Abstract
OBJECTIVES
Endothelial cells and their progenitors play an important role in angiogenesis that is essential for organogenesis and tissue remodelling, as well as for inflammatory responses and carcinogenesis in all periods of life. In the present study, the authors concentrated on the direct effect of beta-carotene (BC) on human umbilical cord-originated endothelial progenitor cells and human umbilical vein endothelial cells.
METHODS
BC uptake was measured using high-performance liquid chromatography. The effect on cell proliferation was measured based on bromodeoxyuridine incorporation. Chemotaxis was performed in a Boyden chamber. The influence on tubular-like structure formation was investigated using a three-dimensional assay in Matrigel (Becton Dickinson, USA) both in an in vitro and in vivo model. Changes in gene expression were analyzed using the microarray hybridization method. Quantitative gene expression was estimated using the real-time polymerase chain reaction method.
RESULTS
It was shown that BC, in the physiological range of concentrations found in human blood, is a potent activator of chemotaxis of endothelial cells. Microarray data analysis revealed that the genes involved in cell-cell and cell-matrix adhesion, matrix reorganization and activation of chemotaxis were the most affected by BC genes in human umbilical vein endothelial cells and endothelial progenitor cells. These results were also confirmed in an in vivo angiogenesis model.
CONCLUSION
BC, in the range of physiological concentrations, stimulates early steps of angiogenic activity of endothelial cells by activation of cellular migration, as well as by matrix reorganization and a decrease in cellular adhesion.
Keywords: Angiogenesis, Beta-carotene, Chemotaxis, Endothelium, Microarray
Vitamin A-derived retinoic acid (RA) is required for regulation of both epithelial and mesenchymal cell differentiation in embryogenesis, as well as in adult life (1–3). RA, in cooperation with fatty acids and their metabolites, activates nuclear receptors such as retinoid/rexinoid receptors or peroxisome proliferation regulatory receptors (4,5). As regulators of transcription, they influence cell differentiation, maturation of tissues and body organization in fetal life, and tissue remodelling in adolescence (2,6,7). Beta-carotene (BC) is the most active provitamin A in humans; however, 15% of the BC that is absorbed in the intestine is not metabolized and reaching target cells (8). The effect of this event on gene expression has not been recognized until now. Some of the large clinical studies undertaken to prove the efficacy of BC supplementation in the prevention of coronary artery disease and cancer revealed that administration of BC or vitamin A may increase the risk of lung cancer, especially in smokers and patients afflicted with asbestosis (the Alpha-Tocopherol Beta-Carotene cancer prevention study [ATBC] [9], the beta-CArotene and Retinol Efficacy Trial [CARET] [10] and the Physician’s Health Study [PHS] [11]).
Angiogenesis plays a central role in a number of physiological and pathological events, including vascular remodelling during growth, revascularization of ischemic tissue, inflammation and diabetic retinopathy, as well as in the promotion of solid tumour growth and invasiveness (12,13). A number of growth factors (eg, vascular endothelial growth factor [VEGF], basic fibroblast growth factor [bFGF], transforming growth factor-beta [TGF-β], platelet-derived growth factor [PDGF] and insulin-like growth factor [IGF]), cell-matrix interaction (integrins), cell-cell interactions (vascular endothelial cadherins, catenins, endoglins, ephrins, their receptors or the Jagged/Notch pathway) and environmental factors (eg, shear stress, nutrients and oxygen supply) regulate the most important steps in angiogenesis, which include detachment, proliferation, migration, homing and differentiation of the vascular wall, mainly endothelial or its progenitor cells (14,15).
Because 15% of ingested BC reaches target cells in the unmetabolized form in humans and because angiogenesis plays an important part in remodelling of ischemic tissue, as well as in solid tumour malignancy, the present study was undertaken to define the direct effects of BC on endothelial cells in terms of angiogenic activity and regulation of gene expression.
METHODS
High-performance liquid chromatography (HPLC)-grade BC (1 mg) stored in nitrogen in vials made of dark glass were kindly provided by Roche (DSM) Vitamins AG (Switzerland).
Primary endothelial cells (human umbilical vein endothelial cells [HUVECs]) and umbilical progenitor cells were isolated and grown according to a previously described protocol (16,17). The suspension of umbilical progenitor cells after the stabilization of CD34, AC133, vascular endothelial growth factor receptor 2, vascular endothelial cadherin and CD14/CD45 antigen expression after the fifth day of growing in proangiogenic conditions was called ‘endothelial progenitor cells (EPCs)’ and used for the study.
