Abstract
BACKGROUND
In the ischemic or hypoxic heart, an impairment of electrical cell-to-cell coupling and a dephosphorylation of the connexins that comprise the gap junction channel were observed. However, it remains to be elucidated whether the dephosphorylation of the connexin during hypoxia is due to alterations in the ionic strength of Ca2+ or H+, and how the activation of protein kinase A (PKA) affects the hypoxia-induced abnormal function of the gap junction.
OBJECTIVES
The effects of hypoxia, intracellular Ca2+ overload and intracellular acidosis on the PKA-mediated phosphorylation of connexin 43 (Cx43) were examined in relation to the function of the cardiac gap junction.
METHODS
Hearts isolated from adult, male guinea pigs were used. The intercellular electrical cell-to-cell coupling was evaluated by the longitudinal internal resistance and the conduction velocity observed in in vitro experiments using isolated muscle strip preparations. The phosphorylation of Cx43 was evaluated by an immunoblot (Western blot). The localization of immunoreactive Cx43 at the intercalated disk was detected using confocal laser scan microscopy.
RESULTS
Cyclic AMP or the activation of PKA promotes the intercellular electrical coupling that accompanies an augmentation of the PKA-mediated phosphorylation of Cx43. Electrical cell-to-cell decoupling and reduction of the PKA-mediated phosphorylation of Cx43 were dependent on the progression of hypoxia. These results agree with those observed in the progression of intracellular Ca2+ overload or intracellular acidosis. Cyclic AMP or the activation of PKA alleviated the electrical cellular decoupling and the hypoxia-, intracellular Ca2+ overload- and intracellular acidosis-induced deteriorated expression of Cx43. These ameliorative effects of cyclic AMP on the function of the gap junction and on the expression of Cx43 weakened as the hypoxia progressed, and as the intracellular ionic strength of Ca2+ and H+ increased.
CONCLUSIONS
In cardiac ventricular muscle cells, cyclic AMP or the activation of PKA promotes electrical cell-to-cell coupling through the gap junction due to an augmentation of the PKA-mediated phosphorylation of Cx43 in the early stage of hypoxia, as well as in normoxia. The suppression of PKA-mediated phosphorylation of Cx43 during hypoxia may be caused by an increase in the intracellular ionic strength of Ca2+ and H+. Thus, the activation of cyclic AMP-dependent PKA may have an antiarrhythmic effect in the early stage of hypoxia.
Keywords: 8-Bromo-cyclic AMP, Cardiac gap junction, Cx43, Hypoxia, PKA-mediated phosphorylation
In cardiac muscle, the gap junctions greatly contribute to electrical cell-to-cell coupling, which is known to play an important role in intercellular impulse conduction (1,2). Arrhythmogenic factors can impair intercellular conductivity. This physiological function of the gap junction depends on the opening and closing of the channels that comprise the gap junction. In turn, the opening and closing of the channels depends on the phosphorylation of the connexins that comprise the channels because the connexins are phosphoproteins.
It is generally accepted that cyclic AMP increases the electrical conductance of the gap junction and, thus, promotes electrical cell-to-cell coupling in cardiac cells (3,4). These events may result from an increased probability of the channels being open due to the protein kinase A (PKA)-mediated phosphorylation of the connexins (3,5), an augmentation of the synthesis of the connexin protein (6) or an increase in the number of channels with a higher conductance (7). On the other hand, Ca2+ (8–14) and H+ (9,11,14) are known to play a major role in decreasing gap junction conductance and impairing electrical cell-to-cell coupling. A disturbance in the conductivity during hypoxia or ischemia is possibly caused by electrical cell-to-cell decoupling, because a progressive increase in intracellular Ca2+ overload and a progressive intracellular acidosis occur during hypoxia or ischemia (15–17). Previously, we reported on experiments on preparations isolated from adult, guinea pig heart, which showed that D-sotalol, which elevates the intracellular cyclic AMP level, prevented and restored hypoxia-induced electrical cell-to-cell decoupling (18). However, these ameliorative effects of cyclic AMP were not observed when hypoxia continued for a long time, specifically, for more than 1 h (19). Such evidence leads to the question of whether the phosphorylation of gap junction connexin is affected by the ionic strength of Ca2+ or H+. On the other hand, the dephosphorylation of connexin has also been reported to be induced by ischemia (20,21). However, the relationship between Ca2+ or H+ and the dephosphorylation of connexin, as well as the effects of the activation of PKA on the dephosphorylated connexin, still remain to be elucidated.
In the present study, the effects of intracellular Ca2+ overload and intracellular acidosis on the PKA-mediated phosphorylation of connexin 43 (Cx43) were examined on the ventricular muscle of adult guinea pig heart in reference to the functions of the gap junction.
Some parts of the present study were previously reported as abstracts from 1997 to 2003 (19,22–27) and were also reviewed in 2004 (28).
METHODS
Animals
Adult male guinea pigs weighing 385±15.5 g (mean ± SEM) were sacrificed by a blow to the head, and the experimental protocol was performed according to the method approved by the Institutional Animal Care and Use Committees of Fukuoka University (Fukuoka, Japan). The animals were heparinized by a intraperitoneal injection of heparin (NOVO Herparin, Novo Nordik A/S, Denmark) at a dose of 1000 U/kg 30 min before they were sacrificed.
Preparations and samples for experiments
Thin endocardial muscle strips (less than 1 mm in thickness, 10 mm to 15 mm in length and 2 mm to 3 mm in width) were isolated from the right ventricular wall after the removal of the heart from the animal, and were used to measure the conduction velocity and the longitudinal internal resistance (ri).
In another series of experiments, the hearts, which were excised quickly from the animal, were mounted on a Langendorff apparatus and were perfused with well-oxygenated standard Krebs solution (130 mM NaCl, 5.4 mM KCl, 11.9 mM NaHCO3, 1.2 mM NaH2PO4, 1.2 mM MgCl2, 2.5 mM CaCl2 and 5.5 mM glucose, and saturated with a mixture of gases [97% O2 and 3% CO2], pH adjusted to 7.4) at a constant pressure (60 mmHg). After 10 min to 15 min of stabilization, the hearts were perfused with the test solutions and Krebs solutions, including reagents. After treatment with either the test solutions or the reagents, ventricular tissue specimens were removed from the Langendorff apparatus and were subjected to Western blotting and histochemistry.
Test solutions
Hypoxic solution
To expose the heart muscle to hypoxia, Krebs solution without glucose, including Na2S2O4 (0.5 mM), was perfused while being saturated by a mixture of gases (97% N2 and 3% CO2). Under these conditions, the partial pressure of O2 (PO2) in the solution was kept at less than 20 mmHg.
Intracellular Ca2+ overload
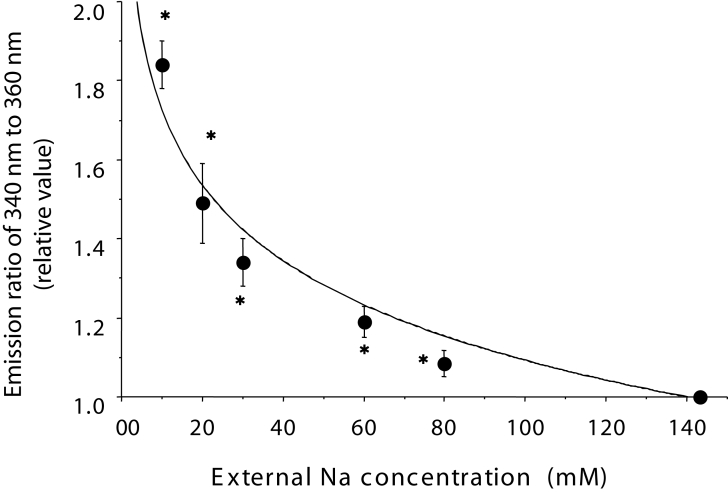
Intracellular Ca2+ overload was induced by a reduction of Na+ in the external perfusing solution substituted with N-methyl-D-glucamine, while adjusting the pH to 7.4 with HCl. The intracelluar Ca2+ level was measured by Fura-2AM (Dojndo, Japan) on ventricular myocytes isolated by collagenase (Figure 1).
