Abstract
Repeated daily application transcutaneous electrical nerve stimulation (TENS) results in tolerance, at spinal opioid receptors, to the anti-hyperalgesia produced by TENS. Since N-Methyl-D-Aspartate (NMDA) receptor antagonists prevent analgesic tolerance to opioid agonists we hypothesized that blockade of NMDA receptors will prevent tolerance to TENS. In rats with knee joint inflammation, TENS was applied for 20 minute daily at high frequency (100 Hz), low frequency (4 Hz), or sham TENS. Rats were treated with the NMDA antagonist MK-801 (0.01 mg/kg-0.1 mg/kg) or vehicle daily before TENS. Paw withdrawal thresholds were tested before and after inflammation, and before and after TENS treatment for 4 days. On day 1 TENS reversed the decreased mechanical withdrawal threshold induced by joint inflammation. On day 4 TENS had no effect on the decreased withdrawal threshold in the group treated with vehicle demonstrating development of tolerance. However, in the group treated with 0.1 mg/kg MK-801, TENS significantly reversed the mechanical withdrawal thresholds on day 4 demonstrating that tolerance did not develop. Vehicle treated animals developed cross-tolerance at spinal opioid receptors. Treatment with MK-801 reversed this cross-tolerance at spinal opioid receptors. In summary, blockade of NMDA receptors prevents analgesic tolerance to daily TENS by preventing tolerance at spinal opioid receptors.
Perspective
Tolerance observed to the clinical treatment of TENS could be prevented by administration of pharmaceutical agents with NMDA receptors activity such as ketamine or dextromethorphan.
Keywords: Inflammation, pain, tolerance, MK-801, TENS, opioid
Transcutaneous Electrical Nerve Stimulation (TENS) is a clinically used non-pharmacological treatment for painful conditions that involves applying electric current through electrodes placed on the skin. Systematic reviews show that TENS reduces pain and improves function in people with knee osteoarthritis 35, rheumatoid arthritis 7 and chronic musculoskeletal pain 21. In animal models of joint, muscle, and cutaneous inflammation both high and low frequency TENS reduces hyperalgesia 1,14,23,38,40,47. Thus, TENS reduces pain in humans and reverses hyperalgesia in animals.
Previous studies show that TENS mediates analgesia through activation of the endogenous opioid system. In an animal model of inflammation, spinal or supraspinal blockade of δ-opioid receptors prevents anti-hyperalgesia produced by high frequency TENS while blockade of μ-opioid receptors prevents anti-hyperalgesia produced by low frequency TENS 22,42. Further, daily administration of high or low frequency TENS results in decreased effectiveness of TENS by the 4th day in a rat model of joint inflammation 8. There was also a reduced effectiveness of intrathecally administered μ- or δ-opioid agonists in animals that were treated with either low or high frequency TENS, respectively 8. Thus, TENS activates specific opioid receptors to produce analgesia, and repeated application of TENS results in tolerance at spinal opioid receptors.
Treatment with the NMDA receptor antagonist MK-801 in rodents prevents the development of analgesic tolerance to exogenously administered opioids. In rats, systemic administration of MK-801, either before or 2 h after administration of the μ-opioid agonist morphine reduces analgesic tolerance to morphine 32,33. Similarly, analgesic tolerance to chronic δ1-opioid agonist DPDPE administration in mice is prevented by pretreating with MK-801 53. Thus, the above studies suggest that the non-competitive NMDA antagonist MK-801 prevents the development of tolerance to μ- and δ-opioid agonists. Given that NMDA receptor antagonists prevent the analgesic tolerance to opioids and that repeated application of TENS results in tolerance at spinal opioid receptors, we hypothesized that blockade of NMDA receptors with MK-801 will prevent analgesic tolerance to repeated application of TENS by preventing tolerance at spinal opioid receptors.
Methods
All experiments were approved by the Animal Care and Use Committee at the University of Iowa and are in accordance with the National Institutes of Health guidelines for care and use of laboratory animals. Male Sprague–Dawley rats (n=107, 225 – 350 g, 2-3 months old, Harlan, Indianapolis, IN) were used for the study.
Behavioral testing
Rats were tested for withdrawal thresholds to mechanical stimuli using von Frey filaments applied to the plantar aspect of the hind paw as previously described 14. Animals were acclimated to the testing room for 30 min after transport to the laboratory from the animal care facilities. They were then placed in transparent Lucite cubicles (24.6 × 7.5 × 7.5 cm3) on an elevated mesh platform and allowed to acclimate for another 30 min prior to testing. To test withdrawal thresholds to mechanical stimuli von Frey filaments with different bending forces were progressively applied perpendicularly to the plantar aspect of the hind paw. Each filament had one trial which consisted of two consecutive applications of the filament. The lowest bending force at which the rat withdrew its paw from one of the two applications was recorded as the paw withdrawal threshold for mechanical hyperalgesia. This testing method has shown significant statistical test–retest reliability 41.
Induction of inflammation
Knee joint inflammation was induced by intra-articular injection of 0.1 ml 3% kaolin and 3% carrageenan dissolved in sterile saline, pH 7.2 into the left knee joint while the rats were anesthetized with 2-4% halothane 43.
Application of TENS
Rats were lightly anesthetized (1-2% halothane) for the 20 min application of TENS 43. Prior studies show that 1) application of halothane without TENS has no effect on the paw withdrawal latency to heat induced by joint inflammation 8, and 2) application of TENS to a non-inflamed knee joint has no effect on the paw withdrawal latency 40. Commercially available EMPI Eclipse+ TENS units (EMPI, Inc, Minneapolis, MN) and electrodes were utilized. Half-inch round pre-gelled surface electrodes were applied to the medial and lateral aspects of the inflamed knee joint in the groups receiving high or low frequency TENS or sham TENS. High (100 Hz) or low (4 Hz) frequency TENS was administered keeping other parameters constant i.e. pulse duration (100 μsec), sensory intensity, 20 min duration. Stimulus intensity was determined by increasing the intensity until a muscle contraction was visibly observed and then reducing the intensity to just below this level, and was defined as sensory intensity. This intensity was between 9 and 10 mA for all animals. The waveform was an asymmetrical biphasic square wave. The parameters were selected to model those used clinically and which produced full inhibition of secondary heat hyperalgesia 40. The sham group was anesthetized with 1-2% halothane and electrodes were placed on their knee joint but did not receive TENS treatment.
