Summary
All microtubules are built from a basic α/β-tubulin building block, yet subpopulations of microtubules can be differentially marked by a number of post-translational modifications. These modifications, conserved throughout evolution, are thought to act individually or in combination to control specific microtubule-based functions, analogous to how histone modifications regulate chromatin functions. Here we review recent studies demonstrating that tubulin modifications influence microtubule-associated proteins such as severing proteins, plus-end tracking proteins, and molecular motors. In this way, tubulin modifications play an important role in regulating microtubule properties, such as stability and structure, as well as microtubule-based functions, such as ciliary beating, cell division, and intracellular trafficking.
Introduction
Microtubules are polar cytoskeletal filaments assembled from head-to-tail and lateral associations of α/β tubulin heterodimers. Most microtubules occur as single tubes and form cellular structures such as the mitotic spindle and the interphase network (Figure 1). A subset of microtubules exist as fused structures where a complete microtubule (A tubule) is fused with one or two incomplete tubules (B and C tubules) to comprise ciliary axonemes (doublet microtubules) or centrioles and basal bodies (triplet microtubules) (Figure 1). How the basic α/β-tubulin building block is used to generate a variety of microtubule structures with organelle-specific properties and functions is not clear. One hypothesis that has gained experimental support recently is that post-translational modifications (PTMs) of the tubulin building block generate functional diversity of microtubules. These modifications may act individually and/or in a combinatorial fashion to recruit specific protein complexes and thus regulate organelle-specific properties of microtubules.
Figure 1. Localization of microtubule structures and modified tubulin subunits in mammalian cells.
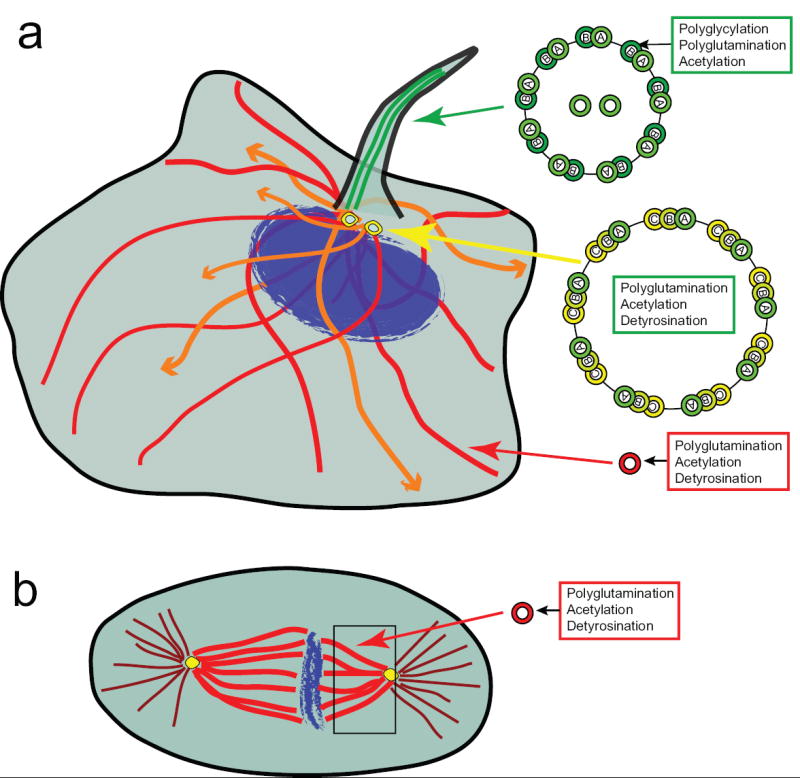
(a) In interphase cells, dynamic microtubules (orange) lack PTMs whereas stable microtubules (red) accumulate PTMs in a time-dependent manner. Ciliary microtubules (green) contain PTMs primarily on the B tubule of the outer doublets. Note that motile cilia contain the 9+2 arrangement of nine doublet microtubules around a central pair (shown) whereas primary cilia lack the central pair (9+0, not shown). PTMs are also prevalent in centriolar/basal body triplet microtubules (yellow). (b) In mitotic cells, spindle microtubules (kinetochore and/or interpolar) microtubules are highly modified whereas astral microtubules are mostly unmodified.
Many tubulin PTMs have been known for decades – detyrosination and the related ∆2 modification, glutamylation, glycylation, acetylation, phosphorylation, and palmitoylation (Figure 2) (for reviews, see [1,2]). Yet their functional roles in different microtubule structures are just beginning to be discovered. Most PTMs occur on microtubules rather than on unpolymerized tubulin and it has long been known that stable microtubules, as compared to dynamic microtubules, accumulate more modifications. The PTMs are postulated to play a role in specific functions of stable microtubules as differences in tubulin PTM patterns can be seen between stable microtubules. For example, in axonemes, although all microtubules are highly stable, the A tubule of the doublet microtubules and the central singlet microtubules are mostly unmodified, whereas the B tubule is highly detyrosinated, polyglycylated, and polyglutamylated (Figure 1). This review will focus on the four best-studied PTMs of tubulin, with a particular focus on recent studies indicating specific functional roles for these modifications in regulating microtubule-based functions in vivo.