Figure 1.
The intracellular Ca2+ level at various external Na+ concentrations ranging from 10 mM to 143.1 mM (standard) was measured with Fura-2AM (Dojndo, Japan) on ventricular myocytes isolated by collagenase. Each plot and vertical bar shows the relative value of the mean ± SEM. A relative value of 1.0 represents the control value at the standard Na+ concentration. Based on the experimental results, the intracellular Ca2+ concentration was calculated to be 1.04±0.06 μM at an Na+ concentration of 20 mM. *P<0.001 versus the value at a standard Na+ concentration
Intracellular acidosis
Intracellular acidosis was induced by an increase in the pressure of CO2. The perfusing solution had a pH of 7.4 (control [normal]), 7.1 to 6.9 (90% O2 and 10% CO2, with a PO2 of 550 mmHg) or 6.1 to 5.9 (50% O2 and 50% CO2, with a PO2 of 400 mmHg), as determined by an alteration in the proportion of O2 and CO2, and was checked by a pH meter (i-STAT Portable Clinical Analyser, i-STAT Corperation, USA). Because intracellular acidosis possibly promotes the inflow of Ca2+ into the cell through the reverse mode of Na+-Ca2+ exchange following Na+-H+ exchange, external Ca2+ was excluded in the present study.
An evaluation of electrical cell-to-cell coupling
Measurement of the conduction velocity
Preparations of muscle were fixed in a three-compartment chamber separated by thin silicon membranes, which were superfused with well-oxygenated standard Krebs solution at a constant flow (5 mL/min) and temperature (37°C). The middle part of the preparation was 2 mm in distance and 0.1 mL in volume. The preparations were electrically stimulated with a suction electrode at 0.5 Hz on one of two sides. The transmembrane action potential was recorded at each part with a conventional glass microelectrode being kept at a constant distance. The middle part of the muscle was superfused with hypoxic solutions or solutions with reagents. The changes in the conduction velocity at the middle of the muscle were estimated by the distance of the middle part divided by the time difference between the two action potentials from both side parts. At the middle part, the maximum rate of depolarization of the action potential (Vmax) was measured.
Measurement of the ri
The method for measuring ri was based on the principle described by Tuganowski et al (29). The preparations were fixed in a three-compartment chamber separated by thin silicon membranes, which were superfused with well-oxygenated standard Krebs solution at a constant flow (5 mL/min) and temperature (37°C). The middle part of the preparation was 0.8 mm in distance and 0.1 mL in volume. The middle part of the muscle was super-fused with reagents and test solutions. The transmembrane action potential was recorded at one of two sides with a conventional glass microelectrode, and the extracellular action potential was recorded with suction electrodes placed on both sides of the preparation. The preparation was electrically stimulated with a suction electrode at 0.5 Hz on one of the two sides. The shunt resistance in the electrical circuit was 50 kΩ. The ri was determined as follows:
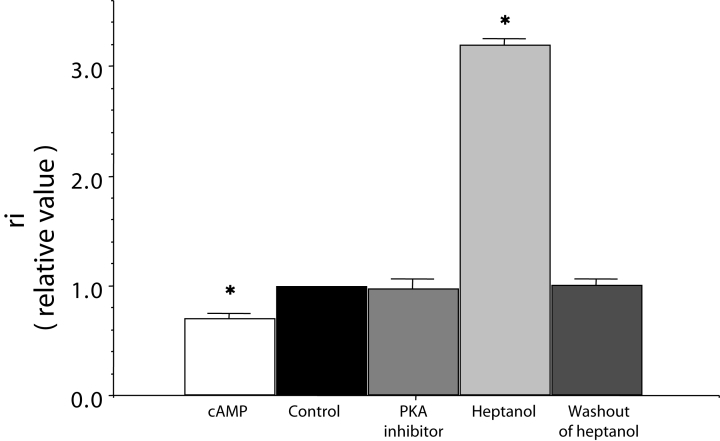
where rs is the shunt resistance in the electrical circuit, Eo is the amplitude of the transmembrane action potential, and E1 and E2 are the amplitude of the extracellular action potentials before and after shunting with rs, respectively. A remarkable increase in ri by heptanol, which closes the gap junction channel, and a complete reversibility by washout of heptanol were demonstrated using this method (Figure 2).
Figure 2.
Effects of cyclic AMP (cAMP), protein kinase A (PKA) inhibitor with cAMP (represented as PKA inhibitor on graph), and heptanol on the longitudinal internal resistance (ri), which is shown as a relative value. A relative value of 1.0 represents the control mean value before treatment of reagents. cAMP (1 μM) and PKA inhibitor (10 μM) were applied for 30 min. Heptanol (1 mM) was applied for 1 min. Each bar corresponds to the mean ± SEM. *P<0.001 versus the control value. Reproduced from reference 38
Immunoblot and an evaluation of the phosphoryation of Cx43
Ventricular samples were frozen quickly in liquid nitrogen. Tissue samples were homogenized in Tris buffer (10 mM Tris-HCl and 150 mM NaCl, with a pH of 7.4) that included phenylmethanesulfonyl fluoride at a final concentration of 1 mM on ice and were centrifuged at 200 g (1200 rpm) for 15 min at 4°C. The supernatants were mixed with 10% Triton X-100 (Nacalai Tesque, Japan) and were centrifuged again at 100,000 g (30,000 rpm) for 30 min and, subsequently, the pellets were subjected to a Western blot analysis. The pellets were solubilized with Laemmli sample dilution buffer (4% sodium dodecyl sulphate [SDS], 125 mM Tris-HCl [pH of 6.8] and 20% glycerol). The protein concentration was measured by a bicinchoninic acid protein assay kit (Pierce, USA). The samples were boiled for 3 min with 2% beta-mercaptoethanol, and 25 μg of total protein was run on a 10% SDS polyacrylamide gel. The separated protein was then electrophoretically transferred to a polyvinylidene fluoride membrane (GE Healthcare Ltd, United Kingdom) in transfer buffer (25 mM Tris, 192 mM glycine and 20% methanol). After the membranes were blocked with 5% skimmed milk in phosphate buffered saline containing 0.1% Tween (Wako, Japan), they were incubated with primary antibody (specific mouse monoclonal anti-Cx43 antibodies, Chemicon International Inc, USA) at a dilution of 1:4000 for 1 h at room temperature. Secondary antibody (antimouse immunoglobulin, GE Healthcare Ltd) was used at a dilution of 1:5000. Cx43 protein-antibody complexes were detected by using chemiluminescence Western blotting detection reagents (ECL Plus [RPN2124], GE Healthcare Ltd), and they were then exposed to chemiluminescence film (Hyperfilm, GE Healthcare Ltd) at room temperature for 5 s to 20 s. The molecular weight was calibrated using a Low Molecular Weight Calibration Kit for SDS electrophoresis (GE Healthcare Ltd).
For the treatment of samples with alkaline phosphatase (from bovine intestinal mucosa, Sigma-Aldrich, USA), the phosphatase was added in the first step of homogenization at a dose of 20 U/mL.
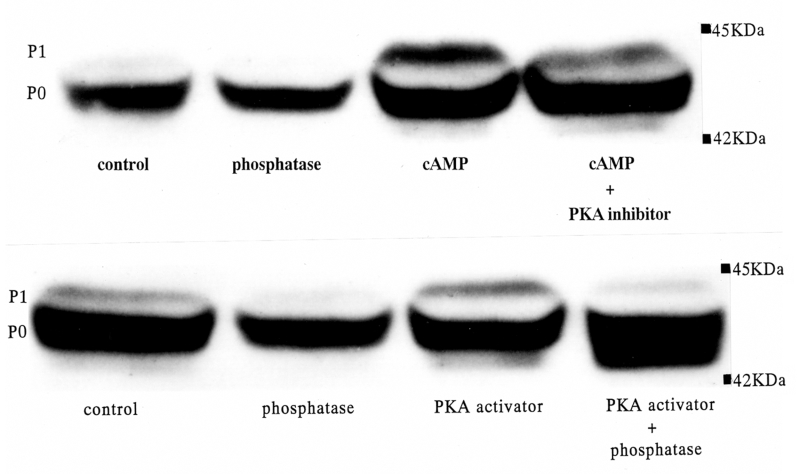
Two isoforms were observed near 43 kDa (Figure 3). The mean density of the higher molecular weight isoform was remarkably reduced by treatment with alkaline phosphatase, and this isoform was determined to be a phosphorylated connexin protein (P1). The lower molecular weight isoform was not affected by alkaline phosphatase and was thought to be the nonphosphorylated connexin (P0). The density ratio of P1 to P0 (P1/P0) was evaluated as the magnitude of the phosphorylation of the connexin.