Intrathecal catheter placement
Chronic indwelling intrathecal (i.t.) catheters (32-gauge polyurethane; 10 cm length; Recathco, Allison Park, PA) were used to administer drugs to the lumbar enlargement of the spinal cord of rats 36. Rats were anesthetized with halothane and a 23-gauge hypodermic needle was inserted into the intervertebral space between L5 and L6 vertebrae. A 32-gauge polyurethane catheter was inserted through the needle and advanced cranially 3.5 – 4.0 cm placing the catheter at the level of the lumbar enlargement of the spinal cord. The external portion of the catheter was secured to the muscle and fascia with 4.0 silk sutures. The free end was then inserted into PE 10 tubing, glued, and tunneled out through the skin at the cervical region. All rats were allowed to recover for 3 - 5 days before behavior testing. Drugs were dissolved in saline and administered in a 10 μl volume. At the end of the experiment 10 μl of lidocaine was injected (i.t.) through the PE 50 tubing. If the rats developed paralysis of the hind limbs it was inferred that the catheters were correctly placed in the (i.t.) space. Before rats were euthanized, methylene blue dye (10 μl) was injected intrathecally. After the animals were euthanized their vertebral columns were dissected to expose the spinal cord, and the correct placement of the catheters was verified by the presence of the dye on the dorsal aspect of the spinal cord in the lumbar enlargement.
Drugs
The NMDA receptor antagonist, MK-801 hydrogen maleate was obtained from Sigma (St Louis, MO) and diluted in sterile saline 52. The δ-opioid receptor agonist, SNC-80 was obtained from Alexis Biochemicals (San Diego, CA) 8. A stock solution of 600 nmol/10 μl SNC-80 was prepared by diluting into DMSO. The solution was then bubbled with carbon dioxide to aid dissolution. Final concentrations were included 24% DMSO. The μ-opioid receptor agonist, DAMGO was obtained from Sigma (St Louis, MO) and diluted in sterile saline30. Saline was used as the vehicle.
Experimental design
Experiment 1
In order to test the hypothesis that daily pretreatment of MK-801 prevents the development of tolerance to TENS the following groups were used: 1) High frequency TENS + MK-801 [0.01 (n=3); 0.03 (n=3); 0.1 (n=6) mg/kg]; 2) Low frequency TENS + MK-801 [0.01 (n=3); 0.03 (n=3); 0.1 (n=6) mg/kg]] ; 3) Sham TENS + MK-801 [0.01 (n=3); 0.03 (n=3); 0.1 (n=6) mg/kg]; 4) High frequency TENS + vehicle (n=6); 5) Low frequency TENS + vehicle (n=6); 6) Sham TENS + vehicle (n=7). The experimental paradigm is outlined in Figure 1A. Briefly, on Day 0 baseline mechanical withdrawal thresholds of the paw were measured and then the rats were injected with 3% kaolin and 3% carrageenan to induce inflammation. Twenty four hours later (Day 1) the rats were tested for mechanical withdrawal thresholds of the paw. Rats were then intraperitoneally injected with MK-801 or vehicle. The dose of MK-801 or vehicle was predetermined and blinded to the examiner. Fifteen min after the injection of MK-801 or vehicle, rats were anesthetized (1-2% halothane) for application of TENS as described above. After 20 min of TENS treatment and recovery from anesthetic, mechanical withdrawal thresholds of the paw were reassessed. The same procedure was done for an additional three consecutive days (Days 2-4). Thus, each animal received 4 days of TENS treatment in combination with either MK-801 or vehicle beginning 24 h after induction of inflammation. We previously determined that by the 4th day rats developed tolerance to TENS 7
Figure 1.
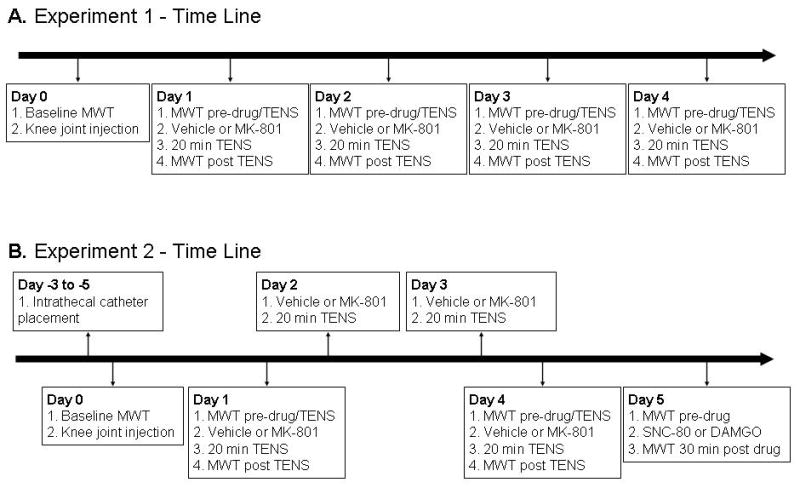
Schematic representation of the experimental paradigm for Experiment 1 (A) and Experiment 1 (B). TENS=transcutaneous electrical nerve stimulation; MWT=mechanical withdrawal threshold
Experiment 2 tested the hypothesis that MK-801 prevents analgesic tolerance to TENS by preventing tolerance at spinal μ- and δ-opioid receptors. The experimental paradigm is outlined in Figure 1B. Intrathecal catheters were placed 3 - 5 days (Day -3 to -5) before behavioral testing to administer drugs directly to the spinal cord. Baseline behavioral testing was performed and the knee joint injected with 3% kaolin and 3% carrageenan on Day 0. Beginning twenty-four hours after induction of inflammation, high frequency, low frequency or placebo TENS was administered daily for a total of 4 days. The groups were subdivided to receive either 0.1 mg/kg MK-801 or vehicle before each TENS treatment on Days 1-4 as outlined above. Behavioral tests were performed before and after TENS on Day 1, and before and after TENS on Day 4 to ensure development of tolerance on Day 4. On Day 5, cumulative dose response curves were done for the μ-opioid receptor agonist DAMGO and the δ-opioid receptor agonist SNC-80. Three different doses of DAMGO (2.34, 7.01 and 23.4 mmol/10 μl, i.t.) and three different doses of SNC-80 (20, 60 and 120 nmol/10 μl, i.t.) were administered in a cumulative manner. Behavioral testing was performed 30 minutes after administration of either DAMGO or SNC-80. Each subsequent dose was given immediately after the 30 minute behavioral test. We therefore tested tolerance at μ-opioid receptors by assessing the effectiveness of the μ-opioid agonist, DAMGO and tolerance at the δ-opioid receptors by assessing the effectiveness of the δ-opioid agonist, SNC-80. All drug dosing was done through intrathecal administration, and with cumulative dose response curves as reported previously 7. Groups are reported in Table 1.