Figure 2. Localization of tubulin PTMs on the α/β-tubulin heterodimer.
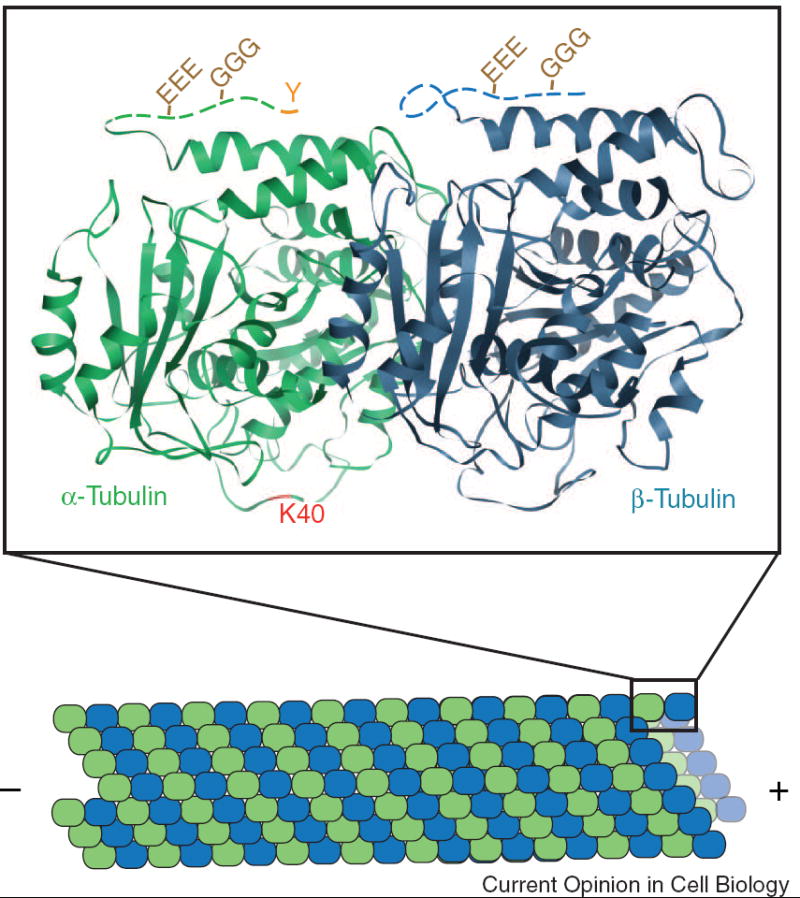
Microtubules are formed by the end-on-end and lateral associations of α- (green) and β- (blue) tubulin heterdimers. The crystal structure (PDB file 1TUB) of one tubulin heterodimer is shown. The CTTs of both α- and β-tubulin, disordered in the structure, are represented in this schematic as dashed lines. Detyrosination entails the removal of the C-terminal tyrosine (Y) from α-tubulin. The resulting detyrosinated α-tubulin is often referred to as Glu tubulin due to the exposed glutamate residue. Polyglutamylation (EEE) and polyglycylation (GGG) occur on the CTTs of α- and β-tubulin. Acetylation of α-tubulin occurs on lysine40 (K40) which is predicated to lie on the luminal face of the microtubule.
Detyrosination/Tyrosination
Detyrosination involves the removal of the gene-encoded C-terminal tyrosine of α-tubulin in microtubule polymers by an unidentified carboxypeptidase (Figure 2) [1,2]. A novel family of cytosolic carboxypeptidases was recently identified whose founding member, Nna1/CCP1, shares some characteristics with the known properties of tubulin carboxypeptidase [3,4]. The reverse tyrosination reaction, or addition of a tyrosine residue to the now C-terminal glutamate residue of α-tubulin, occurs on soluble tubulin heterodimers and is catalyzed by tubulin tyrosine ligase (TTL) [1,2].
The detyrosination/tyrosination cycle differentially recruits two types of microtubule-binding proteins, molecular motors and plus-end tracking proteins (+TIPs). The motor protein Kinesin-1 binds preferentially to detyrosinated microtubules rather than tyrosinated microtubules [5**,6]. This could enable Kinesin-1 to preferentially move various cargoes, including vimentin filaments and transferrin, along detyrosinated microtubules.[6-8]. Tyrosinated microtubules have recently been shown to play a direct role in the recruitment of +TIP proteins that contain a CAP-Gly domain (e.g. CLIP-170, CLIP-115, and p150Glued/dynactin). +TIPs are proteins that localize to and track with the plus-ends of growing microtubules. A pioneering study in S. cerevisiae showed that expression of an α-tubulin gene that lacks the C-terminal phenylalanine (thus resembling detyrosinated tubulin) resulted in mislocalization of Bik1p (CLIP-170 homologue) but not Bim1p (EB1-type +TIP that lacks a CAP-Gly domain), as well as defects in nuclear positioning and spindle dynamics [9**]. Importantly, recruitment of CAP-Gly containing +TIPS by tyrosinated α-tubulin occurs in mammalian cells as well. Fibroblasts isolated from TTL null mice showed a severe reduction in tyrosinated microtubules, mislocalized CLIP170 protein (but not EB1), and misoriented mitotic spindles [10**]. The latter effect may be a direct consequence of the ability of highly tyrosinated astral microtubules to recruit CAP-Gly-containing +TIPs, such as CLIP-170 and p150Glued/dynactin, and promote attachment of microtubule plus-ends to the cell cortex [10**-13]. Recent structural work has shown that a conserved basic region in the CAP-Gly domain is required for targeting to the acidic EEY/F sequence present at the C-terminus of α-tubulin [14,15]. CAP-Gly domains and C-terminal EEY/F motifs also contribute to binding interactions between +TIP proteins ([14-17] and references therein). Thus, multiple interactions between tyrosinated α-tubulin, +TIPs, and the dynein/dynactin complex likely contribute to microtubule-based functions during mitosis and interphase.