Figure 3.
Representative Western blot findings for connexin 43 showing the effects of cyclic AMP (cAMP), protein kinase A (PKA) activator, cAMP with PKA inhibitor, alkaline phosphatase and PKA activator with phosphatase in normoxia. The control was not treated by any reagents. 8-Bromo-cyclic AMP (1 μM), PKA activator (1 μM) and PKA inhibitor (10 μM) were applied for 60 min on Langendorff perfusion. Alkaline phosphatase (20 U/mL) was treated at the first step of the homogenizing procedure (see Methods). P0 Nonphosphorylated connexin; P1 Phosphorylated connexin
Immunohistochemistry of Cx43
For the immunohistochemistry of Cx43, the rabbit polyclonal anti-Cx43 antibody (Zymed Lab Inc, USA) was used at dilutions of 1:200 and the goat antirabbit immunoglobulin G (Alexa Fluor 488, Invitrogen, USA) was used at dilutions of 1:200 as the secondary antibody. Immunofluorescence was detected by a confocal laser scan microscopy (LSM-410, Carl Zeiss Inc, Germany).
Densitometry
The mean density of the Cx43 complex isoforms in the immunoblots and the mean fluorescent intensity of immunoreactive particles for Cx43 on the confocal laser scan micrographs were analyzed by NIH Image Software (National Institutes of Health, USA).
Chemicals and reagents
8-Bromo-cyclic AMP (8-bromo-adenosine 3′,5′-cyclic monophosphate sodium salt, Sigma-Aldrich) (used as a cyclic AMP analogue [cyclic AMP mentioned in the figures and the text represents 8-bromo-cyclic AMP]), PKA activator (Sp-adenosine 3′,5′-cyclic monophosphothioate triethylamine salt, Sigma-Aldrich) and PKA inhibitor (Rp-adenosine 3′,5′-cyclic monophosphothioate triethylamine salt, Sigma-Aldrich) were used.
Statistical analysis
The data are expressed as means ± SEM. The unpaired Student’s t test was used to analyze statistical significance (P<0.001) between means.
RESULTS
The effects of cyclic AMP on the ri and the phosphorylation of Cx43 in normoxia
In normoxia, cyclic AMP (1 μM) decreased the ri by approximately 30% of the control within 30 min. This effect of cyclic AMP was inhibited or abolished by PKA inhibitor (Figure 2).
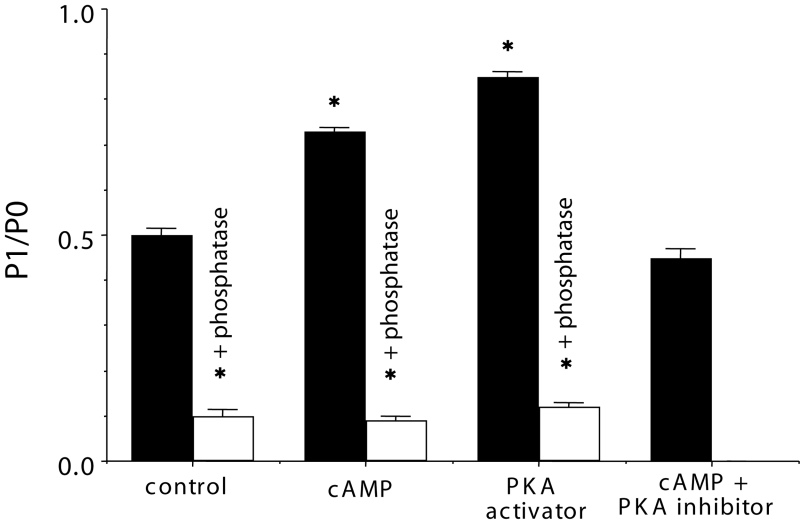
The Western blot findings showed that the density of the higher molecular weight isoform of Cx43 (P1) was augmented by cyclic AMP or PKA activator, and it was inhibited by alkaline phosphatase and PKA inhibitor (Figure 3). The P1/P0 ratio was augmented by cyclic AMP and PKA activator, and suppressed by PKA inhibitor and alkaline phosphatase (Figure 4).
Figure 4.
An analysis of phosphorylated connexin (P1)/nonphosphorylated connexin (P0) from some examples in the same experiment as Figure 3. Shown on the y-axis is the value of the P1/P0 ratio. The control was not treated with any reagents. The white columns represent the value after treatment with alkaline phosphatase. Each bar corresponds to the mean ± SEM. *P<0.001 versus the control value. cAMP Cyclic AMP; PKA Protein kinase A
The effects of cyclic AMP on the conduction velocity and the ri in hypoxia
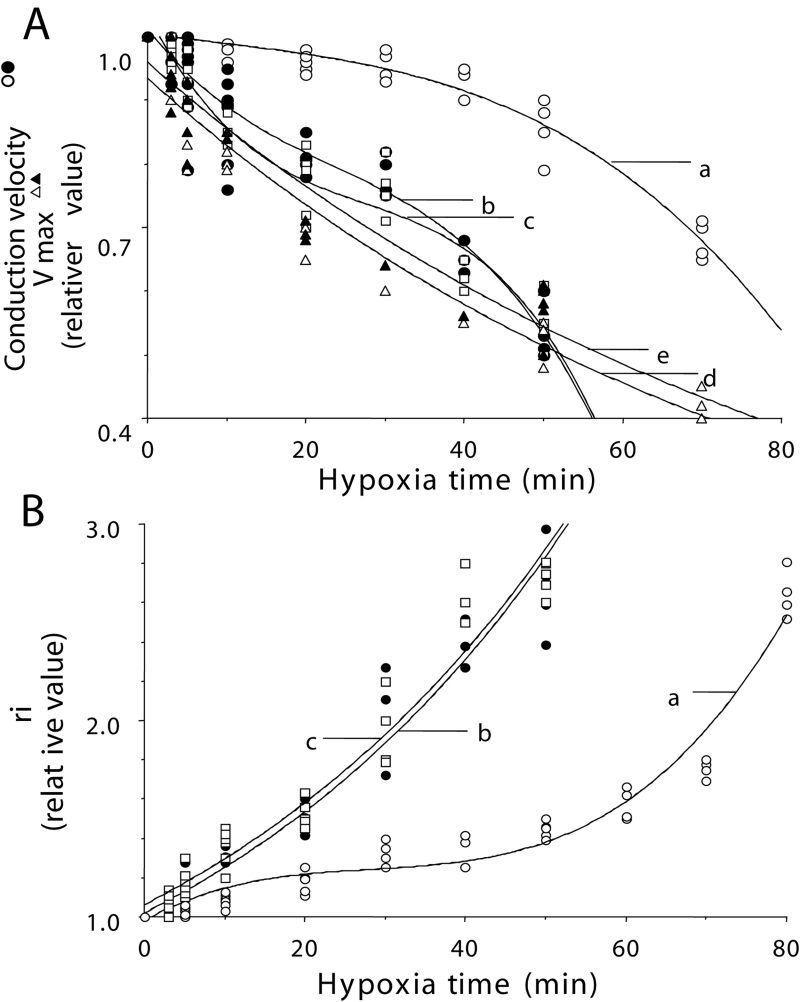
The conduction velocity decreased depending on the exposure time to hypoxia. Within 60 min, the conduction velocity decreased to approximately 40% of the control (before hypoxia), and thus, the conductivity was decreased. In the presence of cyclic AMP (1 μM), the changes in the conduction velocity were alleviated and conductivity was maintained for approximately 70 min to 80 min (Figure 5A). Vmax also decreased in a time-dependent manner during hypoxia. In the presence of cyclic AMP, the Vmax did not recover and, instead, was observed to decrease (Figure 5A).