Table 1.
| High Frequency TENS | Low Frequency TENS | Sham TENS | ||||
|---|---|---|---|---|---|---|
| 0.1 mg/kg MK-801 | Vehicle | 0.1 mg/kg MK-801 | Vehicle | 0.1 mg/kg MK-801 | Vehicle | |
| SNC80 | N=4 | N=6 | N=4 | N=6 | N=4 | N=4 |
| DAMGO | N=4 | N=4 | N=4 | N=4 | N=4 | N=4 |
Statistical analysis
Since the data were not normally distributed, the paw withdrawal thresholds were analyzed for differences between groups with a Kruskal-Wallis ANOVA at each time period. Post hoc testing for differences between individual groups was done with Mann-Whitney and Wilcoxon – Sign rank test. p < 0.05 was considered significant. Data are presented as medians with 25th and 75th percentiles.
Results
Development of Tolerance to TENS
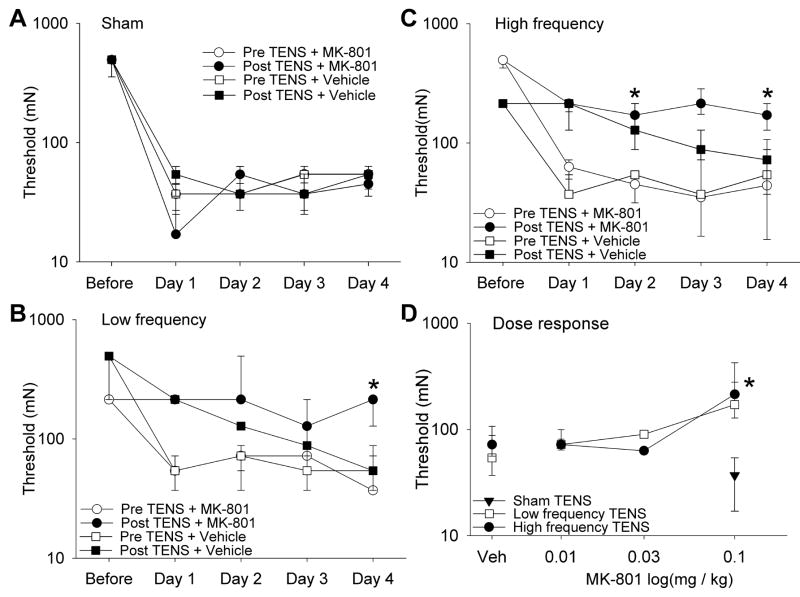
Twenty four hours after induction of inflammation there was a decrease in the mechanical withdrawal threshold of the paw; and there was no difference between groups at these time periods. Significant differences between groups were observed at all time periods immediately after TENS treatment: Day 1 χ2=26.2, p=0.0001; Day 2 χ2=28.9, p=0.0001; Day 3 χ2=25.5, p=0.0001; Day 4 χ2=24.1, p=0.0001. Specifically, treatment with either low (p=0.004) or high frequency (p=0.002) TENS on Day 1 reversed the decreased paw withdrawal threshold induced by joint inflammation when compared to sham treatment (Figure 2A,B,C). Similarly the withdrawal thresholds after TENS were significantly increased when compared to the sham TENS group on Day 2 (LF p=0.004; HF p=0.002) and Day 3 (LF p=0.004; HF p=0.004). However by Day 4 there was no difference after treatment with either low or high frequency TENS when compared to sham TENS. Thus, these data show that there is a loss TENS effectiveness after 4 days of treatment, and is interpreted as tolerance.
Figure 2.
A. Bar graphs represent the ipsilateral mechanical paw withdrawal thresholds before and after TENS treatment for the groups that received vehicle or MK-801 (0.1 mg/kg) in the groups treated with sham (A), low frequency TENS (B), and high frequency TENS (C). Data prior to induction of inflammation is represented as the baseline for both groups. The data before and after TENS for each group is shown for Day 1, Day 2, Day 3 and Day 4 in A, B, C. D. Dose response curves for the withdrawal threshold on Day 4 for the groups treated MK-801 (0.1 mg/kg, 0.03 mg/kg and 0.01 mg/kg) and either high or low frequency TENS. The group treated with vehicle and high or low frequency TENS (Veh), and the group treated with MK-801 and sham TENS, are also shown for comparison. For the time period after TENS treatment, the group treated with MK-801 and TENS were significantly increased from vehicle (*). Data are represented as medians with the 25th and 75th percentiles.
Prevention of tolerance to TENS by blocking NMDA receptors
In the group treated with the NMDA receptor antagonist MK-801 (0.1 mg/kg) daily 15 minutes prior to either low frequency (Figure 2B,D) or high frequency TENS (Figure 2C,D) the mechanical withdrawal thresholds on Day 4 were higher than those treated with vehicle prior to TENS (HF p=0.004; LF p=0.009). There mechanical withdrawal thresholds were also greater on Day 2 for the group treated with low frequency TENS when compared to sham TENS (p=0.017). Further the withdrawal thresholds for the group treated with low frequency TENS and MK-801 were significantly greater than those treated with sham TENS and MK-801 after the first (p=0.001), second p=0.001), third (p=0.001), and fourth (p=0.001) TENS treatment. Similarly, the withdrawal thresholds for the group treated with high frequency TENS and MK-801 were significantly greater than those treated with sham and MK-801 after the first (p=0.001), second (p=0.001), third (p=0.002), and fourth (p=0.001) TENS treatment. There were no differences between the sham group that received vehicle and the sham group that received MK-801 showing that 0.1 mg/kg MK-801 has no effect on the decreased withdrawal threshold produced by inflammation (Figure 2A,D). In the groups with low or high frequency TENS and lower doses of MK-801 i.e. 0.03 mg/kg and 0.01 mg/kg the changes between paw withdrawal thresholds were not significant when compared with vehicle and TENS (Figure 2D).
