Abstract
Complete patient health information that is available where and when it is needed is essential to providers and patients and improves healthcare quality and patient safety. VA and DoD have built on their previous experience in patient data exchange to establish data standards and terminology services to enable real-time bi-directional computable (i.e., encoded) data exchange and achieve semantic interoperability in compliance with recommended national standards and the eGov initiative. The project uses RxNorm, UMLS, and SNOMED CT terminology standards to mediate codified pharmacy and allergy data with greater than 92 and 60 percent success rates respectively. Implementation of the project has been well received by users and is being expanded to multiple joint care sites. Stable and mature standards, mediation strategies, and a close relationship between healthcare institutions and Standards Development Organizations are recommended to achieve and maintain semantic interoperability in a clinical setting.
Introduction
Health information technology, through the important role it plays in improving the quality and effectiveness of the healthcare system, has generated an unprecedented level of public interest and appreciation during the past several years. A major task for health information technology is to enable data exchange, integration, and use of patient data originating from different sources.
“Interoperability” is the ability of two or more health care information systems to exchange information and to use the information that has been exchanged. A recent survey compiled meanings of the term “interoperability” from 100 sources across multiple industries and countries and proposed three distinctions: technical (or functional), semantic, and process interoperability. 1 “Technical interoperability” ensures that these systems reliably exchange information without error. This type of interoperability is often realized with the exchange of textual data. Next, “semantic interoperability” requires the ability to interpret and, therefore, to make effective use of the exchanged information. “Process interoperability” assumes that exchanged information can support a coordinated care workflow.
This paper describes a two-year old project, called “Clinical Data Repository/Health Data Repository” (CHDR), that builds on previous functional interoperability efforts between VA and DoD and, for the first time, enables semantic interoperability. The project offers a general strategy to accomplish semantic interoperability and its applicability in a multi-system clinical setting, an operational maintenance plan to preserve it over time, and baseline mediation success rates achieved in pharmacy and allergy domains.
Background
The Need
Local and federal policies have played a significant role as a catalyst in the development of health information technology and interoperability. The President’s eGov initiative, including Executive Order 13410, and the creation of the Office of the National Coordinator of Health Information Technology to improve health care delivery led to calls for federal agencies (including VA and DoD) to “develop and deploy electronic medical records that are interoperable and standards-based.”
The creation of a complete interoperable patient information record, available to clinicians and consumers, is also driven by economic factors. For instance, it is estimated that fully standardized, nationwide semantic interoperability for exchanging patient data could save $77.8 billion each year in redundant testing and administrative overhead. 2,33
Previous studies suggest that standards-based interoperability will result in a positive long-term return on investment for Electronic Health Records (EHRs). These studies propose various incentives and policy changes, among other things, to increase adoption of standards and make interoperability a requirement for clinical information systems. 3,33
Policies, quality, economic benefits, and organizational proximities have provided VA and DoD with unique incentives perhaps not available to others to make significant progress in interoperability.
The Environment
VA and DoD are both distributed health care delivery systems that share a large patient population. There have been several projects between VA and DoD to enable patient data exchange. Previous efforts, still active, provide uni-directional and real time bi-directional textual data exchange. 4 These programs, installed at all VA sites and many DoD sites, give clinicians access to textual patient data from the other agency, including demographic and clinical domains. However, the data are not computable (i.e., associated with alphanumeric codes that denote meaning) and, as a result, patients do not benefit from health information decision support such as testing for drug-allergy interactions, drug-drug interactions, and duplicative drug therapy. Whereas the previous efforts responded to the technical interoperability need, the work reported here aims to realize semantic interoperability.
DoD and VA purchase 40% of the care they provide to beneficiaries. In order to achieve their goal for comprehensive and longitudinal electronic health record, DoD and VA have to capture and exchange health information with all healthcare partners. Pilot studies are underway in Pensacola, Florida, Spartanburg, South Carolina and as part of the National Health Information Network Trial Implementation to examine the feasibility of sharing clinical data with non-federal partners.
Related Interoperability Efforts
Various organizations and projects have identified interoperability as a key enabling technology, including the National Health Information Infrastructure (NHII), 5,6 the Public Health Information Network (PHIN) preparedness initiative, 7 other secondary uses of health data for bio-surveillance and research, 8 the Commission on Systemic Interoperability, 9 the National Library of Medicine (NLM) “Next 20 Years” Plan, 10 and the World Wide Web Consortium’s (W3C) Semantic Web Health Care and Life Sciences Interest Group. 11
In general, implementation of health information exchange projects has been limited due to lack of funding and the need for a better understanding of technical and organizational issues. 12,13,14 A few projects have demonstrated successful implementation at various semantic levels such as the Indiana Health Network 15 and the Massachusetts MAShare project. 16 Finally, at the international level, several countries including the UK NHS, 17 Canada Infoway, 18 France DMP, 34 and Australia NHETA 19 are engaged in extensive standardization and mediation-based interoperability efforts in the pursuit of a national electronic health record.
In contrast to the VA-DoD project, these efforts did not attempt to standardize the data sources or enable decision support on the data exchanged.
Design Objectives
The project objective is to develop an interface between DoD’s national clinical data store called the Clinical Data Repository (CDR) and VA’s national data store called the Health Data Repository (HDR). This initiative, known as “CHDR,” supports the secure, real time bi-directional exchange of computable health data for “Active Dual Consumer” patients, or those patients who receive treatment at both VA and DoD treatment facilities.
To meet semantic interoperability requirements, VA and DoD have defined the following objectives: (1) Provide the ability to exchange codified data through a standards-based approach, using standards recommended by the federal Consolidated Health Informatics (CHI) initiative, now subsumed by the public-private Health Information Technology Standards Panel (HITSP) organization. 20 (2) Allow health care providers and computers to use the exchanged information in care settings to improve the quality, safety, continuity, and efficiency of care for shared patients. In particular, the aim is to enable decision support processing such as checking for drug-drug interactions, drug allergies, and duplicative therapies on both the data collected locally and the data received from the other agency. (3) Catalog gaps in interoperability standards and identify implementation and maintenance challenges for these standards based on experience from a real-world setting.