Endothelial cells were incubated with solvent (0.075% tetrahydrofuran/ethanol) containing medium (as the control) and BC (3 μM) or arachidonic acid (AA) (3 μM) (Sigma, USA), or both BC (3 μM) and AA (3 μM), for 24 h at 37°C in a 95% CO2/5% O2 humid atmosphere (Jouan IG-150 incubator, Thermo Electron Corporation, USA).
BC uptake following a 24 h incubation of HUVECs and EPCs with the compounds under study was measured by the HPLC method. The whole preparation procedure has been previously described (16). The amount of BC is expressed as picomoles of BC per 106 cells.
Migration of endothelial cells was analyzed using the Boyden chamber assay. The chemotactic activity of the endothelial cells is expressed as the chemotaxis index, which represents the ratio of compound-stimulated migration to that of random, unstimulated migration of EPCs and HUVECs in the tetrahydrofuran/ethanol control sample.
A three-dimensional (3D) in vitro model of tubulogenesis in Matrigel (Becton Dickinson, USA) was used. HUVECs and EPCs were resuspended in Matrigel (containing laminin, collagen IV, entactin and heparan sulfate proteoglycans) to a final concentration of 1×106 cells/mL on ice. The formation of tubules by HUVECs and EPCs, after a 24 h incubation with the investigated factors, was assessed under a light microscope (magnification ×10) and photographed. The lengths of the analyzed tubule-like structures were calculated and expressed as an average sum of the total length of tubules visible under the light microscope.
Analysis of angiogenesis in vivo was performed using a model of subcutaneous injection of Matrigel into a mouse. The protocol was accepted by the local university ethics committee. Female BALB/c mice (n=10 per group) were fed for five weeks with two different diets with BC (Kliba 2415 [Provimi Kliba AG, Switzerland] vitamin A 1400 U/kg plus BC beadlet [10% or 1200 parts per millions of BC]) or without BC (Kliba 2415 plus placebo [ie, vitamin A 1400 U/kg plus 0 parts per million BC]), which were kindly supplied by Roche (DSM Switzerland). After four weeks of the diet, the female BALB/c mice (n=6) received sterile injections of 2×500 μL Matrigel subcutaneously (dorsally). The Matrigel plug contained solvent for bFGF control or bFGF (50 nM). Six days later, the animals were sacrificed and the Matrigel plugs were removed, fixed and immersed in paraffin. Immunohistochemistry was performed using a routine protocol (16). The number of capillaries was counted under the microscope in five different fields in each of the three slices taken from different parts of each of the plugs. The number of capillaries detected in slices from the plugs was expressed as the number of vessels with or without the lumen. Separately, the number of detected separate PECAM-positive, single cells, not connected with capillaries, migrating to Matrigel, was examined.
Following 24 h incubation with the studied compounds, total RNA was isolated from cell lines by the guanidine thiocyanate-cesium chloride method (18) using Trizol (Invitrogen Life Technologies, USA) and purified using the SV Total RNA Isolation System Kit (Promega, USA). The aim of the microarray experiments was to screen the effects of BC on gene expression patterns. Affymetrix HG-U133A GeneChips (Affymetrix Inc, USA) (for cell lines) and 10,000-oligonucleotide custom-made arrays based on the physical deposition of material (50 bp oligonucleotides) on coated glass slides (for removed Matrigel plugs) were used. Changes in relative gene expression were calculated versus controls. Only spots with significant differences in signal intensity (more than 1.4-fold in the in vitro model and more than 2.0-fold in the in vivo model) and only significant differences of P<0.05 were included in the analysis.
To investigate the regulation of the expression of genes important in the proangiogenic activity of BC, a quantitative expression analysis of these genes was performed using real-time polymerase chain reaction (PCR) with glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as the reference gene. Real-time PCR in a reaction mixture containing QuantiTect SYBR Green PCR (Qiagen, Germany) mix and primers was performed using the DNA Engine Opticon II system (MJ Research, Germany). Results were calculated using the software Calculation Matrix for PCR Efficiency REST-XL (Gene Quantification, Germany).
Statistical analysis
Statistical analysis was performed using Microsoft Excel (version 5.0, Microsoft Corporation, USA) and by one-way ANOVA. All results are expressed as means ± SEM. Before statistical analysis, normal distribution and the homogeneity of variables were tested. Parameters that did not fulfill these tests were logarithmically transformed. Statistical comparisons were made by unpaired t tests for comparisons of quantitative variables. P<0.05 was considered significant.