Cross tolerance to δ- and μ-opioid agonist and TENS
For the group treated with vehicle and sham TENS there was an increase in withdrawal thresholds after intrathecal administration of SNC-80 (20, 60 and 120 nmol/10μl) showing an analgesic effect of SNC-80 (Fig. 3A). However in the group treated with high frequency TENS and vehicle the withdrawal thresholds after administration of SNC-80 were significantly less than the group treated with vehicle and sham TENS, showing development of tolerance at spinal δ-opioid receptors. In the group treated with vehicle and sham TENS there was an increase in withdrawal threshold after intrathecal administration of DAMGO (2.4, 7 and 23.4 mmol/10μl) demonstrating an analgesic effect of DAMGO (Fig. 3B). However in the group treated with low frequency TENS and vehicle the withdrawal thresholds after administration of DAMGO were significantly less than the group treated with vehicle and sham TENS, showing development of tolerance at spinal μ-opioid receptors.
Figure 3.
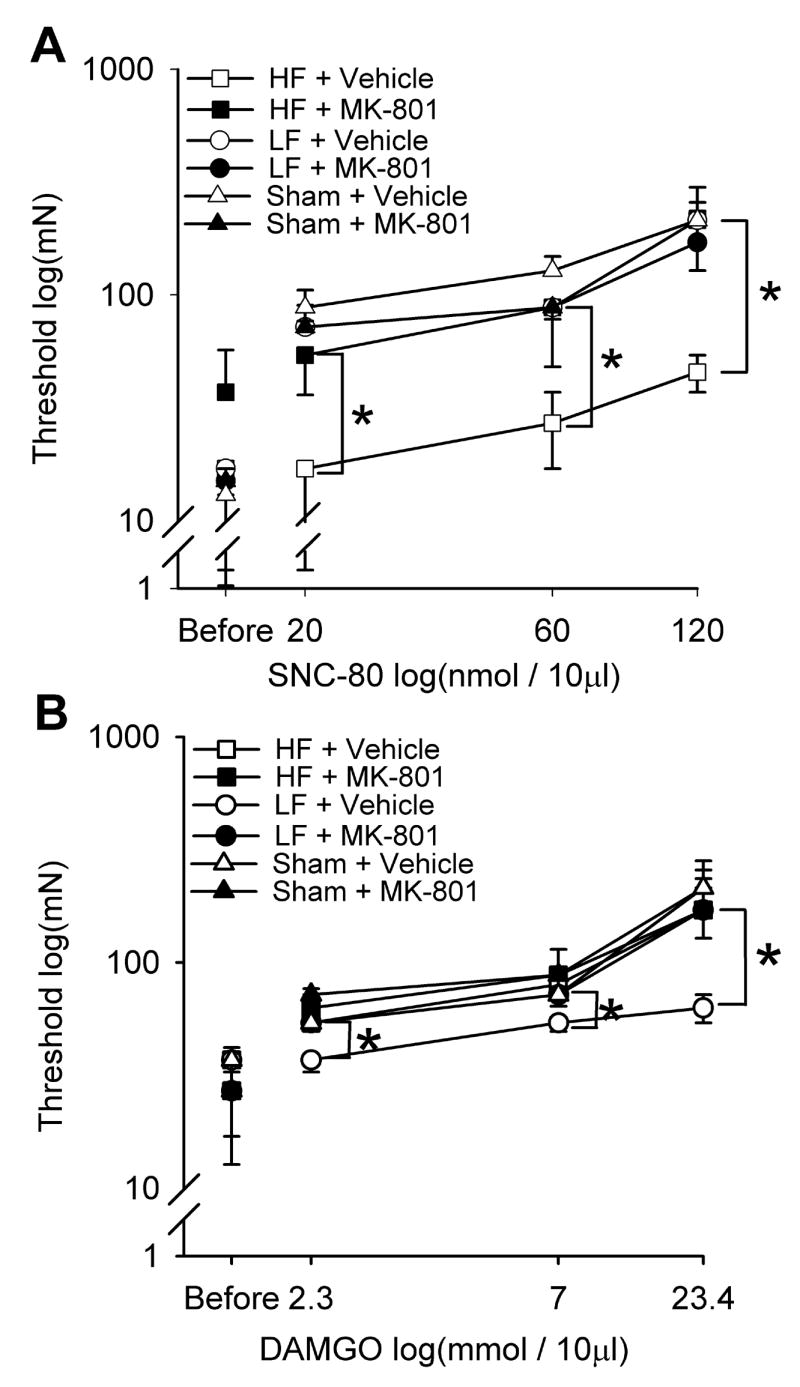
Line graphs representing the mechanical paw withdrawal thresholds after intrathecal injection of increasing doses of SNC-80 (A) or DAMGO (B) in groups treated with low frequency, high frequency or sham TENS. Following administration of SNC-80, the group that received high frequency TENS and vehicle the withdrawal threshold were lower than those that received sham TENS and those that received MK-801 and high frequency TENS (*). Following administration of DAMGO the group that received low frequency TENS and vehicle the withdrawal thresholds were lower than those that received sham TENS and those that received MK-801 and low frequency TENS (*). Data are represented as medians with the 25th and 75th percentiles.
Prevention of cross tolerance at spinal opioid receptors by blockade of NMDA receptors
To test if MK-801 prevented tolerance at opioid receptors in the spinal cord, we tested the effectiveness of the δ-opioid agonist SNC-80 and the μ-opioid agonist DAMGO delivered intrathecally in animals that received 0.1 mg/kg MK-801 and TENS when compared to those that received vehicle and TENS. In animals that were injected with the δ-opioid agonist SNC-80 there was a significant difference between groups (χ2=17.083, p=0.004) (Figure 3A). In the group treated with high frequency TENS with MK-801 group, the withdrawal thresholds following SNC-80 (20, 60 and 120 nmol) were significantly greater than the withdrawal thresholds from the group that received high frequency TENS with vehicle (p=0.004 all doses). In animals that were injected intrathecally with the μ-opioid agonist DAMGO, there was a significant difference between groups (χ2=12.492, p=0.029) (Figure 3B). In the low frequency TENS group treated with MK-801, the withdrawal thresholds following DAMGO (2.34, 7.01 and 23.4 mmol) were significantly greater than the withdrawal thresholds from the group treated with low frequency TENS and vehicle (p=0.029 all doses). Thus, MK-801 in combination with TENS prevented cross-tolerance at spinal opioid receptors.