It is not within the scope of CHDR to attempt to change the DoD and VA existing terminology standards or repositories, which are very different. Rather, CHDR is the middleware that was designed to connect the two systems where they meet. The CHDR project demonstrates in a clinical setting how data standards and a mediation strategy can enable semantic interoperability. Furthermore, it defines and implements a coordinated maintenance plan between VA, DoD, and the Standards Development Organizations (SDOs). Finally, CHDR reports on actual mediation success rates in pharmacy and drug allergies domains based on real patient data.
System Description
Interoperability Framework
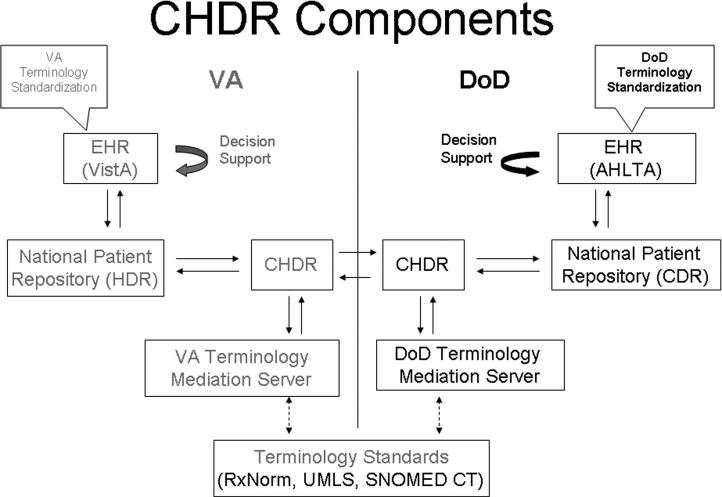
▶ shows the major components that enable the exchange of encoded patient data between VA and DoD.
Figure 1.
Major components of the VA-DoD data exchange CHDR project include the Electronic Health Record (VistA at VA and AHLTA at DoD), terminology standardization programs, national patient data repositories (HDR at VA and CDR at DoD), the CHDR data movement platforms, and the terminology mediation servers—all acronyms are defined in the sections below.
Electronic Health Record (VistA and AHLTA)
VA and DoD have two of the largest clinical information systems in the US: VistA at VA and AHLTA at DoD. AHLTA is deployed at 138 DoD facilities as the electronic record for more than 8.6 million beneficiaries. The VA has more than 1,300 care facilities that care for 7.5 million veterans through 128 VistA instances.
Today after the implementation of the multiple data exchange programs, clinicians at either agency can review data collected by the other agency. The other agency textual data can be accessed under a separate tab. In the CHDR project, shared data are seamlessly integrated (e.g., one medication profile includes prescriptions from both agencies) and order checks are triggered as if all data were collected locally (e.g., a prescription written at VA can trigger an alert because of an interaction with an allergy that was documented at DoD).
Terminology Standardization
Terminology standardization refers to the use of the same set of codes, preferably national standard codes, to encode patient data throughout a system. 21
Both VA and DoD have successfully standardized their terminology for vital signs, allergies, pharmacy, laboratory chemistry and hematology, and note titles with minimal impact to their operational clinical applications. 22 Patient data encoded with non-standard terms such as local compounds and study drugs cannot be exchanged in a codified way and thus cannot trigger decision support.
National Patient Data Repositories (HDR and CDR)
Today after standardization both VA and DoD patient data are collected from the different points of care and assembled into one enterprise health data repository for each agency. Each enterprise repository can then support viewing of the complete patient EHR, population queries, decision support, surveillance studies, and data exchange with external institutions.
At the present time the VA HDR is an HL7 message-based data store. The content includes vitals, allergies, and outpatient pharmacy and will soon include laboratory data and clinical document titles. DoD’s CDR uses HL7 V3 RIM-based models for procedures, diagnoses, encounter notes, allergies, pharmacy, pathology, radiology, microbiology, and immunization data.
Terminology Mediation Servers
VA and DoD have each evolved a terminology maintenance environment based on standard terminologies like SNOMED CT, LOINC, and RxNorm. Although these standard code sets are represented in the terminology servers, they are not directly used by clinical applications but rather mapped to VA or DoD enterprise concepts. Each VA concept is assigned a VA Unique IDentifier (VUID) and each DoD concept is assigned a Numeric Concept ID (NCID). Enterprise-specific terminology content is deployed to clinical applications along with specific services to access terms during runtime. For example, to support mediation, there are translation services that translate from an agency’s internal terminology to the national standard terminologies (e.g., from VUID or NCID to RxNorm CUI). These services were developed conformant with the HL7 specifications for Common Terminology Services. 23
CHDR Patient Data Exchange Gateway
CHDR is the gateway service that enables computable patient data exchange between VA and DoD for shared patients. CHDR incorporates a push and pull model, meaning patient data can be sent automatically upon creation (push) or queried on demand (pull). VA CHDR extracts existing patient data from the HDR, automatically receives subsequent updates, structures data in a commonly agreed-upon HL7 message format, and sends the message to DoD CHDR, where it is unpacked and saved in the DoD CDR along with data created in DoD. Conversely, VA CHDR receives DoD patient data and stores it in the HDR. During these transfers, key clinical elements of the message (e.g., a medication name or an allergen name) are translated from/to the agency vocabulary to/from a commonly agreed-upon mediation terminology.
With this strategy, if a patient is an Active Dual Consumer (i.e., receives care from both VA and DoD), then that patient’s allergies and outpatient pharmacy data are duplicated in both agencies’ national data repositories. When a patient is first designated as an Active Dual Consumer, the data are extracted from one agency and added to the other agency’s national data repository. Then, as new data are created for this patient at either agency’s care sites, the data are immediately and automatically sent to the other agency.
The Mediation Strategy: Mediation-based Interoperability
The two CHDR platforms exchange information using a common syntax and common semantics, in compliance with broad HL7 terminology principles. 24 To achieve semantic interoperability, the two systems could implement the same health terminology. This means that clinicians at both agencies would see the same pick lists for medications, allergies, laboratory tests, etc. when providing and documenting patient care. This may be feasible in the long term, but it requires stable and operational standards that are not in place today; synchronized implementation of these standards; and last, but not least, practice pattern changes at both agencies. An insightful description of this vision of the future is being proposed by a task force on terminology standards. 25
Another practical and cost-effective solution today is for each agency to map its native agency health language to an agreed-upon terminology mediation. This option, mediation-based interoperability, would yield a high success rate, but that rate is unlikely to approach 100 percent from the start, as many practical factors limit it. 26-30 Nonetheless, the coded data that are exchanged are clinically useful. Furthermore, this approach is scalable and superior to direct pair-wise mapping between parties, which is not scalable. The mediation standards, compliant with HITSP recommendations, will enable all federal agencies to “speak the same language” and share that information with minimal impact to clinical operations as detailed in ▶ below, but for many reasons would be imperfect.