RESULTS
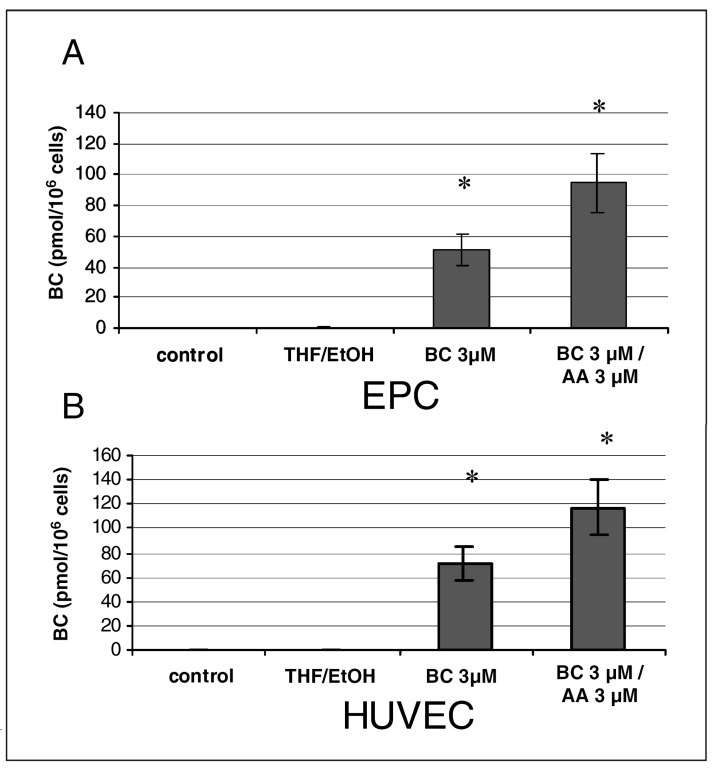
BC uptake by endothelial cells in culture was concentration dependent, and the presence of AA (3 μM) augmented BC uptake (Figure 1). A BC concentration of 3 μM was used for further experiments because higher concentrations of BC caused endothelial cell toxicity (especially in the presence of AA), as evidenced by the lactate dehydrogenase leakage from HUVECs and EPCs after 24 h of incubation (data not shown).
Figure 1).
Beta-carotene (BC) uptake by endothelial progenitor cells (EPCs) (A) and human umbilical vein endothelial cells (HUVECs) (B). Cellular BC content was analyzed using high-performance liquid chromatography after a 24 h incubation of HUVECs and EPC with BC (3 μM) and arachidonic acid (AA) (3 μM). Values are expressed as means ± SEM. *P<0.05 versus control (n=10, performed in triplicates). THF/EtOH Tetrahydrofuran/ethanol
Unlike in the cells treated with proapoptotic staurosporine (1 nM), no proapoptotic activity, as measured by caspase activation, was detected in endothelial cells incubated for 24 h with BC or AA at 3 μM. Both BC and AA, which were used in nontoxic concentrations, did not influence HUVEC and EPC proliferation, as measured by bromodeoxyuridine incorporation (data not shown).
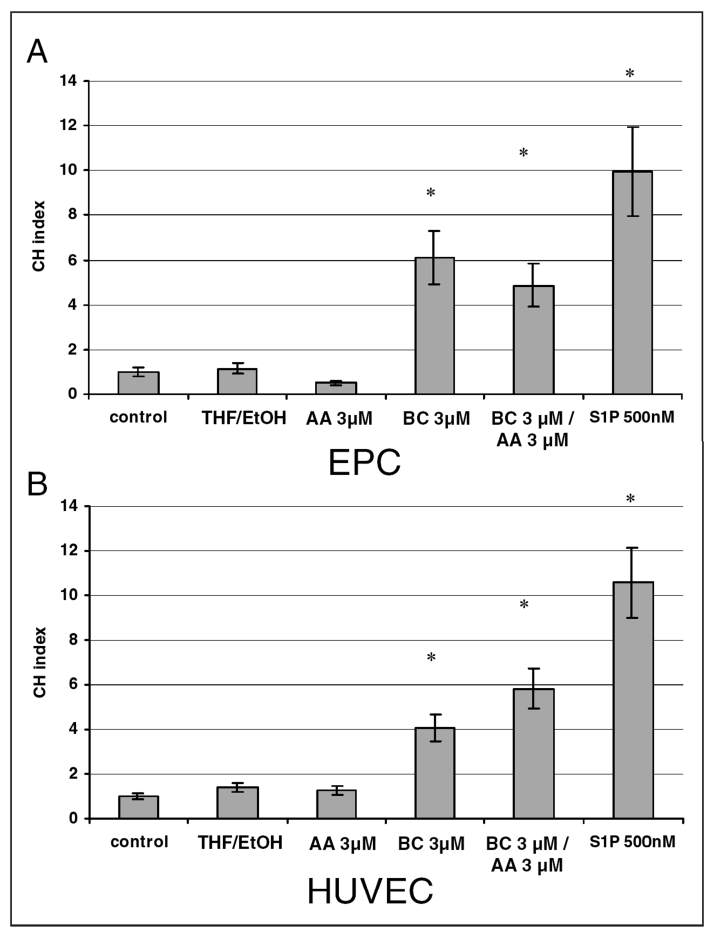
BC (3 μM) increased the migration of HUVECs and EPCs fourfold and fivefold, respectively (Figure 2). AA alone did not stimulate HUVEC migration, but cell migration was further increased (sixfold) when AA was used in combination with BC. In EPCs, AA alone inhibited migration by 50% compared with the migration of control (nonstimulated) cells. In the presence of AA, the mitogenic effect of BC in EPC decreased by 20% (Figure 2). Sphingosine-1-phosphate (S1P), a potent activator of endothelial cell migration, was used as a positive control to confirm the chemotactic potential of the endothelial cells (19).