Discussion
In the current study and in our previous study 8 daily application of either high or low frequency TENS resulted in reduced analgesic effectiveness by the 4th day of treatment. We interpret this reduced effectiveness of TENS by the 4th day as tolerance. We further show that pretreatment with the NMDA receptor antagonist MK-801 prior to TENS prevents the development of this tolerance effect. The tolerance effect of high and low frequency TENS results in cross-tolerance at spinal δ- and μ-opioid receptors, respectively, similar to that observed in our previous study 8. Daily pretreatment with MK-801 prior to high or low frequency TENS also prevents the cross-tolerance at δ- or μ-opioid receptors in the spinal cord. Thus, we conclude that repeated application of TENS produces tolerance at spinal opioid receptors and this tolerance is prevented by blockade of NMDA receptors.
Development of tolerance
The current study shows development of tolerance to TENS, a clinically utilized method of pain treatment, in an animal model of joint inflammation. These results agree with prior studies showing the development of opioid tolerance after induction of inflammation 27,28. In fact, in animals with inflammation there is a larger shift in the morphine ED50 after chronic morphine administration suggesting that inflammation enhances tolerance 27. The current study also shows development of tolerance to TENS by testing mechanical sensitivity of the paw with von Frey filaments. The majority of previous studies examine tolerance to exogenously administered opioids using acute thermal nociceptive responses including tail flick, hot water tail immersion, hot plate and paw withdrawal to radiant heat 4,6,12,26,32,33,45,46. In addition, a recent study, demonstrates opioid tolerance by testing mechanical sensitivity using forceps applied to the paw 28.
The current study shows that there was a cross tolerance to opioid agonists delivered spinally after repeated TENS application. These data led us to conclude that TENS produced analgesic tolerance through release of endogenous opioid receptors within the spinal cord. Prior studies in our laboratory show that the effects of high or low frequency TENS are mediated by release of endogenous opioids acting at δ- or μ-opioid receptors, respectively, in the spinal cord or brainstem 22,42. Analgesic tolerance to endogenously released opioid peptides has also been observed in animals receiving electroacupuncture. Electroacupuncture applied at either high or low frequencies repeatedly results in a reduced effectiveness of the treatment 17,18,19. Similar to the current study, this tolerance-like effect of electroacupuncture is associated with cross-tolerance at spinal opioid receptors 9. The cross tolerance to spinally administered opioids is in agreement with prior studies showing tolerance to exogenously applied opioids including morphine, DAMGO, and DPDE 30,32,33,53. Thus, the current study shows development of analgesic tolerance after endogenous release of opioid peptides induced by repeated TENS treatment in animals with mechanical hyperalgesia induced by joint inflammation.
Blockade of tolerance
The current study demonstrates high and low frequency TENS-induced tolerance is associated with tolerance at δ- and μ-opioid receptors in the spinal cord, respectively. Analgesic tolerance to TENS, and the coincident tolerance at spinal opioid receptors, is prevented by pretreatment with the NMDA receptors antagonist MK-801. These data are in agreement with numerous studies showing that treatment with non-competitive NMDA receptor antagonists prevents development of analgesic tolerance to exogenously administered opioid agonists acting at either δ- and μ-opioid receptors 5,6,11,29,30,32,33,52. Further, competitive NMDA receptor antagonists like LY 235959, AP-5, CGP 39551 attenuate tolerance to exogenously administered μ- and δ-opioid agonists 5,6,13,49 and blockade at the glycine site of the NMDA receptor with L-701,324 prevents tolerance to morphine 26. Additionally, the clinically used NMDA receptor antagonist ketamine prevents tolerance to repeated electroacupuncture treatment in rats 19. Thus, NMDA receptor antagonists, whether competitive or non-competitive, prevent tolerance to exogenous opioids and to clinical treatments that use endogenous opioids.
Systemic delivery of NMDA receptor antagonists prevents development of analgesic tolerance to μ- or δ-opioid agonists administered systemically 16,32,33, supraspinally 5,6,52, spinally 12,30, or peripherally 24,25. Therefore, the site of action of MK-801 in the current study could be supraspinal, spinal or peripheral. We expect that TENS releases opioid peptides both spinally and supraspinally since blockade of μ- and δ-opioid receptors in the RVM or spinal cord prevents anti-hyperalgesia produced by low or high frequency TENS, respectively 22,42. The current study shows that administration of MK-801 prior to daily application of TENS reduces tolerance of spinally administered opioid agonists supporting the conclusion that blockade of NMDA receptors in the spinal cord prevents development of tolerance to TENS. We do not know, however, if TENS also produces tolerance at opioid receptors supraspinally or peripherally and if tolerance is prevented by blockade of those NMDA receptors.
An alternative explanation for the continued effectiveness of TENS during 4 days of TENS treatment with MK-801 is a synergistic interaction between MK-801 and TENS. The synergistic interaction would be presented as an analgesic effect on each day. Indeed NMDA agonists do synergize with opioid agonists, both mu- and delta 10,12,15,37. However, the dose of MK-801, 0.1 mg/kg) used in the current study has no effect on mu-opioid induced analgesia (morphine/fentanyl) when co-administered 37. The current data clearly shows a tolerance effect to spinally administered opioids the on the fifth day after 4 days of TENS and MK-801 co-administration, a time when there was no drug or TENS delivered. We also clearly show that in animals treated with MK-801 and high or low frequency TENS there is a loss of tolerance to δ- or μ-opioid agonists delivered spinally on the fifth day. We further show that on Day 5, 24 h after the last administration of TENS and MK-801, animals still present with hyperalgesia suggesting that the analgesic effects on Day 4 do not last through 24 h. The current data thus argues for a loss of tolerance to TENS when MK-801 is given simultaneously rather than a synergist interaction between TENS and MK-801
Mechanisms of Tolerance
Several mechanisms could explain the action of NMDA receptor antagonists in preventing the development of analgesic tolerance to chronic opioid exposure. Chronic morphine infusion decreases expression of glutamate transporters EAAC1 and GLAST in the rat spinal cord 31. Downregulation of glutamate transporters would result in increase in extracellular and synaptic glutamate. Glutamate increases in the spinal cord after administration of morphine in morphine tolerant rats 48. Conversely, spinal administration of glutamate transporter activator MS-153, increases glutamate uptake, and prevents morphine tolerance 34. The increase in spinal glutamate in morphine tolerant rats is reduced by pretreatment with NMDA receptor antagonist MK-801 48. In parallel, repeated administration of μ-and δ-opioid agonists increases the MK-801 binding affinity both spinally and supraspinally 50,53, supporting the hypothesis that there is a potentiation of NMDA receptors after the development of tolerance. In contrast, there is an increase in expression of vesicular glutamate transporter 1 in the spinal cord following chronic morphine in mice 44. These differences in regulation of glutamate transporters by chronic morphine exposure may be related to differential expression of glutamate transporters 1) in glia or neurons, 2) on presynaptic vs. postsynaptic terminals, 3) or inhibitory vs. excitatory neurons 39. Taken together, these studies suggest an enhancement of excitatory signaling by glutamate and activation of NMDA receptors in the spinal cord accompanies opioid tolerance.