Table 1.
Table 1 Clinical Domains Exchanged with the Mediation Terminology, Version Information, and Mapping Method Used to Create Translation Content
| Domain | Mediation Terminology | Version Information | Mapping Method |
|---|---|---|---|
| Medications | RxNorm, limited to Semantic Clinical Drugs (SCDs) | April 2005 (DoD) and June 2005 (VA) | For VA, the mapping was established by the RxNorm team. For DoD, an algorithm-based method was used initially but since abandoned in favor of the mapping established by the RxNorm team [31]. |
| Drug Allergens | UMLS | UMLS 2005 AA | VA extracted existing mapping from UMLS and then completed the task using the UMLSKS command-line query tools. DoD used an algorithmic approach that was developed by Language & Computing, Inc. |
| Allergy Reactions | SNOMED CT | SNOMED CT, Jan 2005 | VA used Apelon’s TermWorks to map its dictionary to SNOMED CT, while DoD used the Clue Browser. |
In each domain, it was necessary to include an independent review of concepts that are common to both agencies to ensure accurate translation.
In addition to their internal terminology maintenance plans, the two agencies and the terminology mediation organizations (e.g., RxNorm team) identified various joint tasks around which they need to communicate terminology content updates to each other to preserve interoperability over time. These joint tasks address the following four sources of changes that affect terminology content: (1) updates to one agency’s terminology, (2) updates to the other agency’s terminology, (3) exceptions or terms that fail to translate during system operation, and (4) updates to the mediation standards (e.g., RxNorm).
For instance, every two months, VA updates its National Drug File. After an update is finalized at VA, a copy is sent through a file transfer protocol (ftp) site to the NLM team in charge of RxNorm. Then the new release of RxNorm is received by the terminologists who extract the mapping information (VA to RxNorm or DoD to RxNorm) as established by the NLM team and import it into their mediation servers.
Mediation Success Rate Calculations
The mediation success rate defines the percentage of data in one system that is understood and computable by the other system. For each direction of the data exchange, inbound or outbound, there is a different mediation success rate. For mediation to succeed, two translations have to be successful. First, the source agency has to translate from its vocabulary to the mediation terminology. Then, the target agency has to translate from the mediation terminology to its native vocabulary without loss of meaning. Both agencies continuously measure these rates by recording every translation attempt and whether it failed or succeeded.
Prior to implementation, the mediation success rates can be estimated based on the number and frequencies of shared versus unique concepts. If the two agencies share frequent findings (e.g., allergy to penicillin) then the mediation success rate will be higher than if the two agencies share rare findings (e.g., allergy to durian).
Status Report
VA CHDR, HDR, and associated servers run on computer systems located in Austin, TX. DoD’s systems are located in Montgomery, AL. During the last 10 months, the CHDR project was implemented at joint VA-DoD care sites in El Paso, TX; Pensacola, FL; Puget Sound, WA; Augusta, GA; North Chicago, IL; and San Diego, CA. 32 Each clinic has a clinic coordinator who is given training and access to the CHDR administrator Web site. Clinic coordinators use this Web site to enroll patients into the VA-DoD patient data exchange program (i.e., mark them as Active Dual Consumers).
The count of Active Dual Consumer patients at El Paso, TX, during the period from July to November 2006 was 2,464, which amounts to about 110 new patients per week. When patients are initially activated, 5 years’ historical outpatient pharmacy data and all available allergies data are pulled, mediated, and exchanged. Thereafter, all newly-collected data are mediated and exchanged in real time.
Agency-to-agency health message engines and CHDR services provide the interagency data message handling between the agencies’ HDR and CDR data repositories. Actual data synchronization for shared patients is accomplished within 120 seconds. The data exchange on application-triggered data updates (additions and changes) for shared patients occurs within 12 seconds.
From September 11 to September 15, 2006, we conducted a user and administrator survey to evaluate System Acceptance Testing (SAT) criteria for CHDR Version 2.0.090. The SAT examined and considered whether CHDR was sufficiently mature to support a full deployment decision. The scope of the SAT covered all operational aspects of the system. Based on survey responses from 46 clinicians and 13 system administrators and a threshold of 70 percent, the CHDR project demonstrated that it can operate in the field environment as expected, that typical end-users and patient administrators can exercise and sustain the system as intended, that clinicians find it useful, and that logistics support performs as expected.
Mediation Content Development, Validation, and Maintenance
Each agency employed 1 to 1 ½ full-time, active terminologists to support the CHDR project. After the initial development of the mediation strategy and the mediation tables, which took several months for each agency, a mapping verification effort was engaged. In the pharmacy domain, the reviews yielded too many defects, and this resulted in the replacement of the algorithm-based mapping method used by one agency in favor of mapping established by the RxNorm group. In the allergies domain, three reviews by two reviewers, each necessitating 10 hours for a total of 60 experts’ hours identified various discrepancies in about 5 percent of the total number of terms. Examples of serious discrepancies include “CEPHAZOLIN” mapped to “OLEIC ACID” and “MG TRISILICATE/AL HYDROX” mapped to “ALUMINUM HYDROXIDE,” whereas an example of a minor problem is “SODIUM PENTOTHAL” mapped to “PENTOTHAL.” All discrepancies that were found in the mapping tables were corrected.
The two agencies agreed on a quarterly update schedule for the pharmacy domain because of the frequency of pharmacy changes and a semi-annual update schedule for the allergy domain. Typically, on the VA side, a content update will include 200 to 300 new medications and 100 to 150 new ingredients, generics, and drug classes. Collecting these changes, submitting them to the RxNorm team, receiving the new releases, and incorporating the updates into the CHDR mediation tables requires 2 to 3 weeks, not accounting for the time taken by NLM. Verification, documentation, and deployment takes another 2 to 3 weeks. These levels of effort are baseline numbers which are being improved with experience. An import report provides the types and counts of updates that are made to the translation information. Verification uses several data consistency rules, such as one VUID must not map to two mediation codes. However, one mediation code could be mapped to two VUIDs, in which case a preferred translation flag indicates the preferred translation term. Translation services are tested through a J-unit test, which tests every translation record in the mapping tables against its known result. A Web-based portal exposes all terminology services for viewing and testing. The response time of the translation services was also ascertained so that system parameters can be configured to meet the needs of the CHDR project in the clinical setting.