Proper functioning of the detyrosination/tyrosination cycle has also been shown to influence tumorigenesis and neuronal organization. Multiple studies have shown that TTL is downregulated in animal and human cancers. Low levels of TTL protein and tyrosinated tubulin correlate with increased tumorigenisis, tumor invasiveness, and poor prognosis [18-21], suggesting that detyrosinated tubulin provides a growth advantage. The role of tyrosinated tubulin in neuronal organization was highlighted by the fact that TTL null mice undergo normal embryonic development but die perinatally due to neuronal disorganization. Analysis of TTL-/- neurons in culture suggested that TTL activity, and the relative amounts of detyrosinated/tyrosinated tubulin, are important for the regulation of neurite extention and axon specification, perhaps due to the recruitment of CLIP-170 and other +TIPs in growth cones [22*].
Polymodifications—Glutamylation and Glycylation
Glutamylation and glycylation involve the addition of variable numbers of glutamate or glycine residues, respectively, onto glutamate residues in the C-terminal tails (CTTs) of both α-and β-tubulin (Figure 2) [1,2]. Glycylation is mainly limited to tubulin incorporated into axonemes (cilia and flagella) whereas glutamylation is prevalent in neuronal cells, centrioles, axonemes, and the mitotic spindle. Both modifications have been found on the same tubulin CTT and there is cross-talk between the α- and β-tubulin tails regulating the type and level of each modification [23,24].
An important breakthrough in the study of tubulin PTMs was made with the recent identification of the enzymes that catalyze tubulin glutamylation [25**]. The tubulin glutamylases belong to a family of enzymes that contain a tubulin tyrosine ligase-like (TTLL) domain. Eight mammalian TTLL domain-containing proteins (TTLLs 1,4-7,9,11,13) have been shown to be tubulin glutamylases that differ in their specificity for α- or β-tubulin and in preferential chain-initiating or chain-elongating activity ([25**,26**,27*] and J.Gaertig, personal communication). Other TTLL domain-containing proteins (TTLLs 3,8,10,12) are suspected (but not yet shown) to facilitate ligation of glutamates or other amino acids such as glycine to tubulin or other proteins. Both glycylation and glutamylation are reversible reactions, however the enzymes responsible for this reverse reaction are still elusive.
In cilia and flagella, the tubulin CTTs and their polymodifications play a role in the formation and maintenance of axonemal structures. Deletion of the CTTs of either α- or β-tubulin is lethal in Tetrahymena [24]. In Drosophila, β-tubulin lacking its CTT can support assembly of most types of microtubules but fails to assemble into axonemes of sperm [28]. Thus, the tubulin CTTs can influence the properties of assembled microtubules. The exact sequence of the tails is not important as the CTTs of α- and β-tubulin can substitute for each other [24,29]. In the case of the β-tubulin CTT, it is the polymodifications that are critical for ciliary assembly and motility, as mutation of acceptor glutamate residues in the β-tubulin CTT was either lethal or hypomorphic, depending on the site and extent of the mutations. In particular, a severe loss of polymodifications on the β-tubulin CTT resulted in non-motile, short cilia, perhaps due to structural defects (absence of central pair microtubules and loss of B tubules), as well as effects on intraflagellar transport (see below) [23,29-31]. A role for polymodification of the β-tubulin CTT in ciliary assembly and function is supported by a recent study in which knockdown of the TTLL6 polyglutamylase in zebrafish inhibited the formation of olfactory cilia [32]. For the α-tubulin CTT, the polymodifications appear not to play a major role in ciliary assembly and function in Tetrahymena [23,29] but may be critical in mammals, as mice deficient in PGs1, a non-catalytic component of the TTLL1 α-tubulin polyglutamylase complex, have multiple defects including male sterility due to abnormal assembly of sperm flagella [33]. Finally, too much polyglutamylation (specifically chain length) is also detrimental to cilia motility, as overexpression of Tetrahymena TTLL6A, an active β-tubulin elongase, resulted in paralyzed cilia [25**].
