton is active in all cell layers of the leaf. Am J Bot. 1998;85:322–327. [PubMed] [Google Scholar]
- 9.Stewart RN. Camellia + ‘daisy Eagleson’, a graft chimera of camellia sansanqua and c. japonika. Am J Bot. 1072;59:515–524. [Google Scholar]
- 10.Peters WS, Tomos AD. The history of tissue tension. Annals of Botany. 1996;77:657–665. doi: 10.1006/anbo.1996.0082. [DOI] [PubMed] [Google Scholar]
- 11.Thimann KV, Schneider CL. Differential growth in plant tissues. Am J Bot. 1938;25:627–641. [Google Scholar]
- 12.Sachs J. Text-book of botany. London: Oxford: calarendon press; 1875. [Google Scholar]
- 13.Green PB. Organogenesis-A Biophysical View. Annual Review of Plant Physiology and Plant Molecular Biology. 1980;31:51–82. [Google Scholar]
- 14.Kutschera U. The role of the epidermis in the control of elongation growth in stems and coleoptiles. Botanica Acta. 1992;105:246–252. [Google Scholar]
- 15.Green PB. Pattern formation in shoots: A likely role for minimal energy configurations of the tunica. Int J plant Sci. 1992:153. [Google Scholar]
- 16.Cosgrove DJ. Growth of the plant cell wall. Nat Rev Mol Cell Bio. 2005;6:850–861. doi: 10.1038/nrm1746. [DOI] [PubMed] [Google Scholar]
- 17.Cosgrove DJ. Loosening of plant cell walls by expansins. Nature. 2000;407:321–326. doi: 10.1038/35030000. [DOI] [PubMed] [Google Scholar]
- 18.Fleming AJ, McQueen-Mason S, Mandel T, Kuhlemeier C. Induction of leaf primordia by the cell wall protein expansin. Science. 1997;276:1415–1418. [Google Scholar]
- 19.Pien S, Wyrzykowska J, McQueen-Mason S, Smart C, Fleming A. Local expression of expansin induces the entire process of leaf development and modifies leaf shape. Proc Natl Acad Sci U S A. 2001;98:11812–11817. doi: 10.1073/pnas.191380498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reinhardt D, Frenz M, Mandel T, Kuhlemeier C. Microsurgical and laser ablation analysis of interactions between the zones and layers of the tomato shoot apical meristem. Development. 2003;130:4073–4083. doi: 10.1242/dev.00596. [DOI] [PubMed] [Google Scholar]
- 21.Benkova E, Michniewicz M, Sauer M, Teichmann T, Seifertova D, Jurgens G, Friml J. Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell. 2003;115:591–602. doi: 10.1016/s0092-8674(03)00924-3. [DOI] [PubMed] [Google Scholar]
- 22.Reinhardt D, Pesce ER, Stieger P, Mandel T, Baltensperger K, Bennett M, Traas J, Friml J, Kuhlemeier C. Regulation of phyllotaxis by polar auxin transport. Nature. 2003;426:255–260. doi: 10.1038/nature02081. [DOI] [PubMed] [Google Scholar]
- 23**.Bemis SM, Torii KU. Autonomy of cell proliferation and developmental programs during Arabidopsis aboveground organ morphogenesis. Dev Biol. 2007;304:367–381. doi: 10.1016/j.ydbio.2006.12.049. [DOI] [PubMed] [Google Scholar]; The authors examined the extent of growth coordination between the L1 and the inner layers. To this end they attenuated cell division only in the outer layer by expressing cyclin-dependent kinase inhibitor genes under L1 specific promoter. The resulted plants were smaller in size with fewer cells in the epidermis, as predicted. Surprisingly, the number of cells in the subepidermal layer was not changed as compared to wild-type plants. However, they had significantly reduced size and were disorganized. This demonstrates an autonomous behavior during cell division, with lack of L1 control. The smaller size of cells derived from L2 likely reflects a response to a physical constrain.
- 24.De Veylder L, Beeckman T, Beemster GT, Krols L, Terras F, Landrieu I, van der Schueren E, Maes S, Naudts M, Inze D. Functional analysis of cyclin-dependent kinase inhibitors of Arabidopsis. Plant Cell. 2001;13:1653–1668. doi: 10.1105/TPC.010087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang H, Zhou Y, Gilmer S, Whitwill S, Fowke LC. Expression of the plant cyclin-dependent kinase inhibitor ICK1 affects cell division, plant growth and morphology. Plant J. 2000;24:613–623. doi: 10.1046/j.1365-313x.2000.00899.x. [DOI] [PubMed] [Google Scholar]
- 26*.Serralbo O, Perez-Perez JM, Heidstra R, Scheres B. Non-cell-autonomous rescue of anaphase-promoting complex function revealed by mosaic analysis of HOBBIT, an Arabidopsis CDC27 homolog. Proc Natl Acad Sci U S A. 2006;103:13250–13255. doi: 10.1073/pnas.0602410103. [DOI] [PMC free article] [PubMed] [Google Scholar]; HBT encodes for a CDC27 homolog, a component of the APC/C complex, which regulates mitotic progression. The paper describes loss-of-functions sectors (marked by GFP) using the Cre/lox based recombination system in homozygous hbt, complemented with a wild-type HBT. In leaves, excision of HBT impairs cell proliferation and is accompanied by increased cell expansion. Interestingly, in L1 derived sectors (representing excised HBT), cell division was rescued by the underlying layers, but not cell morphology. In contrast, no rescue occurred in sectors spanning all three layers. Thus, inter-layer non-autonomous signal (currently unknown) can compensate for defects in cell proliferation.