Interoperability will require on going support from terminology specialists, although with time we are more efficient. In general, the level of terminology resources will dependent on the size and complexity of the institution, the degree of terminology standardization, and the availability of mediation standards for the domains of data exchanged.
Expected Mediation Success Rates
▶ gives the counts of vocabulary terms per CHDR domain and shows the counts of common and unique terms when the terminologies from the two agencies are compared through their mappings to the same mediation terminology.
Table 2.
Table 2 Common and Unique Concepts Determined by Each Agency Mapping its Domain Terms to the Mediation Terminology
| Totals | VA Unmapped Terms | Mapped Terms Unique to VA | Common Terms | Mapped Terms Unique to DoD | DoD Unmapped Terms | |
|---|---|---|---|---|---|---|
| Pharmacy | VA = 8002 | 2 | 5321 (66%) | 2681 | 2094 (44%) | 0 |
| DoD = 4775 | ||||||
| Drug Allergens | VA = 6821 | 2112 (31%) | 2916 (62%) | 1793 | 6434 (78%) | 0 |
| DoD = 8227 | ||||||
| Allergy Reactions | VA = 344 | 22 (6%) | 25 (8%) | 299 | 47 (13%) | 110 (24%) |
| DoD = 456 |
Each agency mapped its domain terms to the mediation terminology, and the resulting mapping tables were compared to determine common and unique concepts. Typically, unmapped terms are not compared directly between agencies, but rather, are submitted to the mediation terminology group for addition to the next release. The mapped and unmapped percentages shown in parentheses represent the ratios of mapped and unmapped counts to totals.
Before the CHDR project was implemented in a live clinical setting, annual frequency reports for pharmacy and allergy data were obtained from each agency, and the expected mediation success rates were estimated as 96% and 61% respectively.
Actual Mediation Success Rates
After the CHDR project was implemented at three sites, a direct count of translation successes and failures produced the actual translation and mediation success rates as shown in ▶. ▶
Table 3.
Table 3 Actual Translation and Mediation Success Rates in the Pharmacy Domain for Four Time Periods
| 16-Sep to 22-Sep | 23-Sep to 29-Sep | 30-Sep to 13-Oct | 14-Oct to 4-Nov | Totals | |
|---|---|---|---|---|---|
| VA-to-DoD Medications Exchange | |||||
| Total VA-to-RxNorm translation attempts | 43,306 | 37,169 | 17,673 | 58,164 | 156,312 |
| Translation failures ALL | 1,525 | 1,386 | 701 | 2,748 | 6,360 |
| Translation failures – non-standard meds | 1,244 | 1,128 | 381 | 1,681 | 4,434 |
| Translation failures – standard meds | 281 | 321 | 320 | 1,067 | 1,989 |
| Total medications sent to DoD CHDR | 41,781 | 35,783 | 16,972 | 55,416 | 149,952 |
| Translation Success Rate VA-to-RxNorm | 99% | 99% | 98% | 98% | 99% |
| Total medications received from VA | 41,781 | 35,783 | 16,972 | 55,416 | 149,952 |
| Translation failures ALL | 5,281 | 4,208 | 2,487 | 7,099 | 19,075 |
| Translation failures – non RxNorm SCDs | 2,515 | 2,067 | 1,181 | 3,076 | 8,839 |
| Translation failures – RxNorm SCDs | 2,766 | 2,141 | 1,306 | 3,375 | 9,588 |
| Total VA medications stored in DoD CDR | 36,500 | 31,575 | 14,485 | 48,317 | 130,877 |
| Translation Success Rate: RxNorm-to-DoD | 93% | 94% | 92% | 93% | 93% |
| Mediation Success Rate VA-to-DoD | 92% | 93% | 90% | 92% | 92% |
| DoD-to-VA Medications Exchange | |||||
| Total DoD-to-RxNorm translation attempts | 11,545 | 12,030 | 4,809 | 16,295 | 44,679 |
| Translation failures ALL | 1,830 | 1,446 | 1,002 | 2,504 | 6,782 |
| Translation failures – non standard meds | 1,376 | 1,040 | 729 | 1,721 | 4,866 |
| Translation failures – standard meds | 454 | 406 | 273 | 783 | 1,916 |
| Total medications sent to VA CHDR | 9,715 | 10,584 | 3,807 | 13,791 | 37,897 |
| Translation Success Rate: DoD-to-RxNorm | 96% | 96% | 93% | 95% | 95% |
| Total medications received from DoD | 9,715 | 10,584 | 3,807 | 13,791 | 37,897 |
| Translation failures ALL | 1,695 | 1,850 | 625 | 2,614 | 6,784 |
| Translation failures – RxNorm SCDs | 193 | 181 | 27 | 224 | 625 |
| Translation failures – non RxNorm SCDs | 1,502 | 1,669 | 598 | 2,390 | 6,159 |
| Total DoD medications stored in VA HDR | 8,020 | 8,734 | 3,182 | 11,177 | 31,113 |
| Translation success rate: RxNorm-to-VA | 98% | 98% | 99% | 98% | 98% |
| Mediation Success Rate DoD-to-VA | 93% | 94% | 93% | 93% | 93% |
▶ provides an explanation of the rows and counts included in this table.
Table 4.