Polymodification of the α- and β-tubulin CTTs may also influence the transport of structural and membrane components within cilia and flagella, a process known as intra-flagellar transport (IFT) [34]. Cells with mutation of the β-tubulin CTT in Tetrahymena showed accumulation of dense material, presumably IFT particles, between the outer doublets and the ciliary membrane, reminiscent of cells defective in the molecular motors that drive IFT [23]. Yet the relationship between tubulin modifications and IFT is likely complex as illustrated by recent studies on DYF-1. Work in C. elegans identified DYF-1 as a component of the IFT particle that functions to positively regulate the homodimeric OSM-3 motor (a kinesin-2 family member), perhaps by docking OSM-3 onto IFT particles [35]. Mutations in the DYF-1 homolgue in zebrafish, Fleer, resulted in short cilia with a dramatic loss of polyglutamylation and defects in B-tubule structure [32]. Since polyglutamylation levels were unaffected in osm-3 mutants [32], it seems unlikely that DYF-1 regulation of OSM-3 transport promotes tubulin polyglutamylation. Rather, DYF-1 may promote tubulin modifications that influence OSM-3 activity. Clearly, more work is needed to resolve the circular connection between polyglutamylation, IFT transport, and axonemal structural defects.
That different kinesin motors are differentially sensitive to the presence of specific PTMs is supported by recent work [1,2]. Kinesin-1 bound effectively to axonemes from wildtype Tetrahymena and α-tubulin modification site mutants but was unable to effectively bind axonemes from β-tubulin polymodification site mutants [5**]. A reduction in α-tubulin polyglutamylation, due to mutation in the TTLL1 subunit PG1s, resulted in altered distribution of Kif1A (kinesin-3 family) but not Kif3a (kinesin-2 family) or Kif5 (kinesin-1 family) [36**]. Importantly, these mice also displayed decreased density of synaptic vesicles, a Kif1A cargo, in axon terminals [36**]. In a related study, a decrease in β-tubulin glutamylation due to knockdown of TTLL7, a β-tubulin initiating glutamylase, resulted in impaired neurite outgrowth [27]. Whether this effect is also due to impaired function of motors or other microtubule associated proteins requires further study.
Recent work has also shown that polymodifications of the tubulin CTTs could mark specific microtubules for severing and thus influence polymer dynamics and density. In Tetrahymena, mutations of the β-tubulin polymodification sites resulted in an inability to clear cortical microtubules from the cleavage furrow region, preventing cells from completing cytokinesis [30,31]. Interestingly, loss of katanin, a microtubule severing protein, gives almost an exact phenocopy [37*]. In addition, cells lacking katanin accumulate excessive levels of polymodifications on microtubules [37*]. In C. elegans, overexpression of katanin is lethal but can be rescued by mutation of a potential polymodification site on the β-tubulin CTT [38*], suggesting that katanin activity is influenced by modification of β-tubulin. Indeed, the activity of two microtubule severing proteins, spastin and katanin, requires the CTTs of tubulins in vitro [39-41].
Acetylation
Acetylation is unique among the known tubulin modifications in that it occurs on lysine40 of α-tubulin which is postulated to reside on the luminal face of microtubules (Figure 2). It is unclear how the enzymes that carry out acetylation/deacetylation would have access to this site. It is also unclear how this luminal modification could influence microtubule-based functions that occur on the cytoplasmic face of the microtubule. The acetylation enzyme has not been identified, but two enzymes have been shown to deacetylate α-tubulin in vitro and in vivo, namely HDAC6 and Sirt2 [42-44]. Knockdown of either HDAC6 or Sirt2 results in hyperacetyled tubulin suggesting that the two enzymes may be interdependent [42,44].
Acetylation of α-tubulin on lysine40 is fairly common and can be found on stable microtubules in most cell types (Figure 1). However, mutation of lysine40 in Chlamydomonas, Tetrahymena, or C. elegans had no obvious phenotype (discussed in [1,2]). Although tubulin acetylation is not necessary for cell and organism survival, recent work has suggested that α-tubulin plays a positive role in motor-based trafficking in mammals [5**,45,46**]. Kinesin-1 binds with higher affinity to acetylated microtubules in vitro [5**]. Hyperacetylation of microtubules in neuronal cells using small molecule inhibitors of HDAC6 caused Kinesin-1 transport of JIP1 to be redirected from a subset to the majority of neurites and enhanced the anterograde and retrograde transport of BDNF vesicles [5**,46**]. As HDAC6 has recently been shown to deacetylate cytosolic proteins other than tubulin (e.g. cortactin and HSP90) and regulates a variety of other cellular events (e.g. aggresome formation, cell motility, ciliary disassembly, and transcriptional corepression), the role of HDAC6/Sirt2 deactylation of α-tubulin in regulation of microtubule-based functions remain to be clarified (reviews [1,2,47] and also [48-50]).
Concluding remarks
Although PTMs of tubulin subunits within microtubule structures have been known for many years, the cellular functions of the PTMs have only recently begun to be revealed. So far, tubulin PTMs have been shown to affect primarily two microtubule-based properties. First, tubulin PTMs influence the stability and/or structure of microtubule assemblies. Whether this is a direct effect of tubulin modification on microtubule structure or indirectly due to regulation of microtubule-associated proteins, such as severing proteins, will be a fertile area of future research.
