Abstract
It has already been shown that mild to moderate exercise training may protect against the development of atherosclerosis. However, the precise mechanisms behind this protection are still unknown. The hypothesis that exercise training reduces the severity of experimental atherosclerosis in apolipoprotein (apo) E-deficient mice was assessed. Swimming training was conducted three times per week for 20 min on apo E-deficient mice fed a high-fat diet for eight or 16 weeks. Atherosclerotic lesions were evaluated. Fatty streak formation and fibrofatty plaques developed in apo E-deficient mice fed the high-fat diet, and were markedly suppressed in mice that received exercise for eight or 16 weeks compared with in nonexercise mice. Differences in lesion area did not correlate with any significant alterations in serum lipid levels. Thus, exercise therapy markedly suppressed experimental atherosclerosis.
Keywords: Apolipoprotein E-deficient mice, Atherosclerosis, Exercise, Lipids
Exercise is a deterrent of cardiovascular disease, and its anti-atherogenic effects have been described in different animal models (1,2). Exercise can also positively influence risk factors that are associated with cardiovascular diseases, hypertension, diabetes mellitus, obesity, increased plasma lipids and endothelial dysfunction (3). However, the mechanism by which exercise may benefit cardiovascular disease is still unknown.
Since the oxidation hypothesis of atherosclerosis was suggested approximately 15 years ago (4,5), many experiments involving cell culture, animal and human studies have shown that oxidized lipids could exhibit numerous in vitro proatherogenic effects (6–9). Paradoxically, exercise also induces oxidative stress in animals and humans (10,11), and this would appear to be inconsistent with its antiatherogenic effects. To resolve this paradox, some researchers recently proposed, based on human studies, that either the overall beneficial effects of exercise would overwhelm the deleterious effects of oxidative stress, or the exercise-induced oxidative stress may itself be beneficial by inducing arterial antioxidant enzymes (9,10,12). Enzymes associated with anti-oxidant defence, such as manganese superoxide dismutase, heme oxygenase and catalase (13–16), as well as the synthesis of glutathione (17), can be induced by oxidants in cell culture studies.
In the present study of apolipoprotein (apo) E-deficient mice, we have provided evidence for the induction of fatty streaks and fibrofatty streaks in the arterial walls and the lowering effects of experimental atherosclerotic lesions by exercise.
MATERIALS AND METHODS
Experimental atherosclerosis
The apo E-deficient 129Ola × C57BL/6 hybrid mice were generous gifts from Dr Edward M Rubin (University of California, Berkeley, California, USA). These mice were mated with C57BL/6 mice to produce F1 hybrids. The F1 apo E+/− mice were then backcrossed to C57BL/6 mice for 10 generations. Mice homogeneous for the apo E-null allele on a C57BL/6 background were subsequently generated. Male mice were subjected to subsequent experiments. The mice were kept in a temperature-controlled facility on a light-dark cycle of 14 h of light and 10 h of dark, with free access to food and water.
After being weaned at four weeks of age, mice were fed a normal chow diet (NCD) until six weeks, at which time the animals were switched to a high-fat diet (HFD) containing 20% fat and 0.3% cholesterol, as previously described (18).
Animal experiments were performed in accordance with the Declaration of Helsinki and were approved by the authors’ institutional ethics committee for animal experiments.