Table 4 Actual Translation and Mediation Success Rates in the Allergies Domain for Four Time Periods
| 16-Sep to 22-Sep | 23-Sep to 29-Sep | 30-Sep to 13-Oct | 14-Oct to 4-Nov | Totals | |
|---|---|---|---|---|---|
| VA-to-DoD Allergens Exchange | |||||
| Total VA-to-UMLS translation attempts | 259 | 225 | 61 | 266 | 811 |
| Translation failures | 51 | 54 | 12 | 47 | 164 |
| Total allergies sent to DoD CHDR | 208 | 171 | 49 | 219 | 647 |
| Translation Success Rate: VA-to-UMLS | 80% | 76% | 80% | 82% | 80% |
| Total allergies received from VA CHDR | 208 | 171 | 49 | 219 | 647 |
| Translation failures | 50 | 25 | 11 | 31 | 117 |
| Total VA allergies sent to DoD CDR | 158 | 146 | 38 | 188 | 530 |
| Translation Success Rate: UMLS-to-DoD | 76% | 85% | 78% | 86% | 82% |
| Mediation Success Rate VA-to-DoD | 61% | 65% | 62% | 71% | 65% |
| DoD-to-VA Allergens Exchange | |||||
| Total DoD-to-UMLS translation attempts | 203 | 265 | 477 | 749 | 1,694 |
| Translation failures | 45 | 70 | 90 | 197 | 402 |
| Total allergies sent to VA CHDR | 158 | 195 | 387 | 552 | 1,292 |
| Translation Success Rate: DoD-to-UMLS | 78% | 74% | 81% | 74% | 76% |
| Total allergies received from DoD CHDR | 158 | 195 | 387 | 552 | 1,292 |
| Translation failures | 28 | 41 | 86 | 119 | 274 |
| Total DoD allergies sent to VA HDR | 130 | 154 | 301 | 433 | 1,018 |
| Translation success rate: UMLS-to-VA | 82% | 79% | 78% | 78% | 79% |
| Mediation Success Rate DoD-to-VA | 64% | 58% | 63% | 58% | 60% |
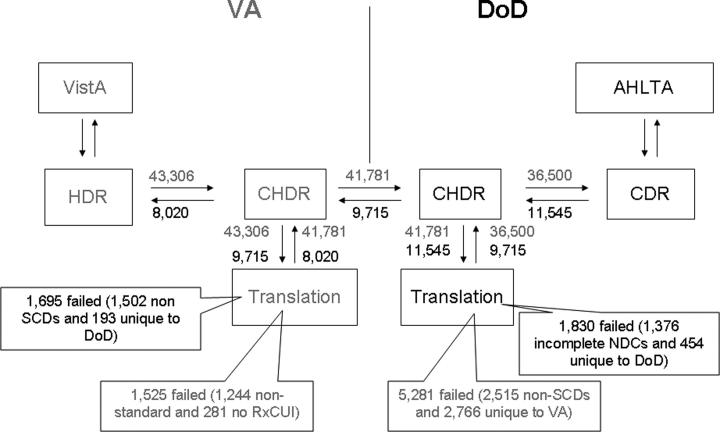
To facilitate the reading of ▶, ▶ takes the first column (16-Sept to 23-Sept) and illustrates the flow of patient data element counts.
Figure 2.
CHDR framework with patient pharmacy data counts shown in both directions for the week of Sept 16 to 22, 2006 with reference to ▶.
The actual mediation success rates are greater than 92 percent in both directions and consistent with initial projections. The difference between expected and actual results may be due to the fact that the frequency data used are from 2005, whereas actual exceptions are from September and October 2006 and prescription patterns may have changed in 2006; and, also, the fact that the frequency data represents national counts whereas the exceptions are from a few care sites. Fluctuations between time periods are likely to be due to fluctuation in patient case mix from week to week.
Like the pharmacy domain, the actual mediation rates for allergies are consistent with initial projections.
Discussion
Mediation Exceptions
Mediation exceptions correspond to all data elements that are not exchanged because of terminology failures. The first category of exceptions corresponds to non-standardized data elements. In the pharmacy domain, for instance, these are data elements without a VUID on the VA side or without an 11-digit NDC code on the DoD side. These are generally investigational drugs (e.g., ACCORD-FUROSEMIDE 20MG OL STUDY DRUG), local compounds (e.g., 300MG PAPAVER/100MCG ALPROST/10MG PHNTOL), or drugs that failed to be matched to the mediation standard in the first place (e.g., CODEINE 10MG/GUAIFENESIN 100MG/5ML (SF & AF) LIQUID). This group of exceptions did not penalize the mediation rates since they were considered to be a limitation of standardization and not mediation. From the data above, one can estimate that non-standard data account for 3 to 11 percent of the total volume of pharmacy data, and for about 71 and 75 percent of the total volume of translation failures. Pharmacy reports of non-standard data are sent to local sites, which are asked to improve their match to the standard. Local sites are motivated to standardize their names as much as possible because only standard pharmacy products are eligible for mail order, thus reducing medication costs.
The second category corresponds to medications or allergens that are unique to each agency. Through a review of exceptions that were encountered during the first 2 months of use, differences were categorized as follows. Actions that have been taken or that will be taken to reduce these differences are shown in parentheses.
Medications
Differences in formularies (which can be partially mitigated using RxNorm relationships—see Smart Mediation section below), incorrect DoD mappings (which have been corrected), incomplete VA mappings (which have been mitigated with a faster and improved process for submission to NLM), and supplies (which can be omitted because they are out of scope for this phase of this project).
Drug Allergens
Brand names vs generics and different salt forms (which can be partially mitigated if we leverage relationships such as “has_tradename” and “has_form” that are present in both UMLS and RxNorm), incorrect DoD mappings (which have been corrected), and incomplete VA mappings (which have been mitigated with a faster and improved process for submission to NLM). Also, DoD has an extensive list of over-the-counter (OTC) drugs, herbals, and dietary supplements; whereas VA has only a small number of these items.
The most frequent pharmacy exceptions to mediation from DoD to VA are Acetaminophen 100 MG/ML Oral Suspension, Guaifenesin 600 MG/Pseudoephedrine 120 MG Extended Release Tablet, Vitamin D2 1.25 MG Oral Capsule, Cetirizine 5 MG/Pseudoephedrine 120 MG 12 hour Extended Release Tablet, and Vitamin E 400 UNT Oral Capsule. Note the challenge with the dosage form differences, “Extended Release” vs. “12 hour Extended Release,” and combination drugs. These top five data elements account for 55 percent of all valid terminology exceptions. The most common pharmacy exceptions to mediation from VA to DoD are OMEPRAZOLE 20MG CAP,SA, MENTHOL 2%/METHYL SALICYLATE 10% OINT,TOP, VARDENAFIL HCL 20MG TAB, BUPROPION HCL 150MG 12HR TAB,SA, and LOSARTAN POTASSIUM 100MG TAB. These top five data elements account for 45 percent of all valid terminology exceptions.