Figure 2).
Beta-carotene (BC)-induced chemotaxis of endothelial progenitor cells (EPCs) (A) and human umbilical vein endothelial cells (HUVECs) (B). Cells treated with sphingosine-1-phosphate (S1P) were used as positive controls. The chemotaxis index (CHI) is the ratio of stimulated migration divided by that of basal unstimulated migration of HUVECs in a control medium sample. Values are expressed as means ± SEM (n=3, performed in triplicates). *Significantly different (P<0.05) from the corresponding control. AA Arachidonic acid; THF/EtOH Tetrahydrofuran/ethanol
The 3D Matrigel assay of tubulogenesis was used to verify the angiogenic properties of BC and AA in vitro. Only a trace of tubulogenic activity in HUVECs was detected in cells cultured in Matrigel covered with medium (without factors). No tubulogenic activity of BC and AA incubated alone or together was observed in the HUVEC or EPC in vitro model (data not shown).The proangiogenic factors VEGF (0.2 nM) and bFGF (0.5 nM) potently increased the number of tubules in the Matrigel HUVEC suspension. Contrary to HUVECs, bFGF and VEGF did not stimulate tubule formation in the EPC in vitro tubulogenesis model (data not shown).
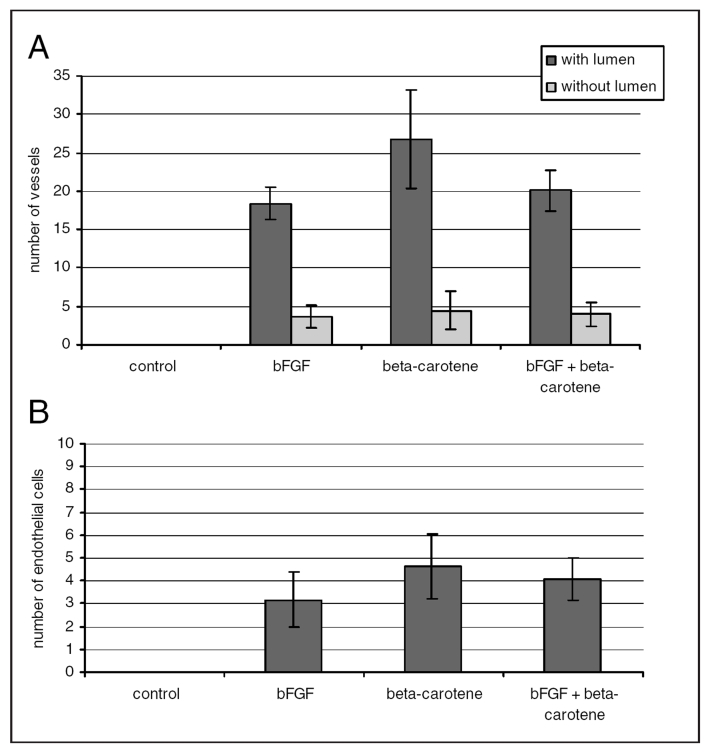
The analysis of the proangiogenic activity of BC in the in vivo mouse model showed that BC supplementation in the diet significantly increased the density of the capillary network (with lumen) and the amount of endothelial cells penetrating into the Matrigel plug (stimulated by BC in the subcutaneously administered Matrigel ) (Figure 3).
Figure 3).
Influence of beta-carotene on tube-like structure formation in the in vivo angiogenesis model. A Formation (length of tubules); B Number of the single cells, most connected with tubules. bFGF Basic fibroblast growth factor
Using the criteria described in the Methods section, genes were identified whose expression changed only in response to stimulation with BC. The analysis of microarray data in both endothelial cell lines, including genes regulated (up or down) from 1.4-fold up to 10-fold, identified significantly regulated genes (915 genes in EPCs and 838 genes in HUVECs) coding for proteins that belong to cellular pathways such as: pathways contributing to proangiogenic activity (cell cycle, adhesion, matrix remodelling and chemotaxis); apoptosis; receptor-mediated signal transduction; transcription factors and regulators of protein synthesis (zinc-finger proteins and ribosomal proteins); xenobiotic metabolism; and inflammatory response. Special attention was paid to the BC-regulated genes that were involved in the cell cycle/proliferation of HUVECs and EPCs. BC weakly upregulated the key genes coding for proteins that participate in cell-cycle regulation connected with the G1/S checkpoint. Among the proteins participating in intracellular signalling pathways in both endothelial cell lines, the most evident changes in BC-induced gene expression were found within the members of G-protein-coupled receptors and the Rho-like small GTPase family or their regulators.