Figure 5.
Time course of changes in the conduction velocity (A: a, b and c), the maximum rate of depolarization (Vmax) (A: d and e) and longitudinal internal resistance (ri) (B: a, b and c) in hypoxia. Each plot indicates the relative value. A relative value of 1.0 represents the value before hypoxia. Closed circles, open circles and open squares represent values in the absence of cyclic AMP, in the presence of cyclic AMP and in the presence of cyclic AMP with PKA inhibitor, respectively (A and B). Closed and open triangles represent Vmax in the absence and presence of cyclic AMP, respectively (A). The coefficients of correlation are r=0.974 (A: a), r=0.953 (A: b), r=0.964 (A: c), r=0.956 (A: d), r=0.973 (A: e), r=0.993 (B: a), r=0.990 (B: b) and r=0.978 (B: c)
The ri increased depending on the exposure time to hypoxia. Within 60 min, the ri increased to approximately 400% of the control (before hypoxia). In the presence of cyclic AMP (1 μM), a rise in the ri was alleviated during approximately 1 h of hypoxia. However, approximately 90 min later in hypoxia, the ri increased to 400% of the control, even in the presence of cyclic AMP (Figure 5B).
The effects of cyclic AMP on the phosphorylation of Cx43 during hypoxia
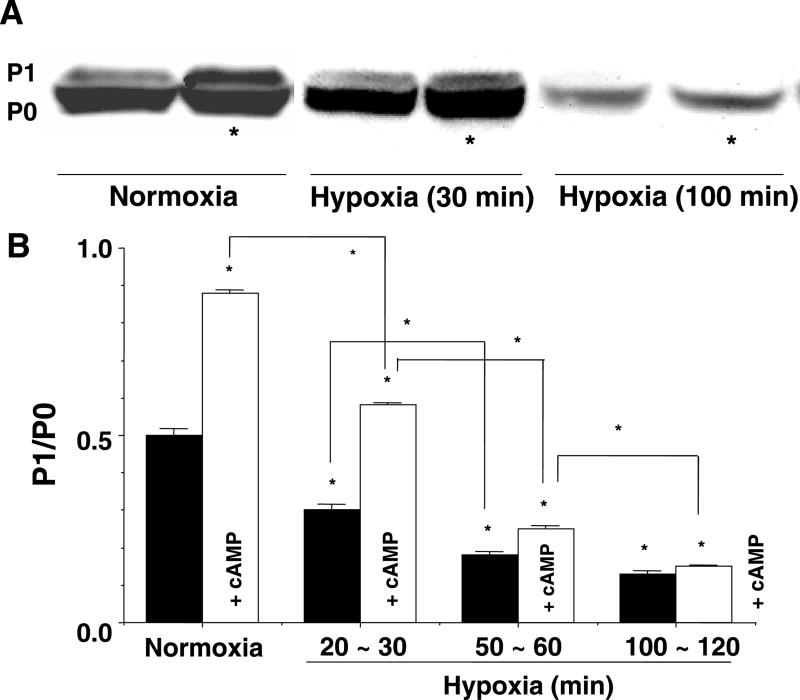
A Western blot and densitometry analysis of the immunoblot showed that PKA-mediated phosphorylation decreased as the hypoxia advanced (Figures 6A and 6B). The P1/P0 ratio decreased in a time-dependent manner after exposure to hypoxia (Figure 6B). In the presence of cyclic AMP, the reduction in the P1/P0 ratio was mitigated during approximately 1 h of hypoxia (Figure 6B). The restorative effects of cyclic AMP were not observed at more than 90 min of hypoxia (Figures 6A and 6B). The effects of PKA activation were the same as those of cyclic AMP (not shown in Figure).
Figure 6.
A Western blot findings for connexin 43 showing the effects of cyclic AMP (cAMP) in normoxia, and at 30 min and 100 min of hypoxia. *The presence of cAMP. B Analysis of phosphorylated connexin (P1)/nonphosphorylated connexin (P0) from some examples from the same experiment as A. The y-axis shows the value of the ratio of P1/P0. White columns represent the value in the presence of cAMP (1 μM). Each bar corresponds to the mean ± SEM. *P<0.001 versus the control, and difference between indicated lines
The effects of cyclic AMP on the expression of Cx43 at the intercalated disk in normoxia and hypoxia
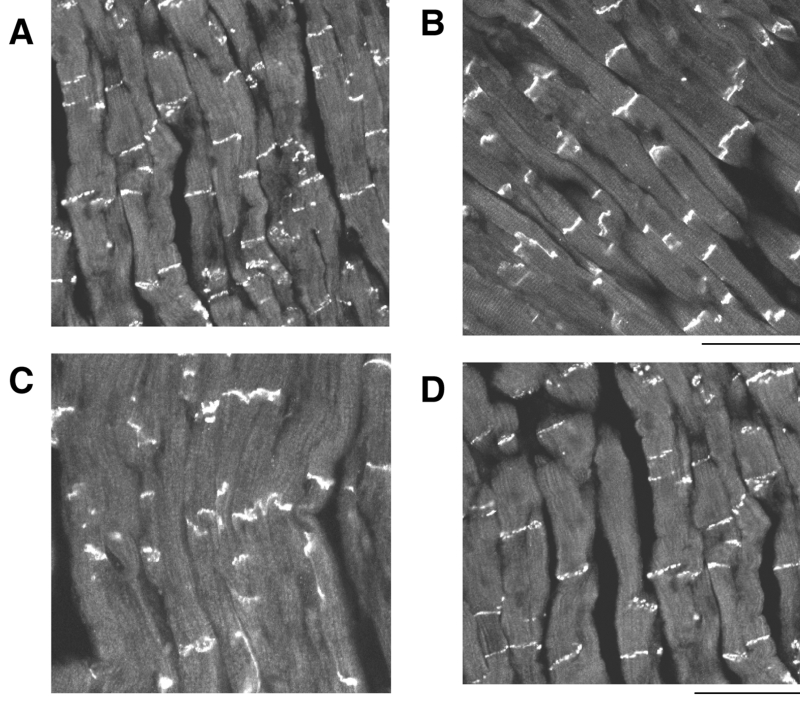
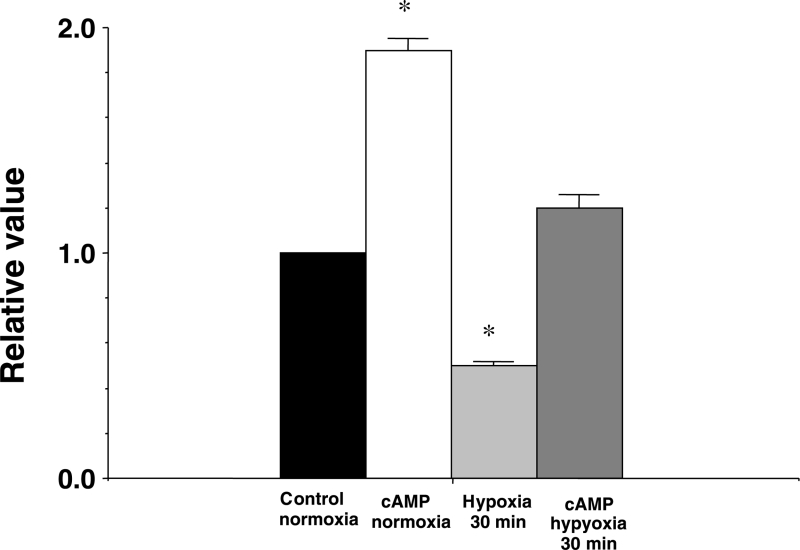
The effects of cyclic AMP on the expression of Cx43 at the intercalated disk in normoxia and hypoxia are shown on the confocal laser scan micrographs of the immunofluorescence of Cx43 using the polyclonal anti-Cx43 antibody in Figure 7, and analysis of the immunoreactive area at the intercalated disk is shown in Figure 8. At 30 min of hypoxia, the immunoreactivity of the phosphorylated Cx43 was scant and inhomogenous (Figure 7C), and the immunoreactive area was reduced (Figure 8). Cyclic AMP (1 μM) augmented the expression of the immunoreactivity of the phosphorylated Cx43 and the immunoreactive area at the intercalated disk in normoxia, as well as in hypoxia of 30 min (Figures 7B, 7D and 8). Recovery of the expression of Cx43 by cyclic AMP was not observed after more than 90 min of hypoxia (not shown in Figure).