The most common allergies exceptions to mediation from DoD to VA are Cipro, Tequin, Lamisil, Avandia, and Motrin. Most of the exceptions are brand name allergens not available as such in the VA allergies vocabulary. These top five entries account for 35 percent of all valid terminology exceptions. The most common allergies exceptions to mediation from VA to DoD are CONTRAST MEDIA, OTHER, INFLUENZA, AMITRIPTYLINE PREPARATION, TOLMETIN PREPARATION, TUBERCULIN, PURIFIED PROTEIN DERIVATIVE PREPARATION. These top five entries account for 34 percent of all valid terminology exceptions.
Note that, because RxNorm SCDs are the recommended standard for clinical drugs and the scope of CHDR is limited to clinical drugs only, non-SCDs such as supplies, brand names, etc., were omitted from the calculations.
Although CHDR has not yet reached 100 percent interoperability, patients whose data are even partially translated receive the benefits of decision support. Any alert or reminder that is triggered has the potential to improve patient safety even if other possible alerts do not occur because of incomplete data exchange. Clinicians at both institutions agreed that this is a benefit that was not there before. For patients whose data are not completely exchanged or are mis-translated, there may be false negative or false positive alerts. Typically physicians review and validate patient’s medication and allergy profiles through patient history taking and other text-based data exchange programs. 4
When interoperability is achieved through mediation, the question of what text is displayed to the clinician user is posed. In the CHDR project, the choice has been made to display the target system text only. An alternative could be to display both the target and the source texts, but this alternative requires screen real estate, and clinicians would be implicitly asked to review translation accuracy and completeness. A second issue for clinicians is whether free text should be exchanged when mediation of coded information fails. At the present time, the CHDR project “drops” data that it cannot mediate, except allergy reactions which are exchanged as free-text when coded mediation fails. VA and DoD clinicians have alternative means through the existing text-based data exchange programs to access the text based pharmacy and allergies record. If CHDR exchanges free text when coded mediation fails, it will be necessary to address the question of how to indicate to the clinician user that some data were mediated in coded form and benefited from decision support services while other data were mediated in textual form only without the screening of decision support.
The preceding discussion describes how full interoperability is limited by mediation exceptions, but interoperability is also limited by other factors. Factors affecting interoperability between a source and target system include: (1) Terminology standardization—If standardization is not accurate and complete, all the data that are collected locally cannot be aggregated within the enterprise database and mediated with partners (e.g., invalid NDC codes, local drug compounds, or investigational drugs). (2) Computability—This refers in part to the decision support services that are available and applicable to one piece of data. VA and DoD systems have different rules for drug-drug interactions, duplicative therapies, drug class memberships, etc., and this results in different uses of the data after they are mediated. (3) Transmission—There could be network data loss problems; data could be incorrectly formatted; or the system could go down during transmission, etc. (4) Translation—Translation is not 100 percent accurate and comprehensive. There could be mapping accuracy problems, and mediation terminologies might not be complete. This means there could be concepts in a given agency’s reference list for which there is no translation. (5) Mediation—If either system fails to translate a piece of data, the result is a failed mediation and failure to exchange that piece of data. The relative importance of these factors remains to be precisely quantified in the CHDR project. However, preliminary data indicate that reliable mediation and terminology standardization are major factors (80 percent), but the other factors are important (20 percent). For instance, VA non-standard pharmacy data elements account for about 5 percent of a patient pharmacy record.
The State of Mediation Terminologies
RxNorm is a drug terminology maintained and distributed by NLM. It is organized around a set of normalized names for drugs that are prescribed to patients. The RxNorm identifiers are linked to identifiers from various drug information suppliers including VA, Food and Drug Administration, SNOMED CT, First DataBank, MicroMedex, Medispan, and Multum. This linkage allows RxNorm identifiers to serve as a translation or mediation between disparate drug vocabularies; in this case, between the VA vocabulary and the First DataBank commercial drug vocabulary that is used by DoD. The CHDR project is by far the largest implementation of RxNorm to date. 31
RxNorm is currently released monthly, with plans to increase release frequency. NLM and RxNorm have had to develop some new processes to address issues that were brought to light by CHDR. In some cases, solutions have focused on training and clarification of RxNorm’s editorial policies. In other cases, detailed analysis of the mappings performed by RxNorm has revealed a small number of incorrect mappings (e.g., DIATRIZOATE MEGLUMINE 52%/DIATRIZOATE NA 8% INJ from the VA formulary has been mis-mapped to Isopropanol 700 MG/ML Injectable Solution), missed mappings (ACETIC ACID 0.9%/OXYQUINOLINE SO4 0.025% GEL,VAG from the VA formulary was mapped to Acetic Acid 0.009 MG/MG Vaginal Gel instead of Acetic Acid 0.009 MG/MG/Oxyquinoline 0.00025 MG/MG Vaginal Gel), and duplicate concepts (e.g., Glucose 250 MG/ML Injectable Solution and Glucose 250 MG Injectable Solution refer to the same clinical product but are present as two different concepts with two different identifiers).
The Unified Medical Language System (UMLS) is a collection of more than 120 dictionary sources (e.g., ICD-9-CM, SNOMED CT, LOINC, CPT, RxNorm, etc.) that are described in the same framework, with representation or mappings of common and related concepts. UMLS is maintained and distributed by NLM, and its content may be used freely unless otherwise specified. UMLS mappings can support various interoperability projects. The process to submit content to UMLS is not as straightforward as it is for RxNorm. UMLS is also missing coverage of some multi-ingredient generics that have resulted in many unmapped VA terms. UMLS utilities to map a single term or batch of terms have proven very useful to VA.
SNOMED CT has excellent coverage of signs and symptoms that can be used to mediate allergy reactions. However, these are not organized in a well-circumscribed subset and, as a result, it is easy for two organizations using SNOMED CT to pick different terms and create mediation exceptions. For example, “Vomiting” can be mapped to two different SNOMED CT codes. VA maps it to 300359004—Finding of vomiting (finding), while DoD maps it to 249497008—Vomiting symptom (finding). Also, SNOMED CT is not organized as a mediation terminology to represent mappings between different vocabularies. Mappings to SNOMED CT terms have to be created and, unless the same mapping rules are adopted by both agencies, quality issues ensue. For example, one mapping rule that VA and DoD had to adopt was to select allergy reactions from the Findings hierarchy of SNOMED CT rather than from the Diseases and Disorders hierarchy. Another mapping rule is to map only to active terms. SNOMED CT is released as part of UMLS, and it offers a process for requesting changes. A few missing allergy reactions were submitted to SNOMED for addition (e.g., SEROTONIN WITHDRAWAL SYNDROME, INCREASED LDL).