The group of BC-regulated genes that code for proteins participating in cell-cell interactions, such as cadherins, catenins and the leukocyte-endothelium adhesion mediating molecules, was generally downregulated by both BC and AA. Contrary to this, the expression of genes coding for proteins associated with cell-extracellular matrix adhesion, such as integrins, was upregulated by BC.
The expression of genes coding for extracellular matrix degrading enzymes and stimulators of chemotaxis, which may regulate matrix degradation, receptor shading and cell migration, was differentially regulated. Downregulation of different types of extracellular matrix components, such as collagens, fibrillin or laminin, was also observed.
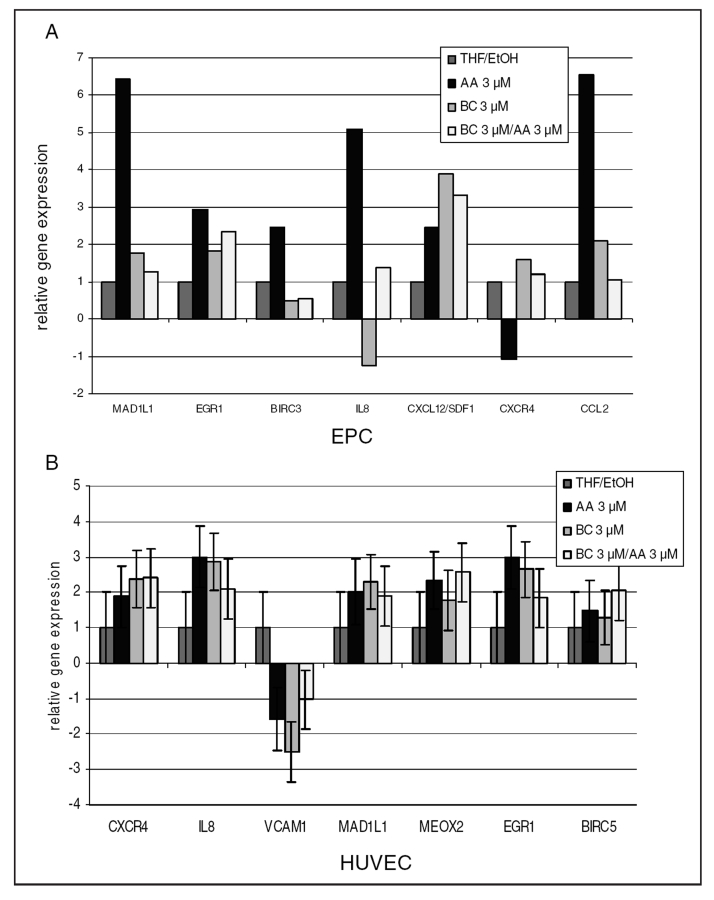
The real-time PCR method confirmed the results of the microarray (Figure 4). In HUVECs after incubation with BC, changes in the expression of mitotic arrest deficient protein 1-like 1 (MAD1L1) and mesenchyme homeobox 2 (MEOX2) genes (regulators of proliferation), and interleukin-8 (IL8), chemokine (CXC motif) receptor 4 (CXCR-4) and vascular cell adhesion molecule 1 (VCAM1) genes (involved in the activation of chemotaxis and homing), as well as early growth response (involved in proangiogenic activity and differentiation) were analyzed. Additionally, the expression of genes related to chemotaxis was analyzed in EPCs. The analyzed genes were early growth response 1 (EGR1) (cell differentiation activator), baculoviral IAP repeat-containing 3 (BIRC3) (inhibitor of apoptosis), IL8 and chemokine (CC motif) ligand 2 (CCL2) (activators of migration), CXCL12 and CXCR4 (activators of homing) (Figure 4).
Figure 4).