Second, tubulin PTMs can influence recruitment of microtubule-associated proteins such as molecular motors and +TIPs. In the case of motor-dependent transport, it is possible that the PTMs serve as “road signs” to direct polarized trafficking such as axonal/dendritic trafficking in neuronal cells. It is also possible that PTMs simply mark stable microtubules for preferential transport. This would enable molecular motors to avoid the undesirable situation in which the microtubule track falls apart before the motor/cargo complex has reached its destination. Future research will determine the functional contribution of tubulin PTMs to each of these possibilities and provide important new information about how the tubulin code regulates microtubule-based functions in cells.
Acknowledgments
We apologize to those authors whose important work we have not cited due to restrictions in number of references and emphasis on publications within the past few years. We thank J Gaertig and members of the Verhey lab for stimulating discussions and reading the manuscript. Work in the author’s laboratory is supported in part by a grant from the National Institutes of Health (GM070862).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Verhey KJ, Gaertig J. The Tubulin Code. Cell Cycle. 2007;6:2152–2160. doi: 10.4161/cc.6.17.4633. [DOI] [PubMed] [Google Scholar]
- 2.Westermann S, Weber K. Post-translational modifications regulate microtubule function. Nat Rev Mol Cell Biol. 2003;4:938–947. doi: 10.1038/nrm1260. [DOI] [PubMed] [Google Scholar]
- 3.Kalinina E, Biswas R, Berezniuk I, Hermoso A, Aviles FX, Fricker LD. A novel subfamily of mouse cytosolic carboxypeptidases. Faseb J. 2007;21:836–850. doi: 10.1096/fj.06-7329com. [DOI] [PubMed] [Google Scholar]
- 4.Rodriguez de la Vega M, Sevilla RG, Hermoso A, Lorenzo J, Tanco S, Diez A, Fricker LD, Bautista JM, Aviles FX. Nna1-like proteins are active metallocarboxypeptidases of a new and diverse M14 subfamily. Faseb J. 2007;21:851–865. doi: 10.1096/fj.06-7330com. [DOI] [PubMed] [Google Scholar]
- **5.Reed NA, Cai D, Blasius TL, Jih GT, Meyhofer E, Gaertig J, Verhey KJ. Microtubule acetylation promotes kinesin-1 binding and transport. Curr Biol. 2006;16:2166–2172. doi: 10.1016/j.cub.2006.09.014. This paper showed that transport of JIP1, a Kinesin-1 cargo, is directed to only a subset of neurites in neuronal cells. The mechanism of polarized trafficking depends on tubulin PTMs as hyperacetylation redirected JIP1 transport to nearly all neurites and Kinesin-1 binds with higher affinity to acetylated microtubules.
- 6.Liao G, Gundersen GG. Kinesin is a candidate for cross-bridging microtubules and intermediate filaments. Selective binding of kinesin to detyrosinated tubulin and vimentin. J Biol Chem. 1998;273:9797–9803. doi: 10.1074/jbc.273.16.9797. [DOI] [PubMed] [Google Scholar]
- 7.Kreitzer G, Liao G, Gundersen GG. Detyrosination of tubulin regulates the interaction of intermediate filaments with microtubules in vivo via a kinesin-dependent mechanism. Mol Biol Cell. 1999;10:1105–1118. doi: 10.1091/mbc.10.4.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin SX, Gundersen GG, Maxfield FR. Export from pericentriolar endocytic recycling compartment to cell surface depends on stable, detyrosinated (glu) microtubules and kinesin. Mol Biol Cell. 2002;13:96–109. doi: 10.1091/mbc.01-05-0224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **9.Badin-Larcon AC, Boscheron C, Soleilhac JM, Piel M, Mann C, Denarier E, Fourest-Lieuvin A, Lafanechere L, Bornens M, Job D. Suppression of nuclear oscillations in Saccharomyces cerevisiae expressing Glu tubulin. Proc Natl Acad Sci U S A. 2004;101:5577–5582. doi: 10.1073/pnas.0307917101. To model the consequences of excessive detyrosinated tubulin in cells, the authors used S. cerevisiae cells expressing α-tubulin lacking the C-terminal aromatic residue. These cells showed decreased association of Bik1p, a yeast ortholog of CLIP-170, with microtubule plus-ends and defects in nuclear oscillations and spindle penetration into the bud.
- **10.Peris L, Thery M, Faure J, Saoudi Y, Lafanechere L, Chilton JK, Gordon-Weeks P, Galjart N, Bornens M, Wordeman L, et al. Tubulin tyrosination is a major factor affecting the recruitment of CAP-Gly proteins at microtubule plus ends. J Cell Biol. 2006;174:839–849. doi: 10.1083/jcb.200512058. This paper showed that +TIPs that contain a CAP-Gly domain bind with higher affinity to microtubules containing tyrosinated α-tubulin in vitro and in vivo. Fibroblasts from TTL null mice showed that tyrosinated tubulin plays a role in cell shape and polarity during interphase and spindle positioning during mitosis.