Clinical relevance
Clinically, TENS is administered for several consecutive days or months likely resulting in tolerance to TENS. Administration of NMDA receptor antagonists at low doses may serve as an adjunct to enhance the efficacy of TENS. Clinically, pharmaceutical agents that block NMDA receptors, i.e. ketamine or dextomethorphan, enhance morphine analgesia, reduce opioid requirements, and reduce opioid side effects 3,20,51 suggesting that NMDA receptor antagonist prevent tolerance-like effects of opioids in human subjects.
Acknowledgments
The authors wish to thank Dr. Takeshi Yokoyama (Kochi Medical School, Japan), Dr. Yumi Maeda, Ms. Jing Danielson, and Ms. Tammy Lisi for technical support. TENS units were donated by EMPI, Inc, Minneapolis, MN. Supported by KO2ARO2201, Carver College of Medicine and AR052316.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ainsworth L, Budelier K, Clinesmith M, Fiedler A, Landstrom R, Leeper BJ, Moeller L, Mutch S, O K, Ross J, Radhakrishnan R, Sluka KA. Transcutaneous electrical nerve stimulation (TENS) reduces chronic hyperalgesia induced by muscle inflammation. Pain. 2005;120:182–187. doi: 10.1016/j.pain.2005.10.030. [DOI] [PubMed] [Google Scholar]
- 2.Allen RM, Granger AL, Dykstra LA. Dextromethorphan potentiates the antinociceptive effects of morphine and the delta-opioid agonist SNC80 in squirrel monkeys. J Pharmacol Exp Ther. 2002;300:435–441. doi: 10.1124/jpet.300.2.435. [DOI] [PubMed] [Google Scholar]
- 3.Bell RF. Low dose subcutaneous ketamine infusion and morphine tolerance. Pain. 1999;83:101–103. doi: 10.1016/s0304-3959(99)00096-2. [DOI] [PubMed] [Google Scholar]
- 4.Ben-Eliyahu S, Marek P, Vaccarino AL, Mogil JS, Sternberg WF, Liebeskind JC. The NMDA receptor antagonist MK-801 prevents long-lasting non-associative morphine tolerance in the rat. Brain Res. 1992;575:304–308. doi: 10.1016/0006-8993(92)90094-p. [DOI] [PubMed] [Google Scholar]
- 5.Bhargava HN, Zhao GM. Effects of N-methyl-D-aspartate receptor antagonists on the analgesia and tolerance to D-Ala2, Glu4 deltorphin II, a delta 2-opioid receptor agonist in mice. Brain Res. 1996;719:56–61. doi: 10.1016/0006-8993(96)00112-6. [DOI] [PubMed] [Google Scholar]
- 6.Bilsky EJ, Inturrisi CE, Sadee W, Hruby VJ, Porreca F. Competitive and non-competitive NMDA antagonists block the development of antinociceptive tolerance to morphine, but not to selective mu or delta opioid agonists in mice. Pain. 1996;68:229–237. doi: 10.1016/s0304-3959(96)03185-5. [DOI] [PubMed] [Google Scholar]
- 7.Brosseau L, Milne S, Robinson V, Marchand S, Shea B, Wells G, Tugwell P. The effectiveness of the transcutaneous electrical nerve stimulation (TENS) for the treatment of chronic low back pain: a meta-analysis. Spine. 2002;27:596–603. doi: 10.1097/00007632-200203150-00007. [DOI] [PubMed] [Google Scholar]
- 8.Chandran P, Sluka KA. Development of opioid tolerance with repeated transcutaneous electrical nerve stimulation administration. Pain. 2003;102:195–201. doi: 10.1016/s0304-3959(02)00381-0. [DOI] [PubMed] [Google Scholar]
- 9.Chen XH, Han JS. Analgesia induced by electroacupuncture of different frequencies is mediated by different types of opioid receptors: another cross-tolerance study. Behavioral Brain Research. 1992;47:143–149. doi: 10.1016/s0166-4328(05)80120-2. [DOI] [PubMed] [Google Scholar]
- 10.Chow LH, Huang EY, Ho ST, Lee TY, Tao PL. Dextromethorphan potentiates morphine antinociception at the spinal level in rats. Can J Anaesth. 2004;51:905–910. doi: 10.1007/BF03018888. [DOI] [PubMed] [Google Scholar]
- 11.Dravolina OA, Belozertseva IV, Sukhotina IA, Bespalov AY. Morphine tolerance and dependence in mice with history of repeated exposures to NMDA receptor channel blockers. Pharmacol Biochem Behav. 1999;63:613–619. doi: 10.1016/s0091-3057(99)00028-3. [DOI] [PubMed] [Google Scholar]
- 12.Dunbar S, Yaksh TL. Concurrent spinal infusion of MK801 blocks spinal tolerance and dependence induced by chronic intrathecal morphine in the rat. Anesthesiol. 1996;84:1177–1188. doi: 10.1097/00000542-199605000-00020. [DOI] [PubMed] [Google Scholar]
- 13.Gonzalez P, Cabello P, Germany A, Norris B, Contreras E. Decrease of tolerance to, and physical dependence on morphine by, glutamate receptor antagonists. Eur J Pharmacol. 1997;332:257–262. doi: 10.1016/s0014-2999(97)01099-6. [DOI] [PubMed] [Google Scholar]
- 14.Gopalkrishnan P, Sluka KA. Effect of varying frequency, intensity and pulse duration of TENS on primary hyperalgesia in inflamed rats. Arch Phys Med Rehabil. 2000;81:984–990. doi: 10.1053/apmr.2000.5576. [DOI] [PubMed] [Google Scholar]
- 15.Grass S, Hoffmann O, Xu XJ, Wiesenfeld-Hallin Z. N-methyl-D-aspartate receptor antagonists potentiate morphine’s antinociceptive effect in the rat. Acta Physiol Scand. 1996;158:269–273. doi: 10.1046/j.1365-201X.1996.566309000.x. [DOI] [PubMed] [Google Scholar]