Figure 7.
Confocal laser scan micrographs of the immunofluorescence of connexin 43. A Normal as control at 30 min normoxia; B At 30 min after the application of cyclic AMP (1 μM) in normoxia; C At 30 min of hypoxia; D At 30 min of hypoxia in the presence of cyclic AMP (1 μM). Scale: 100 μm
Figure 8.
An analysis of the immunofluorescence findings of connexin 43. A comparison of the area of the immunoreactive particles at the intercalated disk between the control in normoxia, cyclic AMP (cAMP) in normoxia (30 min after application of cAMP [1 μM]), at 30 min of hypoxia and at 30 min of hypoxia in the presence of cAMP (1 μM). The columns represent the relative values (1.0 represents the value of the control in normoxia). Each vertical bar corresponds to the mean ± SEM. *P<0.001 versus the control in normoxia
The effects of cyclic AMP on the ri in intracellular Ca2+ overload
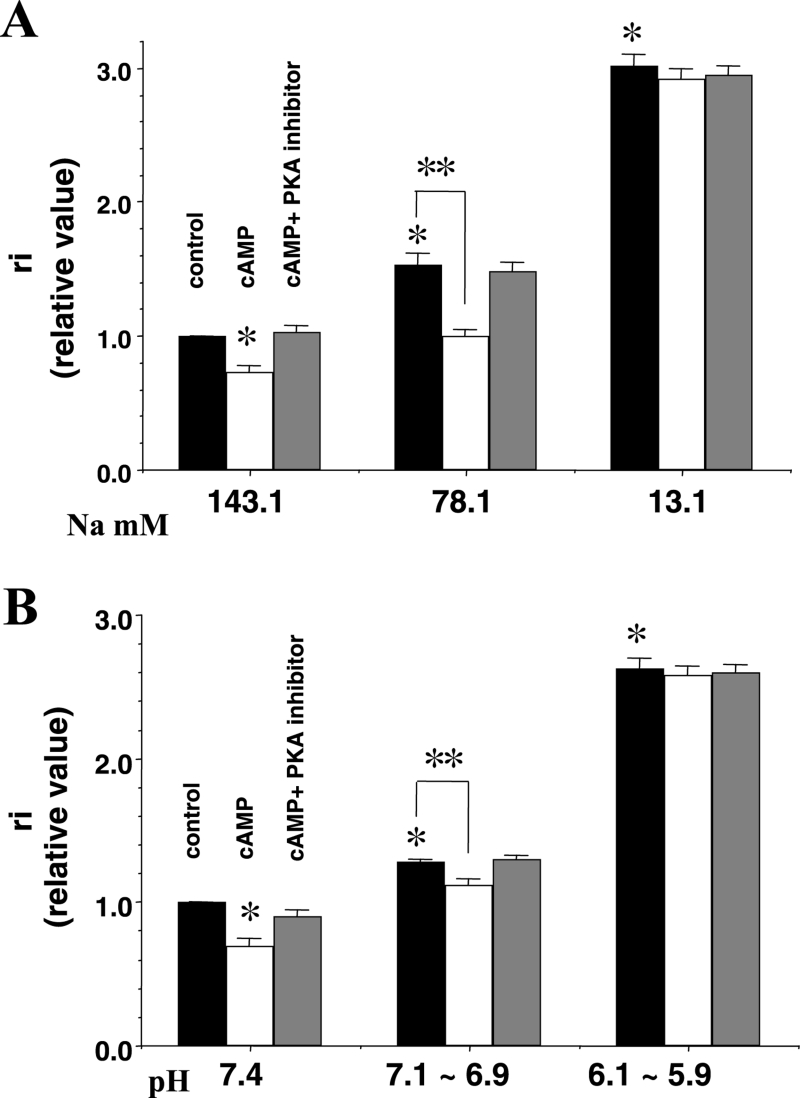
Intracellular Ca2+ overload was induced by a reduction in the external Na+ concentration (see Methods). The ri increased as the external Na+ concentration was reduced from the normal standard concentration of 143.1 mM to lower concentrations of 78.1 mM and 13.1 mM (Figure 9A). In the presence of cyclic AMP at an Na+ concentration of 78.1 mM, the increase in the ri was alleviated. The effects of cyclic AMP were abolished by PKA inhibitor (10 μM) (Figure 9A). However, the ameliorative effects of cyclic AMP on the raised ri were no longer observed at an Na+ concentration of 13.1 mM (Figure 9A).
Figure 9.
The effects of cyclic AMP (cAMP) with protein kinase A (PKA) inhibitor on the longitudinal internal resistance (ri) at low concentrations of external Na+ (A) and low pH (B). The external Na+ concentrations varied from standard (143.1 mM) to low concentrations (78.1 mM and 13.1 mM) and pH varied from a standard pH (7.4) to low pH levels (7.1 to 6.9 and 6.1 to 5.9). The test solutions were applied for 60 min on Langendorff perfusion. Concentrations of cAMP and PKA inhibitor were 1 μM and 10 μM, respectively. The y-axis shows the relative value of ri. A relative value of 1.0 corresponds to the value at the standard Na+ concentration and standard pH without any reagents. The black, white and grey columns represent the control (before treatment of reagents), the presence of cAMP and the presence of cAMP with PKA inhibitor, respectively. Each bar corresponds to the mean ± SEM. *P<0.001 versus the control; **P<0.001. Reproduced from reference 38
The effects of cyclic AMP on the phosphorylation of Cx43 in intracellular Ca2+ overload
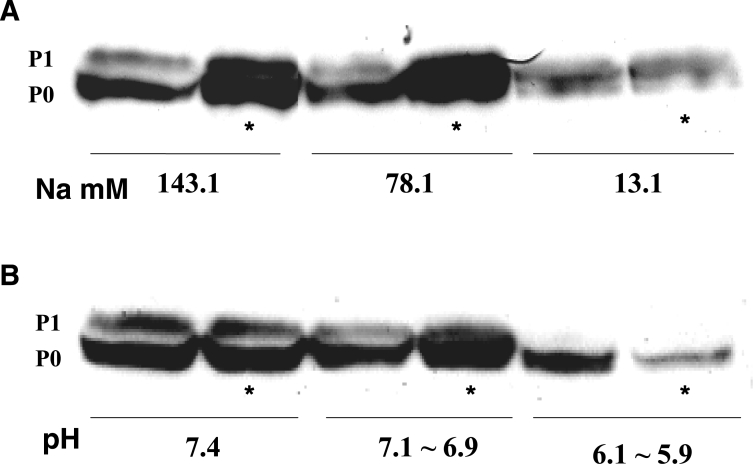
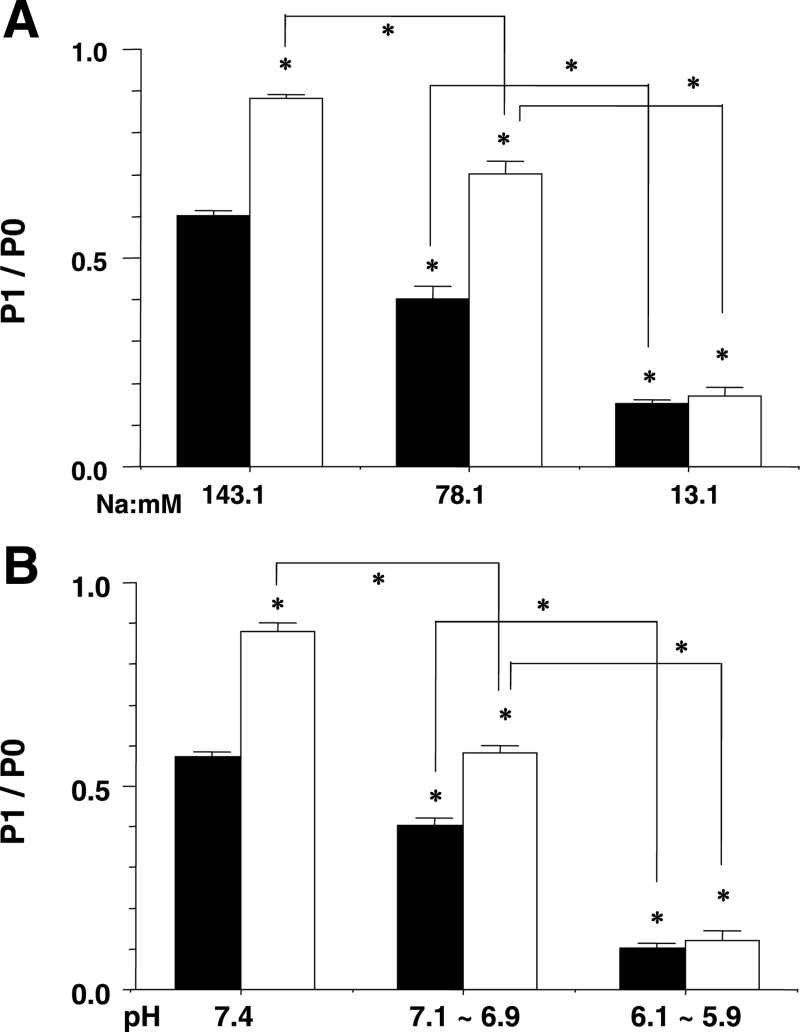
Tissue samples for the Western blot analysis were extracted 60 min after Langendorff perfusion of the low Na+ concentration solution and the test solutions, including cyclic AMP (1 μM). The P1/P0 ratio decreased as the external Na+ concentration was reduced (Figures 10A and 11A). Cyclic AMP (1 μM) augmented the P1/P0 ratio at Na+ concentrations of 143.1 mM and 78.1 mM, but not at 13.1 mM (Figure 10A and 11A).