- 11.Gundersen GG, Bulinski JC. Distribution of tyrosinated and nontyrosinated alpha-tubulin during mitosis. J Cell Biol. 1986;102:1118–1126. doi: 10.1083/jcb.102.3.1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Busson S, Dujardin D, Moreau A, Dompierre J, De Mey JR. Dynein and dynactin are localized to astral microtubules and at cortical sites in mitotic epithelial cells. Curr Biol. 1998;8:541–544. doi: 10.1016/s0960-9822(98)70208-8. [DOI] [PubMed] [Google Scholar]
- 13.O’Connell CB, Wang YL. Mammalian spindle orientation and position respond to changes in cell shape in a dynein-dependent fashion. Mol Biol Cell. 2000;11:1765–1774. doi: 10.1091/mbc.11.5.1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Honnappa S, Okhrimenko O, Jaussi R, Jawhari H, Jelesarov I, Winkler FK, Steinmetz MO. Key interaction modes of dynamic +TIP networks. Mol Cell. 2006;23:663–671. doi: 10.1016/j.molcel.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 15.Mishima M, Maesaki R, Kasa M, Watanabe T, Fukata M, Kaibuchi K, Hakoshima T. Structural basis for tubulin recognition by cytoplasmic linker protein 170 and its autoinhibition. Proc Natl Acad Sci U S A. 2007;104:10346–10351. doi: 10.1073/pnas.0703876104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hayashi I, Plevin MJ, Ikura M. CLIP170 autoinhibition mimics intermolecular interactions with p150(Glued) or EB1. Nat Struct Mol Biol. 2007;14:980–981. doi: 10.1038/nsmb1299. [DOI] [PubMed] [Google Scholar]
- 17.Weisbrich A, Honnappa S, Jaussi R, Okhrimenko O, Frey D, Jelesarov I, Akhmanova A, Steinmetz MO. Structure-function relationship of CAP-Gly domains. Nat Struct Mol Biol. 2007;14:959–967. doi: 10.1038/nsmb1291. [DOI] [PubMed] [Google Scholar]
- 18.Lafanechere L, Courtay-Cahen C, Kawakami T, Jacrot M, Rudiger M, Wehland J, Job D, Margolis RL. Suppression of tubulin tyrosine ligase during tumor growth. J Cell Sci. 1998;111(Pt 2):171–181. doi: 10.1242/jcs.111.2.171. [DOI] [PubMed] [Google Scholar]
- 19.Soucek K, Kamaid A, Phung AD, Kubala L, Bulinski JC, Harper RW, Eiserich JP. Normal and prostate cancer cells display distinct molecular profiles of alpha-tubulin posttranslational modifications. Prostate. 2006;66:954–965. doi: 10.1002/pros.20416. [DOI] [PubMed] [Google Scholar]
- 20.Kato C, Miyazaki K, Nakagawa A, Ohira M, Nakamura Y, Ozaki T, Imai T, Nakagawara A. Low expression of human tubulin tyrosine ligase and suppressed tubulin tyrosination/detyrosination cycle are associated with impaired neuronal differentiation in neuroblastomas with poor prognosis. Int J Cancer. 2004;112:365–375. doi: 10.1002/ijc.20431. [DOI] [PubMed] [Google Scholar]
- 21.Mialhe A, Lafanechere L, Treilleux I, Peloux N, Dumontet C, Bremond A, Panh MH, Payan R, Wehland J, Margolis RL, et al. Tubulin detyrosination is a frequent occurrence in breast cancers of poor prognosis. Cancer Res. 2001;61:5024–5027. [PubMed] [Google Scholar]
- *22.Erck C, Peris L, Andrieux A, Meissirel C, Gruber AD, Vernet M, Schweitzer A, Saoudi Y, Pointu H, Bosc C, et al. A vital role of tubulin-tyrosine-ligase for neuronal organization. Proc Natl Acad Sci U S A. 2005;102:7853–7858. doi: 10.1073/pnas.0409626102. This paper reports the first generation of a mouse model lacking a tubulin PTM enzyme. TTL null mice lack tyrosinated α-tubulin in postmitotic cells and die postnatally, presumably due to defects in neuronal network organization. Neuronal cells cultured from TTL null mice displayed defects in neurite extension and mislocalized CLIP-170 protein.
- 23.Redeker V, Levilliers N, Vinolo E, Rossier J, Jaillard D, Burnette D, Gaertig J, Bre MH. Mutations of tubulin glycylation sites reveal cross-talk between the C termini of alpha- and beta-tubulin and affect the ciliary matrix in Tetrahymena. J Biol Chem. 2005;280:596–606. doi: 10.1074/jbc.M408324200. [DOI] [PubMed] [Google Scholar]
- 24.Duan J, Gorovsky MA. Both carboxy-terminal tails of alpha- and beta-tubulin are essential, but either one will suffice. Curr Biol. 2002;12:313–316. doi: 10.1016/s0960-9822(02)00651-6. [DOI] [PubMed] [Google Scholar]
- **25.Janke C, Rogowski K, Wloga D, Regnard C, Kajava AV, Strub JM, Temurak N, van Dijk J, Boucher D, van Dorsselaer A, et al. Tubulin polyglutamylase enzymes are members of the TTL domain protein family. Science. 2005;308:1758–1762. doi: 10.1126/science.1113010. This paper identified the enzyme(s) responsible for tubulin polyglutamylation and showed that they contain a TTL-like domain. The Tetrahymena proteins TtTTLL1 and TtTTLL6A differed in their subcellular localization as well as their preference for glutamylation of α- or β-tubulin.