- 16.Gutstein HB, Trujillo KA. MK-801 inhibits the development of morphine tolerance at spinal sites. Brain Res. 1993;626:332–334. doi: 10.1016/0006-8993(93)90597-g. [DOI] [PubMed] [Google Scholar]
- 17.Han JS, Li SJ, Tang J. Tolerance to electroacupuncture and its cross tolerance to morphine. Neuropharm. 1981;20:593–596. doi: 10.1016/0028-3908(81)90213-6. [DOI] [PubMed] [Google Scholar]
- 18.Huang C, Long H, Shi YS, Han JS, Wan Y. Nocistatin potentiates electroacupuncture antinociceptive effects and reverses chronic tolerance to electroacupuncture in mice. Neurosci Lett. 2003;350:93–96. doi: 10.1016/s0304-3940(03)00863-2. [DOI] [PubMed] [Google Scholar]
- 19.Huang C, Long H, Shi YS, Han JS, Wan Y. Ketamine enhances the efficacy to and delays the development of tolerance to electroacupuncture-induced antinociception in rats. Neurosci Lett. 2005;375:138–142. doi: 10.1016/j.neulet.2004.10.086. [DOI] [PubMed] [Google Scholar]
- 20.Javery KB, Ussery TW, Steger HG, Colclough GW. Comparison of morphine and morphine with ketamine for postoperative analgesia. Can J Anaesth. 1996;43:212–215. doi: 10.1007/BF03011736. [DOI] [PubMed] [Google Scholar]
- 21.Johnson M, Martinson M. Efficacy of electrical nerve stimulation for chronic musculoskeletal pain: a meta-analysis of randomized controlled trials. Pain. 2007;130(12):157–65. doi: 10.1016/j.pain.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 22.Kalra A, Urban MO, Sluka KA. Blockade of opioid receptors in rostral ventral medulla prevents antihyperalgesia produced by transcutaneous electrical nerve stimulation (TENS) J Pharmacol Exp Ther. 2001;298:257–263. [PubMed] [Google Scholar]
- 23.King EW, Sluka KA. The effect of varying frequency and intensity of transcutaneous electrical nerve stimulation on secondary mechanical hyperalgesia in an animal model of inflammation. J Pain. 2001;2:128–133. doi: 10.1054/jpai.2001.19963. [DOI] [PubMed] [Google Scholar]
- 24.Kolesnikov YA, Pasternak GW. Peripheral blockade of topical morphine tolerance by ketamine. Eur J Pharmacol. 1999;374:R1–R2. doi: 10.1016/s0014-2999(99)00318-0. [DOI] [PubMed] [Google Scholar]
- 25.Kolesnikov YA, Pasternak GW. Peripheral orphanin FQ/nociceptin analgesia in the mouse. Life Sci. 1999;64:2021–2028. doi: 10.1016/s0024-3205(99)00149-6. [DOI] [PubMed] [Google Scholar]
- 26.Kotlinska J. Are glycineB sites involved in the development of morphine tolerance? Pol J Pharmacol. 2004;56:51–57. [PubMed] [Google Scholar]
- 27.Liang DY, Guo T, Liao G, Kingery WS, Peltz G, Clark JD. Chronic pain and genetic background interact and influence opioid analgesia, tolerance, and physical dependence. Pain. 2006;121:232–240. doi: 10.1016/j.pain.2005.12.026. [DOI] [PubMed] [Google Scholar]
- 28.Luis-Delgado OE, Barrot M, Rodeau JL, Schott G, Benbouzid M, Poisbeau P, Freund-Mercier MJ, Lasbennes F. Calibrated forceps: a sensitive and reliable tool for pain and analgesia studies. J Pain. 2006;7:32–39. doi: 10.1016/j.jpain.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 29.Lutfy K, Hurlbut DE, Weber E. Blockade of morphine-induced analgesia and tolerance in mice by MK-801. Brain Res. 1993;616:83–88. doi: 10.1016/0006-8993(93)90195-s. [DOI] [PubMed] [Google Scholar]
- 30.Mao J, Price DD, Lu J, Mayer DJ. Antinociceptive tolerance to the mu-opioid agonist DAMGO is dose-dependently reduced by MK-801 in rats. Neurosci Lett. 1998;250:193–196. doi: 10.1016/s0304-3940(98)00472-8. [DOI] [PubMed] [Google Scholar]
- 31.Mao J, Sung B, Ji RR, Lim G. Chronic morphine induces downregulation of spinal glutamate transporters: implications in morphine tolerance and abnormal pain sensitivity. J Neurosci. 2002;22:8312–8323. doi: 10.1523/JNEUROSCI.22-18-08312.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marek P, Ben Eliyahu S, Gold M, Liebeskind JC. Excitatory amino acid antagonists (kynurenic acid and MK-801) attenuate the development of morphine tolerance in the rat. Brain Res. 1991;547:77–81. doi: 10.1016/0006-8993(91)90576-h. [DOI] [PubMed] [Google Scholar]
- 33.Marek P, Ben-Eliyahu S, Vaccarino AL, Liebeskind JC. Delayed application of MK-801 attenuates development of morphine tolerance in rats. Brain Res. 1991;558:163–165. doi: 10.1016/0006-8993(91)90736-f. [DOI] [PubMed] [Google Scholar]
- 34.Nakagawa T, Ozawa T, Shige K, Yamamoto R, Minami M, Satoh M. Inhibition of morphine tolerance and dependence by MS-153, a glutamate transporter activator. Eur J Pharmacol. 2001;419:39–45. doi: 10.1016/s0014-2999(01)00965-7. [DOI] [PubMed] [Google Scholar]
- 35.Osiri M, Welch V, Brosseau L, Shea B, McGowan J, Tugwell P, Wells G. Transcutaneous electrical nerve stimulation for knee osteoarthritis. Cochrane Database Syst Rev. 2000:CD002823. doi: 10.1002/14651858.CD002823. [DOI] [PubMed] [Google Scholar]