Parallel to the need for strong communication between agencies exchanging patient data is the need for equally strong communication between these agencies with SDOs. For instance, when an SDO releases a new version of its standards, ideally all clients should implement the new version within a given time frame. Except for some “billing” code sets (ICD-9-CM, CPT, DRG, and HCPCS), adoption of SDO data is seldom either timely or automated. Consequently, there will be situations in which two institutions may be using different versions of a mediation standard. While this may have a negative impact on interoperability, it is unlikely a synchronized implementation schedule can be achieved from the start. Over time, VA and DoD have made progress and have recently reached synchronized implementation schedules in pharmacy and will do the same next in allergy.
In the long term, national terminology standards may be implemented natively within each agency’s electronic health record solution, which would eliminate the need for mediation since the two agencies would speak the same health language. This would require that the national terminologies be complete, stable, adaptable by clinical applications, and affordable. Furthermore, the communications between SDOs and consumers of these terminologies need to be strengthened with a shared governance. 25
Lessons Learned
Important success factors learned from the CHDR project, which could be useful to the many health information exchange efforts include:
• Qualified resources including staff skilled in terminology, familiar with the inner workings of SDOs, and expert in domain subject matters. Furthermore, both teams need to have detailed knowledge of both agencies’ clinical information systems, terminology standards, data exchange software behavior, decision support modules, etc.
• Performance measurements to monitor the behavior of the system services need to be anticipated (e.g., exceptions, success rates, percentages of patients whose records are completely mediated).
• A common mapping method is critical to yielding reliable concept matches between the two agencies. Also, a coordinated maintenance plan is needed to preserve a good mediation success rate over time.
• Well coordinated relationships with SDOs; in particular, there is a need for standard formats and tools for submissions, a process for requesting new terms, and a predictable release schedule.
• Regular communications with all stakeholders about mediation strategy, interoperability rates, patient safety issues, resources, QA findings, etc. Management needs to continuously nurture the commitment to collaboration between all the players.
Future Extensions
New domains of patient data will be added to the CHDR project in the future, starting with laboratory chemistry and hematology results, where the LOINC nomenclature is selected as the mediation terminology. Also, new VA and DoD care sites will continue to be added with a full release scheduled in late 2007. In the previous 10 months, sites have been added without significant changes to the current framework. The centralized framework of the data repositories, the CHDR software, and the mediation servers do not change. Privileges are assigned to the new site coordinators and training is provided. General communications are provided to clinician users to inform them of the availability of new sources for patient data.
Today’s mediation is an all-or-nothing mediation in the sense that either two concepts are equivalent or they are not. No partial mapping is given credit. For instance, the VA and DoD medications GUAIFENESIN 600MG/PSEUDOEPHEDRINE 120MG TAB,SA and GUAIFENESIN/PSEUDOEPHEDRINE HCL (AMI-TEX PSE EQ.) TABLET CONTROLLED/SUSTAINED RELEASE 600/120 ORAL do not mediate because they are associated with two different RxCUIs. However, it is easy to see that these drugs share the same clinical components. In the allergy domain, the majority of differences that have been observed between VA and DoD are due to brand vs. generic names or different salt forms of the same therapeutic moiety. In this domain, we calculated that by using RxNorm relationships ‘has_tradename’ and ‘has_form’, there would be a 32 percent increase in mediation success from DoD to VA and a 22 percent gain from VA to DoD.
Finally, after semantic interoperability is achieved, attention will turn to how the data are used to facilitate and improve patient care. This is related to the “process interoperability” that was mentioned earlier. Different decision support systems will generate different alerts and reminders, based on the same patient data. For instance, we have already noticed that, because of drug class memberships being different at the two agencies due to the use of two different drug classifications, the same drug-drug interaction, drug-allergy, and duplicative therapy checks do not occur even when the patient record is fully mediated.
Acknowledgments
This work is collaborative in nature and brings together eclectic teams and organizations all committed to its success, including VA staff and contractors within the VA Office of Information and Technology, in particular, the Standards & Terminology Services team (Holly Miller, Loren Stevenson, Jeff Chevalier, Hayes Murdock, Glen Crandall, Marcia Insley), CHDR (David Seitz, Randall Baylis, Dick Rickard, Ted Baxter), HDR (Tim Cromwell, Steve Martin), Allergy application (David Naber), and Pharmacy application (Don Lees, Lynn Sanders); DoD staff and contractors (Kyle Marchant, Amy Neilson, Mike Morgan, Richard McLean, Cathy Chambers, Richard Van Arsdel, and Drs. Bart Harmon, Henry Gibbs and Mathew Garber); the inter-agency support staff (Laura Megas, Anand Shukla, Robert Fox); and RxNorm team at NLM, in particular, Dr. Stuart Nelson. The authors are also grateful to Drs. Steve Brown, Bonnie Arze, John Carter, and Larry Fagan, and to Barbara Baskett and Kathleen Barnett for their review of this manuscript.
References
- 1.Gibbons P, Arzt N, Burke-Beebe S, Chute C, Dickinson G, Flewelling T, et al. Coming to Terms: scoping interoperability for healthcare. Health Level 7 Electronic Health Record Workgroup. 2007. February.
- 2.Health Research Institute & Global Technology Center Reactive to Adaptive: Transforming Hospitals with Digital Technology, PriceWaterhouseCoopers 2005.