- **26.van Dijk J, Rogowski K, Miro J, Lacroix B, Edde B, Janke C. A targeted multienzyme mechanism for selective microtubule polyglutamylation. Mol Cell. 2007;26:437–448. doi: 10.1016/j.molcel.2007.04.012. The authors demonstrated that six mammalian TTLL proteins are tubulin polyglutamylases. The enzymes differ in their preference for chain initiation and elongation as well as their ability to glutmylate α- or β-tubulin. Thus, the TTLL enzymes are proposed to establish diverse patterns of microtubule polyglutamylation in cells.
- *27.Ikegami K, Mukai M, Tsuchida J, Heier RL, Macgregor GR, Setou M. TTLL7 is a mammalian beta-tubulin polyglutamylase required for growth of MAP2-positive neurites. J Biol Chem. 2006;281:30707–30716. doi: 10.1074/jbc.M603984200. The authors showed that TTLL7 is a β-tubulin polyglutamylase that is highly expressed in the nervous system. Knockdown of TTLL7 in neuronal PC12 cells resulted in decreased neurite outgrowth.
- 28.Fackenthal JD, Turner FR, Raff EC. Tissue-specific microtubule functions in Drosophila spermatogenesis require the beta 2-tubulin isotype-specific carboxy terminus. Dev Biol. 1993;158:213–227. doi: 10.1006/dbio.1993.1180. [DOI] [PubMed] [Google Scholar]
- 29.Xia L, Hai B, Gao Y, Burnette D, Thazhath R, Duan J, Bre MH, Levilliers N, Gorovsky MA, Gaertig J. Polyglycylation of tubulin is essential and affects cell motility and division in Tetrahymena thermophila. J Cell Biol. 2000;149:1097–1106. doi: 10.1083/jcb.149.5.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thazhath R, Jerka-Dziadosz M, Duan J, Wloga D, Gorovsky MA, Frankel J, Gaertig J. Cell context-specific effects of the beta-tubulin glycylation domain on assembly and size of microtubular organelles. Mol Biol Cell. 2004;15:4136–4147. doi: 10.1091/mbc.E04-03-0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thazhath R, Liu C, Gaertig J. Polyglycylation domain of beta-tubulin maintains axonemal architecture and affects cytokinesis in Tetrahymena. Nat Cell Biol. 2002;4:256–259. doi: 10.1038/ncb764. [DOI] [PubMed] [Google Scholar]
- 32.Pathak N, Obara T, Mangos S, Liu Y, Drummond IA. The Zebrafish fleer Gene Encodes an Essential Regulator of Cilia Tubulin Polyglutamylation. Mol Biol Cell. 2007 doi: 10.1091/mbc.E07-06-0537. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Campbell PK, Waymire KG, Heier RL, Sharer C, Day DE, Reimann H, Jaje JM, Friedrich GA, Burmeister M, Bartness TJ, et al. Mutation of a novel gene results in abnormal development of spermatid flagella, loss of intermale aggression and reduced body fat in mice. Genetics. 2002;162:307–320. doi: 10.1093/genetics/162.1.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scholey JM. Intraflagellar transport. Annu Rev Cell Dev Biol. 2003;19:423–443. doi: 10.1146/annurev.cellbio.19.111401.091318. [DOI] [PubMed] [Google Scholar]
- 35.Ou G, Blacque OE, Snow JJ, Leroux MR, Scholey JM. Functional coordination of intraflagellar transport motors. Nature. 2005;436:583–587. doi: 10.1038/nature03818. [DOI] [PubMed] [Google Scholar]
- **36.Ikegami K, Heier RL, Taruishi M, Takagi H, Mukai M, Shimma S, Taira S, Hatanaka K, Morone N, Yao I, et al. Loss of alpha-tubulin polyglutamylation in ROSA22 mice is associated with abnormal targeting of KIF1A and modulated synaptic function. Proc Natl Acad Sci U S A. 2007;104:3213–3218. doi: 10.1073/pnas.0611547104. To examine the effect of polyglutamylation on trafficking in neuronal cells, the authors used mice that lack functional PGs1, a noncatalytic subunit of the TTLL1 α-tubulin glutamylase. Decreased tubulin polyglutamylation in neurons was correlated with decreased levels of Kif1A (kinesin-3 family) in neurites but not Kif3A (kinesin-2 family) or Kif5 (kinesin-1 family). Deficiencies were also found in the axon density of synaptic vesicles (a Kif1A cargo) and in synaptic transmission.