The expression of selected genes verified by quantitative real-time polymerase chain reaction in endothelial progenitor cells (EPCs) (A) and human umbilical vein endothelial cells (HUVECs) (B). The influence of beta-carotene (BC) and arachidonic acid (AA) on the following: chemokine (CC motif) ligand 2 (CCL2); chemokine (CXC motif) ligand 12 (CXCL12; also known as stromal cell-derived factor 1 [SDF1]); CXC receptor 4 (CXCR4; the receptor for SDF1); interleukin-8 (IL8); vascular cell adhesion molecule 1 (VCAM1); mitotic arrest deficient protein 1-like 1 (MAD1L1); mesenchyme homeobox 2 (MEOX2; growth arrest-specific homeobox); early growth response 1 (EGR1); and baculoviral IAP repeat-containing 3 (BIRC3) and BIRC5 (also known as survivin) apoptosis inhibitor. Data are expressed as relative gene expression (mean ± SEM) calculated versus control (HUVECs incubated in medium with 0.075% tetrahydrofuran/ethanol [THF/EtOH]). Significance at P<0.05 (n=3, performed in triplicates)
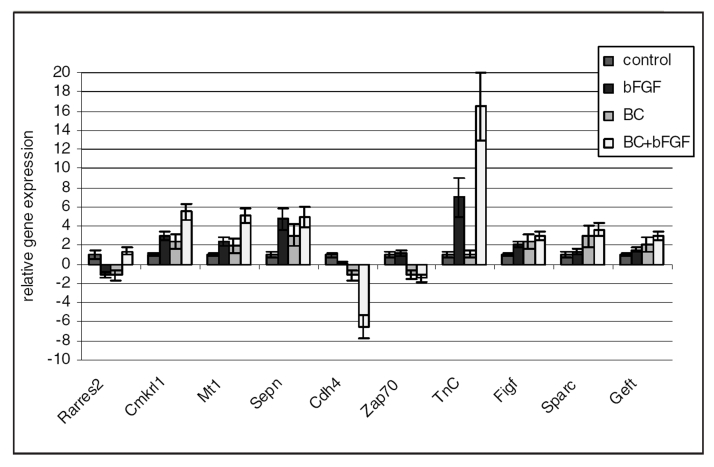
The obtained results of genes expression were also confirmed in cells harvested from Matrigel plugs – the in vivo angiogenesis model. The quantitative analysis of changes in gene expression of cells in Matrigel plugs under the influence of BC was performed using the microarray and verified using real-time PCR methods. The microarray analysis from these cells showed changes in 214 genes (60 genes were changed by BC). The genes with changed expression, as detected in the microarray, were classified according to signalling pathways and cell biological function. BC regulated expression of genes involved in retinoid signalling. Among genes related to proliferation, the activator of the S phase kinase gene expression was significantly upregulated by BC. The microarray data showed changes in the expression of several genes related to adhesion. BC (or BC with bFGF) upregulated the gene expression of proteins participating in cell-cell adhesion, as well as matrix proteins. The upregulation of extracellular matrix degrading enzymes (matrix metalloproteinases) was also observed. To verify the microarray changes in the expression of some selected genes, real-time PCR was used. Genes were chosen based on the magnitude of detected changes in the microarray and based on the confirmation of the expression of genes connected with retinoid signalling (RA receptor responder [Rarres2] and chemokine-like receptor 1 [Cmklr1]), new vascular network formation such as regulating homing of progenitor cells (R-cadherin [Cdh4], zeta-chain [TCR] associated protein kinase [Zap70], tenascin C [TnC] and metallothionein 1 [Mt-1]), angiogenesis related to the VEGF-dependent pathway (c-fos induced growth factor [Figf] and secreted acidic cysteine rich glycoprotein [Sparc]) and regulators of the Ras/Rho/Rac signalling pathway (selenoprotein N 1 [Sepn1], DNA segment, Chr 10 and ERATO Doi 610 [Geft]) – whose activation is connected with cell chemotaxis (Figure 5).
Figure 5).
Changes in the relative expression of genes related to vascular network formation in the angiogenesis model in vivo (real-time polymerase chain reaction). Data are expressed as relative gene expression (mean ± SEM) calculated versus control. Significance at P<0.05 (n=4). BC Beta-carotene; bFGF Basic fibroblast growth factor; Cdh4 R-Cadherin; Cmkrl1 Chemokine-like receptor 1; Figf c-Fos-induced growth factor; Geft ERATO Doi 610; Mt1 Metallothionein 1; Rarres2 Retinoic acid receptor responder; Sepn Selenoprotein N; Sparc Secreted acidic cysteine rich glycoprotein; TnC Tenascin C; Zap70 Zeta-chain (TCR) associated protein kinase
DISCUSSION
BC causes the potent activation of chemotaxis of EPCs, as well as HUVECs, parallelled by changes in gene expression that confirm the observed effect. The expression of several G-protein-coupled receptors and their activators in cells by BC was found. Interaction with the induced extracellular matrix components leads to activation of Rho/Rac/CDC42 small GTPases, which regulate cytoskeletal changes involved in cell motility, shape and contraction (20,21). Rho GTPases, through regulation of the phosphorylation of the myosin light chains (MLCs), promote actin-myosin interaction. MLC phosphorylation is induced by specific MLC kinases, such as MLCK Ca2+-dependent kinase, Rho kinase and p21-activated kinase. Rho kinase also inhibits dephosphorylation of MLC by inhibition of myosin phosphatase type I, which was evidenced by the observed microarray gene expression pattern.