Exercise protocol
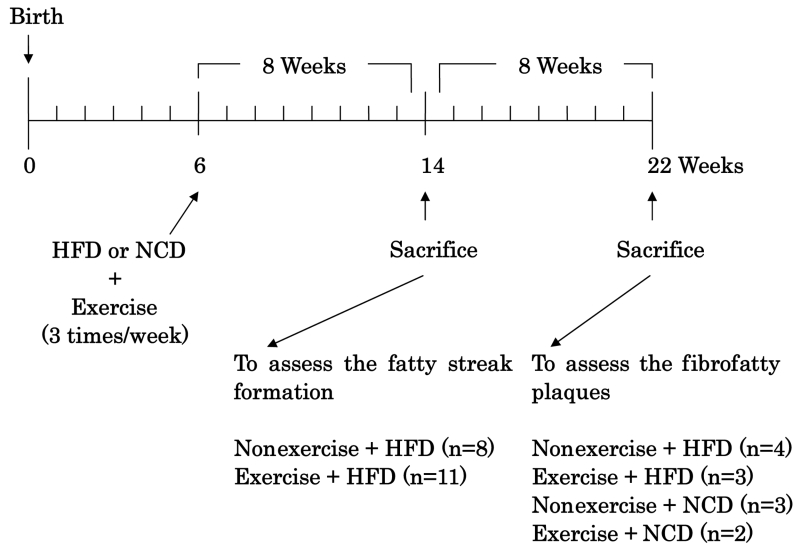
The exercise protocol is shown in Figure 1. At six weeks of age, mice were subjected to a swimming protocol. The mice were forced to swim in a hot water bath at 37°C for 20 min/day, three times per week. This protocol was conducted for eight or 16 weeks. Mice were divided into groups that at day 0 received HFD or NCD during eight weeks of treatment (HFD alone, n=8; HFD plus exercise, n=11) or during 16 weeks of treatment (HFD alone, n=4; HFD plus exercise, n=3; NCD alone, n=3; NCD plus exercise, n=2).
Figure 1.
Treatment protocol. After being weaned at four weeks of age, apolipoprotein E-deficient mice were fed with a normal chow diet (NCD) until six weeks of age, at which time the mice were switched to a high-fat diet (HFD). At six weeks of age, the mice started swimming, and kept with the swimming protocol for eight or 16 weeks
Tissue processing
Mice were killed by bleeding with puncture of the ventricle (19). The blood was collected and allowed to clot. After the serum was separated, lipid profiles were analyzed. The vasculature was perfused with sterile phosphate buffered saline (pH=7.2) and 6.8% sucrose. The root of the aorta was dissected under a macroscope and frozen in optimal cutting temperature embedding medium for serial cryosectioning covering 1.0 mm of the root. The first section was harvested when the first cusp became visible in the lumen of the aorta. Four sections of 6 μm thickness were harvested per slide and, thus, eight slides per mouse were prepared. All sections were immersed for 15 s in 60% isopropanol, stained for 30 min in a saturated oil red O solution at room temperature, counterstained with hematoxylin and then mounted under coverslips with glycerol gelatin.
Lipid analysis
Serum was harvested immediately after it was separated from blood. Serum lipid levels were analyzed with assay kits (Wako, Japan) according to the manufacturer’s instructions on total cholesterol and triglyceride in trained and nontrained mice in all groups.
Statistical analysis
Values are expressed as means ± SD. Statistical analysis of the data was performed by using Student’s t test. P<0.05 was considered statistically significant.
RESULTS
Effects of exercise on organ weights
No significant changes were found for body weight, heart weight and heart weight to body weight ratio at eight or 16 weeks (Table 1).
TABLE 1.
Effects of exercise on body weight and heart weight
| Regimen | n | Body weight (g) | Heart weight (mg) | Heart weight/ body weight (mg/g) |
|---|---|---|---|---|
| 8 weeks | ||||
| Nonexercise plus HFD | 8 | 24.29±2.65 | 140±40 | 5.78±1.54 |
| Exercise plus HFD | 11 | 26.92±4.57 | 140±30 | 5.22±0.69 |
| 16 weeks | ||||
| Nonexercise plus HFD | 4 | 28.38±1.09 | 160±20 | 5.55±0.51 |
| Exercise plus HFD | 3 | 26.37±1.63 | 160±20 | 5.98±0.94 |
| Nonexercise plus NCD | 3 | 31.50±3.20 | 140±10 | 4.58±0.43 |
| Exercise plus NCD | 2 | 26.65±1.91 | 140±20 | 5.05±0.43 |
Results are expressed as means ± SD. The results of body weight, heart weight and heart weight/body weight at eight and 16 weeks are shown. No significant difference was found in all parameters. HFD High-fat diet; NCD Normal chow diet
Effects of exercise on fatty streaks and fibrofatty plaque formation
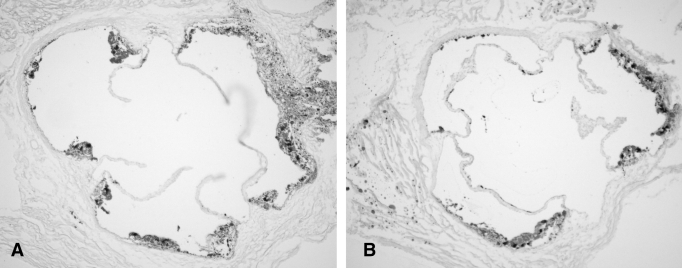
Apo E-deficient mice were forced to swim or not, and were kept on a cholesterol-rich diet for eight weeks to induce fatty streak formation and for 16 weeks to form fibrofatty plaques. The surface area covered by fatty streak and fibrofatty plaque lesions was quantified in oil red O-stained samples, and specimens from exercise-treated mice were compared with controls that did not receive exercise. Controls developed extensive atherosclerotic lesions in the root of the aorta in the fatty streak stage and the fibrofatty streak-formation stage (Figures 2 and 3). In mice treated with swimming, the fraction area of lesion was markedly reduced, as shown in Figure 3 (eight weeks: nonexercise group 8.77±0.90% [n=8] versus exercise group 5.20±1.86% [n=11], P<0.01; 16 weeks: nonexercise group 28.42±3.01% [n=4] versus exercise group 12.75±1.42% [n=3], P<0.01) (Figures 2 and 3, and Table 2).