Figure 10.
Representative Western blot findings for connexin 43 showing the effects of cyclic AMP at low concentrations of external Na+ (A) and low pH (B). The external Na+ concentrations varied from standard (143.1 mM) to low concentrations (78.1 mM and 13.1 mM) and the pH varied from a standard pH (7.4) to lower pH levels (7.1 to 6.9 and 6.1 to 5.9). The test solutions were applied for 60 min on Langendorff perfusion. The concentration of cyclic AMP used was 1 μM. *The presence of cyclic AMP. P0 Nonphosphorylated connexin; P1 Phosphorylated connexin
Figure 11.
An analysis of phosphorylated connexin (P1)/nonphosphorylated connexin (P0) from some examples in the same experiment as Figure 10. The y-axis shows the P1/P0 ratio and x-axis shows the external Na+ concentration (A) or the pH (B). The white columns represent the value in the presence of cyclic AMP. Each bar corresponds to the mean ± SEM. *P<0.001 versus the control, and difference between indicated lines
The effects of cyclic AMP on the ri in intracellular acidosis
The ri increased as the external pH was decreased from the standard concentration (7.4) to lower concentrations (7.1 to 6.9 and 6.1 to 5.9) (Figure 9B). The increase in the ri was alleviated by cyclic AMP (1 μM) at a pH range of 7.1 to 6.9. The effects of cyclic AMP were abolished by PKA inhibitor (10 μM). The restorative effects of cyclic AMP on the raised ri were no longer observed at a pH range of 6.1 to 5.9 (Figure 9B).
The effects of cyclic AMP on the phosphorylation of Cx43 in intracellular acidosis
Tissue samples for a Western blot analysis were extracted 60 min after Langendroff perfusion of the low pH solutions and the test solutions, including cyclic AMP (1 μM) (Figures 10B and 11B). The P1/P0 ratio was compared between each pH level in several examples (Figure 11B). The P1/P0 ratio decreased as the pH was decreased. Cyclic AMP augmented the P1/P0 ratio at the normal standard pH of 7.4 and at the pH range of 7.1 to 6.9, but not at the range of 6.1 to 5.9 (Figures 10B and 11B).
DISCUSSION
In the present study, we examined the influence of cyclic AMP or the activation of PKA on impulse conductivity in normoxia and hypoxia, especially in reference to the function of the gap junction.
Two isoforms of Cx43 were distinctly detected according to the Western blot findings (Figure 3). As described in the Methods section, the ratio of the mean density of the higher molecular weight isoform (P1) to the lower molecular weight isoform (P0), the P1/P0 ratio, was available to evaluate the phosphorylation of Cx43. We used monoclonal anti-Cx43 antibody. It has been reported that in rat heart, the phosphorylated isoform of Cx43 can be detected by polyclonal anti-Cx43 antibody, but not by monoclonal anti-Cx43 antibody (20). In another report (30), it was mentioned that some phosphorylated isoforms of Cx43 near 41 kDa can be detected with the monoclonal antibody. We did not observe a definite difference in the P1/P0 ratio between the monoclonal and polyclonal anti-Cx43 antibodies (data not shown). Thus, it was concluded that monoclonal anti-Cx43 antibody can be used for the detection of the phosphorylated isoform of Cx43 in the guinea pig heart. P1 was augmented by cyclic AMP or PKA activator and suppressed by PKA inhibitor. As a result, P1 was determined to be an isoform of Cx43 that had undergone PKA-mediated phosphorylation. The P1/P0 ratio can be evaluated as the magnitude of the PKA-mediated phosphorylation of Cx43.
It is generally accepted that cyclic AMP increases the macroscopic electrical conductance of the cardiac gap junction due to the activation of PKA (3–5). The present study showed that cyclic AMP decreased the ri (specifically, it promoted electrical cell-to-cell coupling), and the effects of cyclic AMP were inhibited by the PKA inhibitor. Therefore, it is probable that the action of cyclic AMP on the gap junction is mediated by the activation of PKA. There have been observations that cyclic AMP or the activation of PKA can increase the quantity of Cx43 (6,25–27), increase the area of immunoreactive particles for Cx43 at the intercalated disk (Figures 7B and 8) (25–27) and enhance the gap junction assembly (31). These results may indicate that cyclic AMP or the activation of PKA can increase the number of gap junction channels.
In the present study, it was shown by Western blot analysis that cyclic AMP or PKA activator augmented phosphorylation of Cx43, and this effect was suppressed by PKA inhibitor. Such evidence suggests that the promotive effect of cyclic AMP on the gap junction function is attributed to an augmentation of the PKA-mediated phosphorylation of Cx43.
Thus, it is suggested that an augmentation of the PKA-mediated phosphorylation of Cx43 upregulates the function of the gap junction through an increase in the probability of the gap junction channel being open (3,5), a delay in the proteolytic degradation of the connexin or acceleration of the synthesis of the connexin protein (6).
A deterioration in the conduction velocity and the ri during hypoxia was alleviated by cyclic AMP. The effects of cyclic AMP were inhibited by PKA inhibitor. As a result, it is probable that the effects of cyclic AMP were caused by the activation of PKA. The Vmax tended to decrease in the presence of cyclic AMP during hypoxia. This can be explained by evidence that Vmax was suppressed by the activation of beta-adrenergic receptors in the depolarized membrane (32). Therefore, our findings suggest that the effects of cyclic AMP are caused by an amelioration of the passive property of the membrane, namely, the gap junction. The immunohistochemistry results (Figure 7D) support this idea. However, our results also showed that the ameliorative effects of cyclic AMP on gap junction function were transiently exhibited at the early stage, within 60 min of hypoxia, and later on, they were modified by the ionic strength of Ca2+ and H+. This theory is supported by the fact that intracellular Ca2+ overload or acidosis is induced by severe hypoxia (15–17).