The real-time PCR results confirmed the microarray results. The activation of the important genes for homing (CXCL12; also known as stromal cell-derived factor 1 [SDF1]) and for chemotaxis (IL8 and CCL2) argues for the stimulatory effect of BC on the chemotactic activity in HUVECs and EPCs that is observed in cell culture experiments. Despite the fact that the cell fate-determinant genes Jagged-1 and Notch-4 were down-regulated (17) (which is an argument for the promotion of the cell differentiation [22]), the effect of BC on von Willebrand factor expression and endothelial nitric oxide synthase (17) (the markers of endothelial cell maturation) was almost none, thus confirming the lack of end-differentiation. The lack of BC influence on tubulogenesis in the in vitro 3D Matrigel model confirms the observed lack of cell differentiation under BC treatment (no effect on tubulogenesis). BC also did not influence the proliferation of the investigated endothelial cells, which may add to the understanding of the absence of tubulogenesis in vitro (because the proliferation of endothelial cells plays an important role in this event) (23).
We have demonstrated that BC, in the physiological range of concentrations found in human blood, is a potent activator of chemotaxis of EPCs and HUVECs, which is accompanied by the induction of the expression of genes mediating cell adhesion and homing, but not by the final markers of endothelial differentiation. The results obtained for the cells harvested from Matrigel in the in vivo model showed that the proangiogenic activity of BC seems to be related not to cell proliferation, but to the inhibition of apoptosis and stimulation of chemotaxis/homing.
CONCLUSIONS
Our results originally documented the chemotactic effect of BC on differentiated and undifferentiated endothelial cells (umbilical cord progenitors), which may be an important factor in angiogenesis and cell homing. The regulation of the most important steps in angiogenesis – such as detachment, proliferation, migration, homing and differentiation of the vascular wall – by BC and the augmentation of the effects by AA were confirmed by several methods, including by microarray analysis. The present study originally points to the prochemotactic and homing activity of BC in endothelial cells, which may suggest a possible role of this carotenoid in progenitor cell therapy aimed at angiogenesis and tissue repair.
ACKNOWLEDGEMENTS
This work was supported by the F5 EU DLARFID project QLK1-CT-2001-00183. The authors thank Drs R Goralczyk, K Wertz and G Riss (DSM Nutritional Products, Human Nutrition and Health, Carotenoid Section, Basel, Switzerland) for the kind supply of HPLC-grade beta-carotene, as well as for training in the HPLC methodology of measuring carotenoid levels.
REFERENCES
- 1.Dimberg A, Oberg F. Retinoic acid-induced cell cycle arrest of human myeloid cell lines. Leuk Lymphoma. 2003;44:1641–50. doi: 10.1080/1042819031000083316. [DOI] [PubMed] [Google Scholar]
- 2.Bonet ML, Ribot J, Felipe F, Palou A. Vitamin A and the regulation of fat reserves. Cell Mol Life Sci. 2003;60:1311–21. doi: 10.1007/s00018-003-2290-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chew BP, Park JS. Carotenoid action on the immune response. J Nutr. 2004;134:257S–61S. doi: 10.1093/jn/134.1.257S. [DOI] [PubMed] [Google Scholar]
- 4.Boulanger A, McLemore P, Copeland NG, et al. Identification of beta-carotene 15, 15’-monooxygenase as a peroxisome proliferator-activated receptor target gene. FASEB J. 2003;17:1304–6. doi: 10.1096/fj.02-0690fje. [DOI] [PubMed] [Google Scholar]
- 5.Napoli JL. Interactions of retinoid binding proteins and enzymes in retinoid metabolism. Biochim Biophys Acta. 1999;1440:139–62. doi: 10.1016/s1388-1981(99)00117-1. [DOI] [PubMed] [Google Scholar]
- 6.Ross SA, McCaffery PJ, Drager UC, De Luca LM. Retinoids in embryonal development. Physiol Rev. 2000;80:1021–54. doi: 10.1152/physrev.2000.80.3.1021. [DOI] [PubMed] [Google Scholar]