Figure 2.
The effects of exercise on atherosclerosis (eight weeks). Histopathological pictures were obtained and the effects of swimming training for eight weeks were analyzed. Cryosections were obtained from trained and nontrained mice, and stained with oil red O solution. The plaque size of a nonexercise mouse (A) is larger than that of an exercise mouse (B). Original magnification × 40
Figure 3.
The effects of exercise on atherosclerosis (16 weeks). Representative histopathological pictures were obtained and the effects of swimming training for 16 weeks were analyzed. Large plaques (arrows) developed in a nonexercise mouse (A); however, relatively smaller plaques developed in an exercise mouse (B). Original magnification × 40
TABLE 2.
Atherosclerotic plaque size
| Regimen | n | Plaque size (%) |
|---|---|---|
| 8 weeks | ||
| Nonexercise plus high-fat diet | 8 | 8.77±0.90 |
| Exercise plus high-fat diet | 11 | 5.20±1.86* |
| 16 weeks | ||
| Nonexercise plus high-fat diet | 4 | 28.42±3.01 |
| Exercise plus high-fat diet | 3 | 12.75±1.42* |
| Nonexercise plus normal chow diet | 3 | 5.65±2.46 |
| Exercise plus normal chow diet | 2 | 8.70±5.70 |
Results are expressed as means ± SD. The percentages of plaque size were calculated from oil red O-stained tissue. The plaque size of the exercise group was smaller than that of the nonexercise group at eight and 16 weeks. The plaque size was reduced by exercise.
P<0.01
Effects of exercise on lipid profiles
No significant difference in serum total cholesterol or triglycerides was found between the exercise and nonexercise mice (Table 3).
TABLE 3.
Lipid profiles
| Regimen | n | Total cholesterol (mg/dL) | Triglyceride (mg/dL) |
|---|---|---|---|
| 8 weeks | |||
| Nonexercise plus HFD | 5 | 986.0±245.8 | 51.1±50.5 |
| Exercise plus HFD | 5 | 1182.8±237.7 | 53.3±33.5 |
| 16 weeks | |||
| Nonexercise plus HFD | 4 | 992.8±197.1 | 32.0±6.4 |
| Exercise plus HFD | 3 | 1031.7±155.3 | 33.0±12.1 |
| Nonexercise plus NCD | 3 | 822.3±88.7 | 45.3±13.5 |
| Exercise plus NCD | 2 | 825.0±32.5 | 45.0±21.2 |
Results are expressed as means ± SD. No significant difference was found in the total cholesterol or triglyceride concentrations in the sera of the exercise group compared with the nonexercise group. HFD High-fat diet; NCD Normal chow diet
DISCUSSION
In the present study, it was shown that mild to moderate exercise treatment suppressed the development of experimental atherosclerosis in apo E-deficient mice.
