Abstract
Many neurons of the central nervous system display multiple high voltage-activated Ca2+ currents, pharmacologically classified as L-, N-, P-, Q-, and R-type. Of these current types, the R-type is the least understood. The leading candidate for the molecular correlate of R-type currents in cerebellar granule cells is the α1E subunit, which yields Ca2+ currents very similar to the R-type when expressed in heterologous systems. As a complementary approach, we tested whether antisense oligonucleotides against α1E could decrease the expression of R-type current in rat cerebellar granule neurons in culture. Cells were supplemented with either antisense or sense oligonucleotides and whole-cell patch clamp recordings were obtained after 6–8 days in vitro. Incubation with α1E antisense oligonucleotide caused a 52.5% decrease in the peak R-type current density, from −10 ± 0.6 picoamperes/picofarad (pA/pF) (n = 6) in the untreated controls to −4.8 ± 0.8 pA/pF (n = 11) (P < 0.01). In contrast, no significant changes in the current expression were seen in sense oligonucleotide-treated cells (−11.3 ± 3.2 pA/pF). The specificity of the α1E antisense oligonucleotides was supported by the lack of change in estimates of the P/Q current amplitude. Furthermore, antisense and sense oligonucleotides against α1A did not affect R-type current expression (−11.5 ± 1.7 and −11.7 ± 1.7 pA/pF, respectively), whereas the α1A antisense oligonucleotide significantly reduced whole cell currents under conditions in which P/Q current is dominant. Our results support the hypothesis that members of the E class of α1 subunits support the high voltage-activated R-type current in cerebellar granule cells.
Individual nerve cells in the vertebrate nervous system express several types of voltage-gated Ca2+ channel (1–4), as many as five or six channel types distinguishable in some neurons (5). These channels work together to support fundamental cellular activities such as membrane excitation, neurotransmitter release, neurite outgrowth, and gene expression (6, 7). Considerable advances have been made in the understanding of the relationship between channel types, defined by their biophysical and pharmacological characteristics, and their underlying α1 subunits isolated by biochemistry and molecular biology (8–12). It is clear that L-type currents are supported by dihydropyridine-sensitive α1C or α1D subunits (13, 14) and N-type currents are generated by ω-conotoxin-GVIA-sensitive α1B subunits (15). Likewise, P- and Q-type currents are likely to arise from ω-Aga-IVA and ω-CTx-MVIIC-sensitive α1A subunits (16–21).
Among the major categories of Ca2+ channels uncovered so far, R-type channels were the most recently defined and remain the least well-understood. R-type currents were first identified in rat cerebellar granule neurons (22, 23) and were found to be pharmacologically and kinetically distinguishable from L-, N-, P-, and Q-type currents in the same cells (5). The importance of R-type channels for dendritic Ca2+ entry and synaptic transmission has been demonstrated in recent experiments (24–27). In contrast to other high voltage-activated Ca2+ channels, the molecular basis of R-type currents is not settled completely. One obstacle has been the lack of a potent and selective inhibitor for R-type current that spares its better-characterized counterparts. The leading candidate for the molecular correlate of R-type currents is the α1E subunit (28–30). When expressed in Xenopus oocytes and HEK293 cells, α1E subunits induced a prominently inactivating, fast-deactivating current that was highly sensitive to block by Ni2+ (28, 29, 31) and ω-Aga-IIIA (32), similar to R-type current in cerebellar granule neurons (5, 22, 33). However, it also has been suggested that α1E might support a low voltage-activated Ca2+ channel instead of R-type currents (34–37).
To test whether α1E underlies the expression of the R-type current in cerebellar granule cells, we turned to an antisense strategy. Here, we show that antisense oligonucleotides against α1E specifically decrease the expression of R-type currents in cultured cells. Thus, α1E subunits support the high voltage-activated R-type current in cerebellar granule cells.
METHODS
Cell Culture.
Cerebellar neurons were obtained by using a modification of the procedure described by Malgaroli and Tsien (38). Cerebella were removed from the brains of 2- to 5-day-old rat pups. The cerebella were cut into small pieces and rinsed with Ca2+ and Mg2+-free Hank’s solution (Sigma) supplemented with 350 mg/ml NaHCO3, 1 mM Hepes, and 10% fetal bovine serum (FBS, HyClone). The tissue was then digested in saline solution containing 13 mM NaCl, 5 mM KCl, 7 mM Na2HPO3, 10 mg/ml trypsin (type XI, Sigma), and 5 mg/ml DNase (type IV, Sigma) for 5 min at room temperature. The cells were washed with 10% FBS-Hank’s solution and gently dispersed with a fire-polished pasteur pipette in Hank’s solution containing 12 mM MgSO4 and 5 mg/ml DNase. Cells were spun down and resuspended two times in 10% FBS-Hanks solution and plated onto coverslips precoated with Matrigel (Collaborative Research). Cell cultures were kept in a 5% CO2-humidified atmosphere at 37°C in MEM (5.3 mM KCl, GIBCO) supplemented with 5 g/liters glucose, 100 mg/liters transferrin, 25 mg/liters insulin, 300 mg/liters glutamine, 2% B-27 (GIBCO), and 10% FBS. Seventy-five percent of the medium’s volume was replaced after 1 day in vitro with 4 μM cytosine arabinoside-MEM to a final concentration of 3 μM cytosine arabinoside, 2.5% FBS-MEM. Cells were kept in this medium until recordings were made.
Oligonucleotide Treatment.