- 7.Palozza P, Serini S, Torsello A, et al. Regulation of cell cycle progression and apoptosis by beta-carotene in undifferentiated and differentiated HL-60 leukemia cells: Possible involvement of a redox mechanism. Int J Cancer. 2002;97:593–600. doi: 10.1002/ijc.10094. [DOI] [PubMed] [Google Scholar]
- 8.Goodman DS, Blomstrand R, Werner B, Huang HS, Shiratori T. The intestinal absorption and metabolism of vitamin A and beta-carotene in man. J Clin Invest. 1966;45:1615–23. doi: 10.1172/JCI105468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Albanes D, Heinonen OP, Taylor PR, et al. Alpha-Tocopherol and beta-carotene supplements and lung cancer incidence in the alpha-tocopherol, beta-carotene cancer prevention study: Effects of base-line characteristics and study compliance. J Natl Cancer Inst. 1996;88:1560–70. doi: 10.1093/jnci/88.21.1560. [DOI] [PubMed] [Google Scholar]
- 10.Omenn GS, Goodman G, Thornquist M, et al. The beta-CArotene and Retinol Efficacy Trial (CARET) for chemoprevention of lung cancer in high risk populations: Smokers and asbestos-exposed workers. Cancer Res. 1994;54(Suppl 7):2038S–43S. [PubMed] [Google Scholar]
- 11.Cook NR, Le IM, Manson JE, Buring JE, Hennekens CH. Effects of beta-carotene supplementation on cancer incidence by baseline characteristics in the Physicians’ Health Study (United States) Cancer Causes Control. 2000;11:617–26. doi: 10.1023/a:1008995430664. [DOI] [PubMed] [Google Scholar]
- 12.Carmeliet P. Angiogenesis in health and disease. Nat Med. 2003;9:653–60. doi: 10.1038/nm0603-653. [DOI] [PubMed] [Google Scholar]
- 13.Khan ZA, Chakrabarti S. Growth factors in proliferative diabetic retinopathy. Exp Diabesity Res. 2003;4:287–301. doi: 10.1155/EDR.2003.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bazzoni G, Dejana E, Lampugnani MG. Endothelial adhesion molecules in the development of the vascular tree: The garden of forking paths. Curr Opin Cell Biol. 1999;11:573–81. doi: 10.1016/s0955-0674(99)00023-x. [DOI] [PubMed] [Google Scholar]
- 15.Gerhart J. 1998 Warkany lecture: Signaling pathways in development. Teratology. 1999;60:226–39. doi: 10.1002/(SICI)1096-9926(199910)60:4<226::AID-TERA7>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 16.Dembinska-Kiec A, Polus A, Kiec-Wilk B, et al. Proangiogenic activity of beta-carotene is coupled with the activation of endothelial cell chemotaxis. Biochim Biophys Acta. 2005;1740:222–39. doi: 10.1016/j.bbadis.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 17.Kiec-Wilk B, Polus A, Grzybowska J, et al. beta-Carotene stimulates chemotaxis of human endothelial progenitor cells. Clin Chem Lab Med. 2005;43:488–98. doi: 10.1515/CCLM.2005.087. [DOI] [PubMed] [Google Scholar]
- 18.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–9. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 19.Okamoto H, Yatomi Y, Ohmori T, Satoh K, Matsumoto Y, Ozaki Y. Sphingosine 1-phosphate stimulates G(i)- and Rho-mediated vascular endothelial cell spreading and migration. Thromb Res. 2000;99:259–65. doi: 10.1016/s0049-3848(00)00251-6. [DOI] [PubMed] [Google Scholar]
- 20.van Nieuw Amerongen GP, van Hinsbergh VW. Cytoskeletal effects of rho-like small guanine nucleotide-binding proteins in the vascular system. Arterioscler Thromb Vasc Biol. 2001;21:300–11. doi: 10.1161/01.atv.21.3.300. [DOI] [PubMed] [Google Scholar]
- 21.van Nieuw Amerongen GP, Koolwijk P, Versteilen A, van Hinsbergh VW. Involvement of RhoA/Rho kinase signaling in VEGF-induced endothelial cell migration and angiogenesis in vitro. Arterioscler Thromb Vasc Biol. 2003;23:211–7. doi: 10.1161/01.atv.0000054198.68894.88. [DOI] [PubMed] [Google Scholar]
- 22.Miele L, Osborne B. Arbiter of differentiation and death: Notch signaling meets apoptosis. J Cell Physiol. 1999;181:393–409. doi: 10.1002/(SICI)1097-4652(199912)181:3<393::AID-JCP3>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 23.Rumpold H, Wolf D, Koeck R, Gunsilius E. Endothelial progenitor cells: A source for therapeutic vasculogenesis? J Cell Mol Med. 2004;8:509–18. doi: 10.1111/j.1582-4934.2004.tb00475.x. [DOI] [PMC free article] [PubMed] [Google Scholar]