Many studies have reported the induction of antioxidant enzymes by exercise in different species, including humans (20). Kita et al (21) have shown that probucol suppresses the development of atherosclerosis in Watanabe heritable hyper-lipidemic rabbits. An antioxidant system such as superoxide dismutase exchanges hydrogen peroxide to water and oxygen, and it controls the production of radicals and oxidized low density lipoprotein (22). In human studies, exercise modifies the products of adipocytokines such as interleukin-6 and tumour necrosis factor-alpha. The expression of intercellular adhesion molecule-1 and vascular adhesion molecule-1 is regulated by these cytokines (23). Although further studies are necessary to prove the hypothesis, exercise-induced stress may have induced an aortic antioxidant response in the present study, which in turn could eventually be beneficial against atherosclerosis. As a result, using apo E-deficient mice on an HFD, we have shown that exercise can reduce atherosclerotic lesions. That is, oil red O stain analysis showed that the plaque size of the exercise group was smaller than that of the control group.
Exercise may increase the level of plasma oxidized fatty acids in HFD-fed mice and further upregulate the endothelial isoform of nitric oxide synthase (14). The HFD itself seemed to stimulate endothelial nitric oxide synthase expression, which could be attributed to the presence of plasma lipid peroxides (14). The benefits of exercise on atherosclerosis cannot exclusively be attributed to the induction of aortic antioxidant defences because several risk factors for coronary heart disease are favourably modified by physical activities (3).
In conclusion, mild to moderate exercise training protects against experimental atherosclerosis in apo E-deficient mice.
ACKNOWLEDGEMENTS
Supported in part by research grants from the Japanese Ministry of Education, Science, and Culture (14570656), the Shimizu Immunology Foundation, the Chronic Disease and Rehabilitation Research Foundation, and the Cardiovascular Research Foundation
REFERENCES
- 1.Froelicher VF. Animal studies of effect of chronic exercise on the heart and atherosclerosis: A review. Am Heart J. 1972;84:496–506. doi: 10.1016/0002-8703(72)90473-5. [DOI] [PubMed] [Google Scholar]
- 2.Kramsch DM, Aspen AJ, Abramowitz BM, Kreimendahl T, Hood WB., Jr Reduction of coronary atherosclerosis by moderate conditioning exercise in monkeys on an atherogenic diet. N Engl J Med. 1981;305:1483–9. doi: 10.1056/NEJM198112173052501. [DOI] [PubMed] [Google Scholar]
- 3.Shephard RJ, Balady GJ. Exercise as cardiovascular therapy. Circulation. 1999;99:963–72. doi: 10.1161/01.cir.99.7.963. [DOI] [PubMed] [Google Scholar]
- 4.Steinberg D, Parthasarathy S, Carew TE, Khoo JC, Witztum JL. Beyond cholesterol. Modifications of low-density lipoprotein that increase its atherogenicity. N Engl J Med. 1989;320:915–24. doi: 10.1056/NEJM198904063201407. [DOI] [PubMed] [Google Scholar]
- 5.Meilhac O, Ramachandran S, Chiang K, Santanam N, Parthasarathy S. Role of arterial wall antioxidant defense in beneficial effects of exercise on atherosclerosis in mice. Arterioscler Thromb Vasc Biol. 2001;21:1681–8. doi: 10.1161/hq1001.097106. [DOI] [PubMed] [Google Scholar]
- 6.Hessler JR, Morel DW, Lewis LJ, Chisolm GM. Lipoprotein oxidation and lipoprotein-induced cytotoxicity. Arteriosclerosis. 1983;3:215–22. doi: 10.1161/01.atv.3.3.215. [DOI] [PubMed] [Google Scholar]
- 7.Quinn MT, Parthasarathy S, Fong LG, Steinberg D. Oxidatively modified low density lipoproteins: A potential role in recruitment and retention of monocyte/macrophages during atherogenesis. Proc Natl Acad Sci USA. 1987;84:2995–8. doi: 10.1073/pnas.84.9.2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rajavashisth TB, Andalibi A, Territo MC, et al. Induction of endothelial cell expression of granulocyte and macrophage colony-stimulating factors by modified low-density lipoproteins. Nature. 1990;344:254–7. doi: 10.1038/344254a0. [DOI] [PubMed] [Google Scholar]