- 3.Middleton B, Hammond WE, Brennan PF, Cooper GF. Accelerating U.S. EHR Adoption: How to Get There From Here. Recommendations Based on the 2004 ACMI Retreat. J. Am. Med. Inform. Assoc 2005;12(1):13-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Donham GW, Mallia T. Architecture of the FHIE J AHIMA 2006;77(7):60-64Jul-Aug, 66. [PubMed] [Google Scholar]
- 5.Stead WW, Kelly BJ, Kolodner RM. Achievable Steps Toward Building a National Health Information Infrastructure in the United States J. Am. Med. Inform. Assoc 2005;12(2):113-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yasnoff WA, Humphreys BL, Overhage JM, Detmer DE, Brennan PF, Morris RW, Middleton B, Bates DW, Fanning JP. A Consensus Action Agenda for Achieving the National Health Information Infrastructure J. Am. Med. Inform. Assoc 2004;11(4):332-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Loonsk JW, McGarvey SR, Conn LA, Johnson J. The Public Health Information Network (PHIN) Preparedness Initiative J. Am. Med. Inform. Assoc 2006;13(1):1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Safran C, Bloomrosen M, Hammond WE, Labkoff S, Markel-Fox S, Tang PC, Detmer D. Toward a National Framework for the Secondary Use of Health Data: An American Medical Informatics Association White Paper J. Am. Med. Inform. Assoc 2007;14(1):1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Commission on Systemic Interoperability. Ending the document game. Connecting and transforming your health care through information technology. Available at: http://endingthedocumentgame.gov/report.html. Accessed on Jul 15, 2007. [PubMed]
- 10. NLM Long Range Plan September 2006http://www.nlm.nih.gov/pubs/plan/lrpdocs.html 2007. Accessed on Jul 15, 2007.
- 11.World Wide Web Consortium’s (W3C) Semantic Web Health Care and Life Sciences Interest Grouphttp://www.w3.org/2001/sw/hcls 2007. Accessed on Jul 15, 2007.
- 12.Overhage JM, Evans L, Marchibroda J. Communities’ Readiness for Health Information Exchange: The National Landscape in 2004 J. Am. Med. Inform. Assoc 2005;12(2):107-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.CalRHIO ED Linking to Information Project Survey of Vendor Solutions. October 2006http://www.calrhio.org/crweb-files/docs-vendor/20061116%20Vendor%20Solutions.pdf 2005. Accessed on Jul 15, 2007.
- 14. 2006 Third Annual Survey of Health Information Exchange at the State, Regional and Community levelshttp://toolkits.ehealthinitiative.org/assets/Documents/eHI2006HIESurveyReportFinal09.25.06.pdf 2005. Accessed on Jul 15, 2007.
- 15.McDonald JC, Overhage JM, Barnes M, Schadow G, Blevins L, Dexter PR, Mamlin B. The Indiana Indiana Health Network: A Local Health Information Infrastructure. An example of a working infrastructure collaboration that links data from five health systems and hundreds of millions of entries. Health Affairs 2005;24(5):1214-1220. [DOI] [PubMed] [Google Scholar]
- 16.Halamka J, Aranow M, Ascenzo C, Bates D, Debor G, Glaser J, Goroll A, Stowe J, Tripathi M, Vineyard G. Health Care IT Collaboration in Massachusetts: The Experience of Creating Regional Connectivity J. Am. Med. Inform. Assoc 2005;12(6):596-601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.UK NHShttp://www.connectingforhealth.nhs.uk/technical/standards/ 2005. Accessed on Jul 15, 2007.
- 18.Canada Infowayhttp://www.infoway-inforoute.ca/en/Home/home.aspx 2005. Accessed on Jul 15, 2007.
- 19.NEHTA (Australia): National E-Health Transition Authorityhttp://www.nehta.gov.au/content/view/1/103/ 2005. Accessed on July 2007.
- 20.Health Information Technology Standards Panel (HITSP)http://www.ansi.org/hitsp/ 2005. Accessed on July 2007.
- 21.Lau LM, Shakib S. Towards Data Interoperability: Practical Issues in Terminology Implementation and Mapping 2005. Clinical Vocabulary Mapping Methods Institute, 77th AHIMA Convention and Exhibit, October.
- 22.Bouhaddou O, Lincoln M, Maulden S, Murphy H, Warnekar P, Nguyen V, Lam S, Brown S, Frankson F, Crandall G, Hughes C, Sigley R, Insley M, Graham G. A Simple Strategy for Implementing Standard Reference Terminologies in a Distributed Healthcare Delivery System with Minimal Impact to Existing Applications AMIA Annu Symp Proc 2006;2006:76-80. [PMC free article] [PubMed] [Google Scholar]
- 23.HL7 CTS specificationshttp://www.hl7.org 2006. Accessed on Jul 15, 2007.
- 24.Bakken S, Campbell KE, Cimino JJ, Huff SM, Hammond WE. Toward Vocabulary Domain Specifications for Health Level 7—coded Data Elements J Am Med Inform Assoc 2000;7(4):333-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Healthcare Terminologies and Classifications: An Action Agenda for the United States 2006. Terminology and Classification Policy Task Force November.
- 26.Hardiker NR. Mediating between nursing intervention terminology systems Proc AMIA Symp 2001:239-243. [PMC free article] [PubMed]
- 27.Lau LM, Banning PD, Monson K, Knight E, Wilson PS, Shakib SC. Mapping Department of Defense laboratory results to Logical Observation Identifiers Names and Codes (LOINC) Proc AMIA Symp 2005:430-434. [PMC free article] [PubMed]
- 28.Choi J, Jenkins ML, Cimino JJ, White TM, Bakken S. Toward semantic interoperability in home health care: formally representing OASIS items for integration into a concept-oriented terminology J Am Med Inform Assoc 2005;12(4):410-417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mougin F, Burgun A, Bodenreider O. Using WordNet to Improve the Mapping of Data Elements to UMLS for Data Sources Integration Proc AMIA Symp 2006:574-578. [PMC free article] [PubMed]
- 30.Sun JY, Sun Y. A system for automated lexical mapping Am Med Inform Assoc 2006;13(3):334-343May–Jun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parrish F, Do N, Bouhaddou O, Warnekar P. Implementation of RxNorm as a Terminology Mediation Standard for Exchanging Pharmacy Medication between Federal Agencies AMIA Annu Symp Proc 2006;2006:1057. [PMC free article] [PubMed] [Google Scholar]
- 32.The Government Accounting Office report on VA-DoD CHDR projecthttp://www.gao.gov/new.items/d07554r.pdf visited on 7/20/07. Accessed on.
- 33.Walker J, Pan E, Johnston D, Adler-Milstein J, Bates DW, Middleton B. The Value of Health Care Information Exchange and Interoperability Health Affairs 2005. 10.1377/hlthaff.w5.10. [DOI] [PubMed]
- 34.Dossier Médical Personnel-DMP (France)http://www.d-m-p.org/ 2005. Accessed on Jan 7, 2008.