- *37.Sharma N, Bryant J, Wloga D, Donaldson R, Davis RC, Jerka-Dziadosz M, Gaertig J. Katanin regulates dynamics of microtubules and biogenesis of motile cilia. J Cell Biol. 2007;178:1065–1079. doi: 10.1083/jcb.200704021. In this paper, the activity of the Kat1 (katanin) severing protein is shown to correlate with the levels of tubulin polymodification and density of microtubule structures. Both loss of Kat1 activity and loss of polymodification sites in the β-tubulin CTT result in short paralyzed cilia and a defect in end-stage cytokinesis. The authors suggest a function for katanin in recycling older, polymodified microtubule segments.
- *38.Lu C, Srayko M, Mains PE. The Caenorhabditis elegans microtubule-severing complex MEI-1/MEI-2 katanin interacts differently with two superficially redundant beta-tubulin isotypes. Mol Biol Cell. 2004;15:142–150. doi: 10.1091/mbc.E03-06-0418. In C. elegans, ectopic expression of katanin results in lethality during mitosis. The authors show that lethality can be rescued by substitution of a glutamate residue in the CTT of ß-tubulin, supporting the idea that the polymodified tail of ß-tubulin regulates katanin activity
- 39.Roll-Mecak A, Vale RD. The Drosophila homologue of the hereditary spastic paraplegia protein, spastin, severs and disassembles microtubules. Curr Biol. 2005;15:650–655. doi: 10.1016/j.cub.2005.02.029. [DOI] [PubMed] [Google Scholar]
- 40.McNally FJ, Vale RD. Identification of katanin, an ATPase that severs and disassembles stable microtubules. Cell. 1993;75:419–429. doi: 10.1016/0092-8674(93)90377-3. [DOI] [PubMed] [Google Scholar]
- 41.White SR, Evans KJ, Lary J, Cole JL, Lauring B. Recognition of C-terminal amino acids in tubulin by pore loops in Spastin is important for microtubule severing. J Cell Biol. 2007;176:995–1005. doi: 10.1083/jcb.200610072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hubbert C, Guardiola A, Shao R, Kawaguchi Y, Ito A, Nixon A, Yoshida M, Wang XF, Yao TP. HDAC6 is a microtubule-associated deacetylase. Nature. 2002;417:455–458. doi: 10.1038/417455a. [DOI] [PubMed] [Google Scholar]
- 43.Matsuyama A, Shimazu T, Sumida Y, Saito A, Yoshimatsu Y, Seigneurin-Berny D, Osada H, Komatsu Y, Nishino N, Khochbin S, et al. In vivo destabilization of dynamic microtubules by HDAC6-mediated deacetylation. Embo J. 2002;21:6820–6831. doi: 10.1093/emboj/cdf682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.North BJ, Marshall BL, Borra MT, Denu JM, Verdin E. The human Sir2 ortholog, SIRT2, is an NAD+-dependent tubulin deacetylase. Mol Cell. 2003;11:437–444. doi: 10.1016/s1097-2765(03)00038-8. [DOI] [PubMed] [Google Scholar]
- 45.Bulinski JC. Microtubule modification: acetylation speeds anterograde traffic flow. Curr Biol. 2007;17:R18–20. doi: 10.1016/j.cub.2006.11.036. [DOI] [PubMed] [Google Scholar]
- **46.Dompierre JP, Godin JD, Charrin BC, Cordelieres FP, King SJ, Humbert S, Saudou F. Histone deacetylase 6 inhibition compensates for the transport deficit in Huntington’s disease by increasing tubulin acetylation. J Neurosci. 2007;27:3571–3583. doi: 10.1523/JNEUROSCI.0037-07.2007. The authors showed that hyperacetylation of tubulin in neuronal cells can compensate for the reduced transport of BDNF-containing vesicles seen in Huntington Disease. The mechanism was due to effects of tubulin acetylation on increased recruitment of the motors cytoplasmic dynein and Kinesin-1 to microtubules.
- 47.Boyault C, Sadoul K, Pabion M, Khochbin S. HDAC6, at the crossroads between cytoskeleton and cell signaling by acetylation and ubiquitination. Oncogene. 2007;26:5468–5476. doi: 10.1038/sj.onc.1210614. [DOI] [PubMed] [Google Scholar]
- 48.Zhang X, Yuan Z, Zhang Y, Yong S, Salas-Burgos A, Koomen J, Olashaw N, Parsons JT, Yang XJ, Dent SR, et al. HDAC6 modulates cell motility by altering the acetylation level of cortactin. Mol Cell. 2007;27:197–213. doi: 10.1016/j.molcel.2007.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pugacheva EN, Jablonski SA, Hartman TR, Henske EP, Golemis EA. HEF1-dependent Aurora A activation induces disassembly of the primary cilium. Cell. 2007;129:1351–1363. doi: 10.1016/j.cell.2007.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tran AD, Marmo TP, Salam AA, Che S, Finkelstein E, Kabarriti R, Xenias HS, Mazitschek R, Hubbert C, Kawaguchi Y, et al. HDAC6 deacetylation of tubulin modulates dynamics of cellular adhesions. J Cell Sci. 2007;120:1469–1479. doi: 10.1242/jcs.03431. [DOI] [PubMed] [Google Scholar]