- 9.Parthasarathy S, Santanam N, Ramachandran S, Meilhac O. Oxidants and antioxidants in atherogenesis. An appraisal. J Lipid Res. 1999;40:2143–57. [PubMed] [Google Scholar]
- 10.Shern-Brewer R, Santanam N, Wetzstein C, White-Welkley J, Parthasarathy S. Exercise and cardiovascular disease: A new perspective. Arterioscler Thromb Vasc Biol. 1998;18:1181–7. doi: 10.1161/01.atv.18.7.1181. [DOI] [PubMed] [Google Scholar]
- 11.Leaf DA, Kleinman MT, Hamilton M, Deitrick RW. The exercise-induced oxidative stress paradox: The effects of physical exercise training. Am J Med Sci. 1999;317:295–300. doi: 10.1097/00000441-199905000-00005. [DOI] [PubMed] [Google Scholar]
- 12.Wetzstein CJ, Shern-Brewer RA, Santanam N, Green NR, White-Welkley JE, Parthasarathy S. Does acute exercise affect the susceptibility of low density lipoprotein to oxidation? Free Radic Biol Med. 1998;24:679–82. doi: 10.1016/s0891-5849(97)00320-1. [DOI] [PubMed] [Google Scholar]
- 13.Kinscherf R, Deigner HP, Usinger C, et al. Induction of mitochondrial manganese superoxide dismutase in macrophages by oxidized LDL: Its relevance in atherosclerosis of humans and heritable hyperlipidemic rabbits. FASEB J. 1997;11:1317–28. doi: 10.1096/fasebj.11.14.9409551. [DOI] [PubMed] [Google Scholar]
- 14.Ramasamy S, Parthasarathy S, Harrison DG. Regulation of endothelial nitric oxide synthase gene expression by oxidized linoleic acid. J Lipid Res. 1998;39:268–76. [PubMed] [Google Scholar]
- 15.Stocker R. Induction of haem oxygenase as a defence against oxidative stress. Free Radic Res Commun. 1990;9:101–12. doi: 10.3109/10715769009148577. [DOI] [PubMed] [Google Scholar]
- 16.Meilhac O, Zhou M, Santanam N, Parthasarathy S. Lipid peroxides induce expression of catalase in cultured vascular cells. J Lipid Res. 2000;41:1205–13. [PubMed] [Google Scholar]
- 17.Darley-Usmar VM, Severn A, O’Leary VJ, Rogers M. Treatment of macrophages with oxidized low-density lipoprotein increases their intracellular glutathione content. Biochem J. 1991;278:429–34. doi: 10.1042/bj2780429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murayama T, Yokode M, Kataoka H, et al. Intraperitoneal administration of anti-c-fms monoclonal antibody prevents initial events of atherogenesis but does not reduce the size of advanced lesions in apolipoprotein E-deficient mice. Circulation. 1999;99:1740–6. doi: 10.1161/01.cir.99.13.1740. [DOI] [PubMed] [Google Scholar]
- 19.Yuan Z, Kishimoto C, Sano H, Shioji K, Xu Y, Yokode M. Immunoglobulin treatment suppresses atherosclerosis in apolipoprotein E-deficient mice via the Fc portion. Am J Physiol Heart Circ Physiol. 2003;285:H899–906. doi: 10.1152/ajpheart.00926.2002. [DOI] [PubMed] [Google Scholar]
- 20.Shern-Brewer R, Santanam N, Wetzstein C, White-Welkley J, Price L, Parthasarathy S. The paradoxical relationship of aerobic exercise and the oxidative theory of atherosclerosis. In: Sen CK, Packer L, Hanninen O, editors. Handbook of Oxidants and Antioxidants in Exercise. New York: Elsevier; 2000. pp. 1053–67. [Google Scholar]
- 21.Kita T, Nagano Y, Yokode M, et al. Probucol prevents the progression of atherosclerosis in Watanabe heritable hyperlipidemic rabbit, an animal model for familial hypercholesterolemia. Proc Natl Acad Sci USA. 1987;84:5928–31. doi: 10.1073/pnas.84.16.5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parthasarathy S, Young SG, Witztum JL, Pittman RC, Steinberg D. Probucol inhibits oxidative modification of low density lipoprotein. J Clin Invest. 1986;77:641–4. doi: 10.1172/JCI112349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ziccardi P, Nappo F, Giugliano G, et al. Reduction of inflammatory cytokine concentrations and improvement of endothelial functions in obese women after weight loss over one year. Circulation. 2002;105:804–9. doi: 10.1161/hc0702.104279. [DOI] [PubMed] [Google Scholar]