In hypoxic cardiac muscle, abnormalities of the intercellular impulse conductivity are often observed. When cardiac muscle is exposed to hypoxia, intracellular Ca2+ overload and intracellular acidosis are induced (15–17). Because Ca2+ and H+ are major factors that decrease gap junction conductance (8–14), it is possible to conclude that the hypoxia-induced, cell-to-cell decoupling is explainable by the influence of the intracellularly elevated Ca2+ or H+, or both, on the gap junction.
In the present study, the electrical cell-to-cell coupling was shown to be impaired by intracellular Ca2+ overload induced by a reduction in the external Na+ concentration. When intracellular Ca2+ overload was moderate at an external Na+ concentration of 78.1 mM, the Ca2+-induced cell-to-cell decoupling was recovered by cyclic AMP, and the ameliorative effect of cyclic AMP was suppressed by PKA inhibitor. However, when the Ca2+ overload was extremely high, such as that achieved at an external Na+ concentration of 13.1 mM, the ameliorative effect of cyclic AMP was no longer observed. The phosphorylation of Cx43 by PKA was slightly impaired at an external Na+ concentration of 78.1 mM and remarkably impaired at 13.1 mM. During intracellular Ca2+ overload, the effects of cyclic AMP on electrical cell coupling was consistent with those found with cyclic AMP on the phosphorylation of Cx43. These findings suggest that the PKA-mediated phosphorylation of Cx43 is affected by the ionic strength of Ca2+; specifically, it is suppressed by a higher ionic strength of Ca2+.
Intracellular acidosis (pH) is equivalent to extracellular acidosis induced by an elevation in the partial pressure of CO2 (33,34). Intracellular acidosis possibly promotes an inflow of Ca2+ into the cell through activation of the reverse mode of Na+-Ca2+ exchange following Na+-H+ exchange. In our model, the effects of intracellular acidosis were found to depend only on the ionic strength of H+, because external Ca2+ was removed. The increase in the ri at a pH range of 7.1 to 6.9 was alleviated by cyclic AMP, but not at a pH range of 6.1 to 5.9. The phosphorylation of Cx43 by PKA was suppressed depending on the magnitude of acidity. Cyclic AMP augmented the phosphorylation at a pH range of 7.1 to 6.9, but not at the pH range of 6.1 to 5.9. The effect of cyclic AMP on intercellular electrical coupling is consistent with that found on the phosphorylation of Cx43. Such evidence suggests that the PKA-mediated phosphorylation of Cx43 is affected by the ionic strength of H+. In our experiments, the ameliorative effects of cyclic AMP on the electrical decoupling and the dephosphorylation of Cx43 were not observed at a pH below 6.5.
As mentioned above, the PKA-mediated phosphorylation of Cx43 decreased during hypoxia; specifically, it was possibly dephosphorylated by Ca2+ or H+, or both. The activation of protein phosphatase type 1 may mediate the dephosphorylation of Cx43 (21).
It is frequently mentioned that cyclic AMP or the activation of beta-adrenergic receptors is arrhythmogenic due to the promotion of the Ca2+ channels in the active membrane (35,36). On the other hand, it is generally accepted that cyclic AMP promotes intercellular electrical coupling (3–7). As shown in the present study, cyclic AMP prevents disturbances in impulse conductivity during hypoxia due to an augmentation of the PKA-mediated phosphorylation of Cx43. It is understandable that the gap junctional uncoupling is an arrhythmogenic factor in acute ischemia (37). As a result, it is probable that cyclic AMP has an antiarrhythmic effect due to the maintenance or promotion of the gap junction function. However, this antiarrhythmic effect of cyclic AMP may be exhibited at an early stage of hypoxia.
CONCLUSIONS
Cyclic AMP promotes intercellular electrical coupling and gap junctional communication via an augmentation of the PKA-mediated phosphorylation of Cx43. The reduction in the PKA-mediated phosphorylation of Cx43 that accompanies deterioration of the intercellular electrical coupling increased as hypoxia advanced. The same results were also observed with a progressive increase in the intracellular ionic strength of Ca2+ and H+. The activation of cyclic AMP-dependent PKA had protective or ameliorative effects on the hypoxia-, intracellular Ca2+ overload- and intracellular acidosis-induced dephosphorylation of Cx43, as well as on alterations in the function of the gap junction. However, these protective or ameliorative effects were not exhibited in the later stage of hypoxia and in the extreme increase in the ionic strength of Ca2+ or H+. Thus, further studies are required to elucidate the mechanisms of the dephosphorylation of the PKA-mediated phosphorylation of Cx43 induced by Ca2+ and H+.
Regarding the pathophysiological significance of these findings, it is suggested that in the early stage of hypoxia, an increase in sympathetic activity or an activation of cyclic AMP-dependent PKA has antiarrhythmogenic effects due to the prevention or amelioration of gap junctional decoupling, whereas in the later stage of hypoxia, it has an arrhythmogenic effect due to promotive effects on Ca2+ channel activity rather than due to protective effects on the gap junction.
ACKNOWLEDGEMENTS
The present study was supported by a Grant-in-Aid from the Japanese Ministry of Education (1995–1996, No 07670070), a grant from The Vehicle Racing Commemorative Foundation (1999–2001) and a grant from The Central Research Institute of Fukuoka University (2001–2003, No 016010). The authors thank Dr B Quinn for correction of English
REFERENCES
- 1.Weidmann S. The diffusion of radiopotassium across intercalated disks of mammalian cardiac muscle. J Physiol. 1966;187:323–42. doi: 10.1113/jphysiol.1966.sp008092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Imanaga I, Kameyama M, Irisawa H. Cell-to-cell diffusion of fluorescent dyes in paired ventricular cells. Am J Physiol. 1987;252:H223–H32. doi: 10.1152/ajpheart.1987.252.1.H223. [DOI] [PubMed] [Google Scholar]
- 3.Burt JM, Spray DC. Inotropic agents modulate gap junctional conductance between cardiac myocytes. Am J Physiol. 1988;254:H1206–H10. doi: 10.1152/ajpheart.1988.254.6.H1206. [DOI] [PubMed] [Google Scholar]
- 4.De Mello WC. Further studies on the influence of cAMP-dependent protein kinase on junctional conductance in isolated heart cell pairs. J Mol Cell Cardiol. 1991;23:371–9. doi: 10.1016/0022-2828(91)90073-u. [DOI] [PubMed] [Google Scholar]
- 5.De Mello WC. Increase in junctional conductance caused by isoproterenol in heart cell pairs is suppressed by cAMP-dependent protein-kinase inhibitor. Biochem Biophys Res Commun. 1988;154:509–14. doi: 10.1016/0006-291x(88)90169-6. [DOI] [PubMed] [Google Scholar]
- 6.Dodge SM, Beardslee MA, Darrow BJ, Green KG, Beyer EC, Saffitz JE. Effects of angiotensin II on expression of the gap junction channel protein connexin43 in neonatal rat ventricular myocytes. J Am Coll Cardiol. 1998;32:800–7. doi: 10.1016/s0735-1097(98)00317-9. [DOI] [PubMed] [Google Scholar]
- 7.Kwak BR, Jongsma HJ. Regulation of cardiac gap junction channel permeability and conductance by several phosphorylating conditions. Mol Cell Biochem. 1996;157:93–9. doi: 10.1007/BF00227885. [DOI] [PubMed] [Google Scholar]