- 27.Blilou I, Frugier F, Folmer S, Serralbo O, Willemsen V, Wolkenfelt H, Eloy NB, Ferreira PC, Weisbeek P, Scheres B. The Arabidopsis HOBBIT gene encodes a CDC27 homolog that links the plant cell cycle to progression of cell differentiation. Genes Dev. 2002;16:2566–2575. doi: 10.1101/gad.237302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iwakawa H, Shinmyo A, Sekine M. Arabidopsis CDKA;1, a cdc2 homologue, controls proliferation of generative cells in male gametogenesis. Plant J. 2006;45:819–831. doi: 10.1111/j.1365-313X.2005.02643.x. [DOI] [PubMed] [Google Scholar]
- 29.Nowack MK, Grini PE, Jakoby MJ, Lafos M, Koncz C, Schnittger A. A positive signal from the fertilization of the egg cell sets off endosperm proliferation in angiosperm embryogenesis. Nat Genet. 2006;38:63–67. doi: 10.1038/ng1694. [DOI] [PubMed] [Google Scholar]
- 30.Peters WS, Tomos AD. The epidermis still in control? Botanica Acta. 1996;109:264–267. [Google Scholar]
- 31.Bishopp A, Mahonen AP, Helariutta Y. Signs of change: hormone receptors that regulate plant development. Development. 2006;133:1857–1869. doi: 10.1242/dev.02359. [DOI] [PubMed] [Google Scholar]
- 32**.Savaldi-Goldstein S, Peto C, Chory J. The epidermis both drives and restricts plant shoot growth. Nature. 2007;446:199–202. doi: 10.1038/nature05618. [DOI] [PubMed] [Google Scholar]; The authors addressed the role of the epidermis in coordinating cell expansion growth. To this end they targeted the expression of brassinosteroid (BR) biosynthetic, catabolic and signaling components to the epidermis of their corresponding dwarf mutant. A BR biosynthesis gene CPD and the BR receptor BRI1 were able to fully rescue dwarfism of their related mutants. In addition, cell size in both the epidermis and the mesophyll exhibit wild-type size. However, targeted expression to the inner vasculature tissue had close to no effect. This indicates that the epidermis signals non-autonomously to the inner layers. In addition, to study whether the epidermis can also restrict growth, a BR catabolic gene was similarly expressed in the epidermis of wild-type plants. The resulted plants were semi-dwarf.
- 33.Fujioka S, Yokota T. Biosynthesis and metabolism of brassinosteroids. Annu Rev Plant Biol. 2003;54:137–164. doi: 10.1146/annurev.arplant.54.031902.134921. [DOI] [PubMed] [Google Scholar]
- 34.Belkhadir Y, Wang X, Chory J. Brassinosteroid signaling pathway. Sci STKE. 2006;2006:cm4. doi: 10.1126/stke.3642006cm4. [DOI] [PubMed] [Google Scholar]
- 35.Kurata T, Okada K, Wada T. Intercellular movement of transcription factors. Curr Opin Plant Biol. 2005;8:600–605. doi: 10.1016/j.pbi.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 36.Nemhauser JL, Hong F, Chory J. Different plant hormones regulate similar processes through largely nonoverlapping transcriptional responses. Cell. 2006;126:467–475. doi: 10.1016/j.cell.2006.05.050. [DOI] [PubMed] [Google Scholar]
- 37.Boss PK, Thomas MR. Association of dwarfism and floral induction with a grape ‘green revolution’ mutation. Nature. 2002;416:847–850. doi: 10.1038/416847a. [DOI] [PubMed] [Google Scholar]
- 38*.Dumais J. Can mechanics control pattern formation in plants? Curr Opin Plant Biol. 2007;10:58–62. doi: 10.1016/j.pbi.2006.11.014. [DOI] [PubMed] [Google Scholar]; This review discusses and brings our attention to the relevance of mechanics in controlling development.
- 39.Kessler S, Townsley B, Sinha N. L1 division and differentiation patterns influence shoot apical meristem maintenance. Plant Physiol. 2006;141:1349–1362. doi: 10.1104/pp.105.076075. [DOI] [PMC free article] [PubMed] [Google Scholar]