- 36.Pogatzki EM, Zahn PK, Brennan TJ. Lumbar catheterization of the subarachnoid space with a 32-gauge polyurethane catheter in the rat. Eur J Pain. 2000;4:111–113. doi: 10.1053/eujp.1999.0157. [DOI] [PubMed] [Google Scholar]
- 37.Redwine KE, Trujillo KA. Effects of NMDA receptor antagonists on acute mu-opioid analgesia in the rat. Pharmacol Biochem Behav. 2003;76:361–372. doi: 10.1016/j.pbb.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 38.Resende MA, Sabino GG, Candido CRM, Pereira LSM, Francischi JN. Transcutaneous electrical stimulation (TENS) effects in experimental inflammatory edema and pain. Eur J Pharmacol. 2004;504:217–222. doi: 10.1016/j.ejphar.2004.09.055. [DOI] [PubMed] [Google Scholar]
- 39.Rothstein JD, Martin L, Levey AI, Dykes-Hoberg M, Jin L, Wu D, Nash N, Kuncl RW. Localization of neuronal and glial glutamate transporters. Neuron. 1994;13:713–725. doi: 10.1016/0896-6273(94)90038-8. [DOI] [PubMed] [Google Scholar]
- 40.Sluka KA, Bailey K, Bogush J, Olson R, Ricketts A. Treatment with either high or low frequency TENS reduces the secondary hyperalgesia observed after injection of kaolin and carrageenan into the knee joint. Pain. 1998;77:97–102. doi: 10.1016/S0304-3959(98)00090-6. [DOI] [PubMed] [Google Scholar]
- 41.Sluka KA, Christy MR, Peterson WL, Rudd SL, Troy SM. Reduction of pain-related behaviors with either cold or heat treatment in an animal model of acute arthritis. Arch Phys Med Rehabil. 1999;80:313–317. doi: 10.1016/s0003-9993(99)90143-0. [DOI] [PubMed] [Google Scholar]
- 42.Sluka KA, Deacon M, Stibal A, Strissel S, Terpstra A. Spinal blockade of opioid receptors prevents the analgesia produced by TENS in arthritic rats. J Pharmacol Exp Ther. 1999;289:840–846. [PubMed] [Google Scholar]
- 43.Sluka KA, Westlund KN. An experimental arthritis model in rats: the effects of NMDA and non-NMDA antagonists on aspartate and glutamate release in the dorsal horn. Neurosci Lett. 1993;149:99–102. doi: 10.1016/0304-3940(93)90357-q. [DOI] [PubMed] [Google Scholar]
- 44.Suzuki M, Narita M, Narita M, Suzuki T. Chronic morphine treatment increases the expression of vesicular glutamate transporter 1 in the mouse spinal cord. Eur J Pharmacol. 2006;535:166–168. doi: 10.1016/j.ejphar.2006.01.033. [DOI] [PubMed] [Google Scholar]
- 45.Trujillo KA. Are NMDA receptors involved in opiate-induced neural and behavioral plasticity? A review of preclinical studies. Psychopharmacology (Berl) 2000;151:121–141. doi: 10.1007/s002130000416. [DOI] [PubMed] [Google Scholar]
- 46.Trujillo KA, Akil H. Inhibition of opiate tolerance by non-competitive N-methyl-D-aspartate receptor antagonists. Brain Res. 1994;633:178–188. doi: 10.1016/0006-8993(94)91538-5. [DOI] [PubMed] [Google Scholar]
- 47.Vance CG, Radhakrishnan R, Skyba DA, Sluka KA. Transcutaneous electrical nerve stimulation at both high and low frequencies reduces primary hyperalgesia in rats with joint inflammation in a time-dependent manner. Phys Ther. 2007;87:44–51. doi: 10.2522/ptj.20060032. [DOI] [PubMed] [Google Scholar]
- 48.Wen ZH, Chang YC, Cherng CH, Wang JJ, Tao PL, Wong CS. Increasing of intrathecal CSF excitatory amino acids concentration following morphine challenge in morphine-tolerant rats. Brain Res. 2004;995:253–259. doi: 10.1016/j.brainres.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 49.Wong CS, Cherng CH, Luk HN, Ho ST, Tung CS. Effects of NMDA receptor antagonists on inhibition of morphine tolerance in rats: binding at mu-opioid receptors. Eur J Pharmacol. 1996;297:27–33. doi: 10.1016/0014-2999(95)00728-8. [DOI] [PubMed] [Google Scholar]
- 50.Wong CS, Hsu MM, Chou YY, Tao PL, Tung CS. Morphine tolerance increases [3H]MK-801 binding affinity and constitutive neuronal nitric oxide synthase expression in rat spinal cord. Br J Anaesth. 2000;85:587–591. doi: 10.1093/bja/85.4.587. [DOI] [PubMed] [Google Scholar]
- 51.Yang CY, Wong CS, Chang JY, Ho ST. Intrathecal ketamine reduces morphine requirements in patients with terminal cancer pain. Can J Anaesth. 1996;43:379–383. doi: 10.1007/BF03011718. [DOI] [PubMed] [Google Scholar]
- 52.Zhao GM, Bhargava HN. Effect of antagonism of the NMDA receptor on tolerance to [D-Pen2,D-Pen5]enkephalin, a delta 1 opioid receptor agonist. Peptides. 1996;17:233–236. doi: 10.1016/0196-9781(95)02095-0. [DOI] [PubMed] [Google Scholar]
- 53.Zhao GM, Bhargava HN. Effects of multiple intracerebroventricular injections of [D-Pen2,D-Pen5]enkephalin and [D-Ala2,Glu4]deltorphin II on tolerance to their analgesic action and on brain delta-opioid receptors. Brain Res. 1997;745:243–247. doi: 10.1016/s0006-8993(96)01156-0. [DOI] [PubMed] [Google Scholar]