- 8.Burt JM, Frank JS, Berns MW. Permeability and structural studies of heart cell gap junctions under normal and altered ionic conditions. J Membr Biol. 1982;68:227–38. doi: 10.1007/BF01872267. [DOI] [PubMed] [Google Scholar]
- 9.Burt JM. Block of intercellular communication: Interaction of intracellular H+ and Ca2+ Am J Physiol. 1987;253:C607–C12. doi: 10.1152/ajpcell.1987.253.4.C607. [DOI] [PubMed] [Google Scholar]
- 10.Maurer P, Weingart R. Cell pairs isolated from adult guinea pig and rat hearts: Effects of [Ca2+]i on nexal membrane resistance. Pflugers Arch. 1987;409:394–402. doi: 10.1007/BF00583793. [DOI] [PubMed] [Google Scholar]
- 11.Noma A, Tsuboi N. Dependence of junctional conductance on proton, calcium and magnesium ions in cardiac paired cells of guinea-pig. J Physiol. 1987;382:193–211. doi: 10.1113/jphysiol.1987.sp016363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tuganowski W, Korczynska I, Wasik K, Piatek G. Effects of calmidazolium and dibutyryl cyclic AMP on the longitudinal internal resistance in sinus node strips. Pflugers Arch. 1989;414:351–3. doi: 10.1007/BF00584638. [DOI] [PubMed] [Google Scholar]
- 13.Toyama J, Sugiura H, Kamiya K, Kodama I, Terasawa M, Hidaka H. Ca2+-calmodulin mediated modulation of the electrical coupling of ventricular myocytes isolated from guinea pig heart. J Mol Cell Cardiol. 1994;26:1007–15. doi: 10.1006/jmcc.1994.1121. [DOI] [PubMed] [Google Scholar]
- 14.Firek L, Weingart R. Modification of gap junction conductance by divalent cations and protons in neonatal rat heart cells. J Mol Cell Cardiol. 1995;27:1633–43. doi: 10.1016/s0022-2828(95)90623-1. [DOI] [PubMed] [Google Scholar]
- 15.Kléber AG, Riegger CB, Janse MJ. Electrical uncoupling and increase of extracellular resistance after induction of ischemia in isolated, arterially perfused rabbit papillary muscle. Circ Res. 1987;61:271–9. doi: 10.1161/01.res.61.2.271. [DOI] [PubMed] [Google Scholar]
- 16.Steenbergen C, Murphy E, Levy L, London RE. Elevation in cytosolic free calcium concentration early in myocardial ischemia in perfused rat heart. Circ Res. 1987;60:700–7. doi: 10.1161/01.res.60.5.700. [DOI] [PubMed] [Google Scholar]
- 17.Dekker LR, Fiolet JW, VanBavel E, et al. Intracellular Ca2+, intercellular electrical coupling, and mechanical activity in ischemic rabbit papillary muscle. Effects of preconditioning and metabolic blockade. Circ Res. 1996;79:237–46. doi: 10.1161/01.res.79.2.237. [DOI] [PubMed] [Google Scholar]
- 18.Manoach M, Tribulová N, Imanaga I. The protective effect of D-sotalol against hypoxia-induced myocardial uncoupling. Heart Vessels. 1996;11:281–8. doi: 10.1007/BF01747187. [DOI] [PubMed] [Google Scholar]
- 19.Imanaga I, Matsumura K. Effects of c-AMP on disturbance of impulse propagation during hypoxia in myocardium. J Mol Cell Cardiol. 1997;29:A6. (Abst) [Google Scholar]
- 20.Beardslee MA, Lerner DL, Tadros PN, et al. Dephosphorylation and intracellular redistribution of ventricular connexin43 during electrical uncoupling induced by ischemia. Circ Res. 2000;87:656–62. doi: 10.1161/01.res.87.8.656. [DOI] [PubMed] [Google Scholar]
- 21.Jeyaraman M, Tanguy S, Fandrich RR, Lukas A, Kardami E. Ischemia-induced dephosphorylation of cardiomyocyte connexin-43 is reduced by okadaic acid and calyculin A but not fostriecin. Mol Cell Biochem. 2003;242:129–34. [PubMed] [Google Scholar]
- 22.Imanaga I, Mayama T. Effects of c-AMP on intercellular electrical decoupling induced by intracellular Ca2+-overload and acidosis in myocardium. J Mol Cell Cardiol. 1998;30:A306. (Abst) [Google Scholar]
- 23.Imanaga I, Hirosawa N, Matsumura K, Mayama T. Effects of Ca ions and acidosis on phosphorylation of cardiac gap junction connexin43 in adult guinea pig heart. J Mol Cell Cardiol. 2000;32:A119. (Abst) [Google Scholar]
- 24.Imanaga I, Hirosawa N, Lin H, Matsumura K, Mayama T. Factors influencing phosphorylation of connexin of the cardiac gap junction – with special reference to intercellular impulse conductivity. J Mol Cell Cardiol. 2001;33:A50. (Abst) [Google Scholar]
- 25.Imanaga I, Lin H, Ogawa K. Significance of PKA and PKC in connexin 43 of the cardiac gap junction. Jpn J Physiol. 2002;52(Suppl):S83. (Abst) [Google Scholar]
- 26.Imanaga I, Lin H, Uehara A. Phosphorylation of connexin43 of the cardiac gap junction and its relation to functional significance. J Mol Cell Cardiol. 2002;34:A26. (Abst) [Google Scholar]
- 27.Imanaga I, Lin H, Ogawa K. Phosphorylation of connexin43 and regulation of gap junction in the cardiac muscle cells. Jpn J Physiol. 2003;53(Suppl):S57. (Abst) [Google Scholar]
- 28.Imanaga I, Lin H, Ogawa K, Matsumura K, Mayama T. Phosphorylation of connexin in functional regulation of the cardiac gap junction. Exp Clin Cardiol. 2004;9:161–4. [PMC free article] [PubMed] [Google Scholar]
- 29.Tuganowski W, Bukowski M, Korczynska I, Wójcik B, Wasik K. A method of measurement of longitudinal resistances in an isolated cardiac and smooth muscle preparation. Pflugers Arch. 1986;406:232–3. doi: 10.1007/BF00586688. [DOI] [PubMed] [Google Scholar]
- 30.Cruciani V, Mikalsen SO. Stimulated phosphorylation of intracellular connexin43. Exp Cell Res. 1999;251:285–98. doi: 10.1006/excr.1999.4574. [DOI] [PubMed] [Google Scholar]
- 31.TenBroek EM, Lampe PD, Solan JL, Reynhout JK, Johnson RG. Ser364 of connexin43 and the upregulation of gap junction assembly by cAMP. J Cell Biol. 2001;115:1307–18. doi: 10.1083/jcb.200102017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ono K, Kiyosue T, Arita M. Isoproterenol, DBcAMP, and forskolin inhibit cardiac sodium current. Am J Physiol. 1989;256:C1131–7. doi: 10.1152/ajpcell.1989.256.6.C1131. [DOI] [PubMed] [Google Scholar]
- 33.Reber WR, Weingart R. Ungulate cardiac purkinje fibres: The influence of intracellular pH on the electrical cell-to-cell coupling. J Physiol. 1982;328:87–104. doi: 10.1113/jphysiol.1982.sp014254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pressler ML. Effects of pCai and pHi on cell-to-cell coupling. Experientia. 1987;43:1084–91. doi: 10.1007/BF01956044. [DOI] [PubMed] [Google Scholar]
- 35.Vogel S, Sperelakis N. Induction of slow action potentials by microiontophoresis of cyclic AMP into heart cells. J Mol Cell Cardiol. 1981;13:51–64. doi: 10.1016/0022-2828(81)90228-5. [DOI] [PubMed] [Google Scholar]
- 36.Huang XD, Wong TM. Arrhythmogenic effect of forskolin in the isolated perfused rat heart: Influence of nifedipine or reduction of external calcium [corrected] Clin Exp Pharmacol Physiol. 1989;16:751–7. doi: 10.1111/j.1440-1681.1989.tb01513.x. [DOI] [PubMed] [Google Scholar]
- 37.De Groot JR, Coronel R. Acute ischemia-induced gap junctional uncoupling and arrhythmogenesis. Cardiovasc Res. 2004;64:323–34. doi: 10.1016/j.cardiores.2004.01.033. [DOI] [PubMed] [Google Scholar]
- 38.Imanaga I, Hirosawa N, Lin H, Sakamoto Y, Matsumura K, Mayama T. Phosphorylation of connexin 43 and regulation of cardiac gap junction function. In: DeMello WC, Janse MJ, editors. Heart Cell Coupling and Impulse Propagation in Health and Disease. Boston: Kluwer Academic Publishers; 2002. pp. 185–205. [Google Scholar]