Oligonucleotides (ON) were diluted in the replacement medium cytosine arabinoside-MEM to a final ON concentration of 4 μM and added to the culture medium after 1 day in vitro. In some experiments, the ON medium was replaced every two days to maintain ON concentration.
We used an ON against nucleotides 582–599 of the α1E subunit, a region located at repeat I between the S3 and S4 transmembrane domains (28). This particular region of the protein was chosen for its lack of similarity to L-type Ca2+ calcium channel sequences and its low homology with non-L-type channel subunits present in cerebellar granule cells, such as α1A or α1B (27% and 38%, respectively). The antisense ON sequence used was 5′-CGTGGGTGTTGAAATG-3′ and the sense ON was 5′-CATTTCAACACCCACG TG-3′. ON uptake by the cells was monitored with fluorescence microscopy by using antisense ON tagged with fluorescein at the 3′ end of the sequence (data not shown). The α1A antisense ON sequence was 5′-CATCGACTGCTTGTACAT-3′, and the sense sequence was 5′-ATGTACAAGCAGTCGATG-3′; these ONs targeted nucleotides 145–162 of the rat α1A sequence (39).
Electrophysiology.
Thirty minutes before recording, single coverslips were removed from the incubator and placed in a Petri dish containing Tyrode solution (in mM: 119 NaCl, 5 KCl, 2 CaCl2, 1 MgCl2, 30 glucose, 25 Hepes-NaOH (pH 7.3); 305 milliosmolar) supplemented with 1 μM TTX (Sankyo), 1 μM ω-Conotoxin GVIA (Peninsula Laboratories), 0.5 μM ω-Conotoxin MVIIC (Peninsula), 10 μM nimodipine (Research Biochemicals), and 100 μg/ml cytochrome c. The ω-Conotoxin MVIIC was omitted from the solution when P/Q current components were tested. Patch pipettes were made from borosilicate glass with resistance values ranging from 4 to 7 MΩ when measured in the presence of recording solutions. The pipette capacitance was compensated electronically. Cell capacitance and series resistance were measured from the current transient elicited by a hyperpolarizing voltage pulse from −80 to −90 mV and compensated electronically. Mean cell capacitance for each experimental group was (in pF): untreated cells, R-type, 6.1 ± 0.3 (n = 6); untreated cells, P/Q + R-type, 6.1 ± 0.5 (n = 5); antisense α1E-treated, R-type, 6.5 ± 0.4 (n = 11); antisense α1E-treated, P/Q + R-type, 6.2 ± 0.4 (n = 7); sense α1E-treated, R-type, 5.2 ± 1.1 (n = 5); antisense α1A-treated, R-type, 5.1 ± 0.4 (n = 6); antisense α1A-treated, P/Q + R- type, 7.1 ± 0.8 (n = 6); sense α1A-treated, R-type, 4.1 ± 0.7 (n = 5); and sense α1A-treated, P/Q + R-type 4.1 0.7 (n = 5). Mean series resistance was 23 ± 1 MΩ (n = 44). Ba2+ currents were recorded by using whole-cell patch clamp technique and elicited from a holding potential (Vhold) of −80 mV to various test potentials (Vtest) from −70 to +50 mV. Test pulse duration was 100 ms with a 3-sec pulse interval. Current traces were corrected for linear capacitive leak with on-line P/4 trace subtraction following the test pulse. Signals were acquired at 10 KHz and filtered at 2 KHz by using an Axopatch 200A patch clamp amplifier (Axon Instruments, CA) interfaced to a personal computer. The recording chamber solution contained (in mM): 160 tetraethylammonium Cl, 10 BaCl2, and 10 Hepes-CsOH (pH 7.3); 305 milliosmolar and supplemented with 1 μM ω-Conotoxin GVIA, 0.5 μM ω-Conotoxin MVIIC, 10 μM nimodipine, and 100 μg/ml cytochrome c. The intracellular solution contained (in mM): 108 MeSO3− CsOH, 4.5 MgCl2, 9 EGTA, 4 ATP-Mg, 0.3 GTP-Na, and 24 Hepes (pH 7.4); 295 milliosmolar. All experiments were performed at room temperature (22–24°C). Calcium current amplitude did not substantially change in neurons cultured between days in vitro 6–8 and thus were analyzed together. When appropriate, data are reported as the mean ± SEM. Statistical significance was tested by using single factor ANOVA, with P < 0.05 as the limit for statistical significance.
RESULTS
R-type Currents Are Specifically Decreased by α1E Antisense Oligonucleotide.
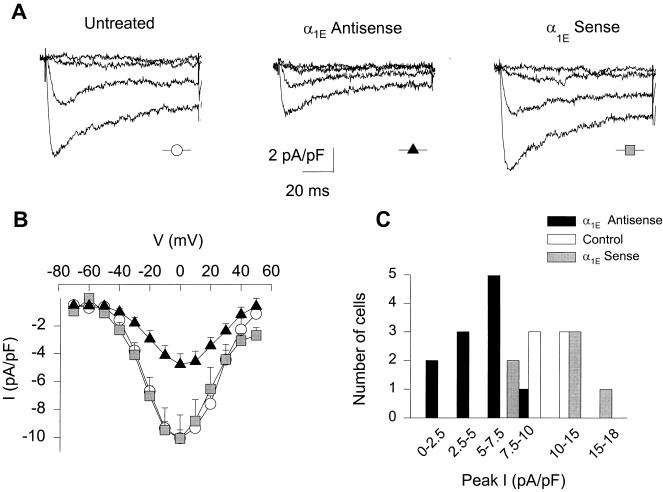
Incubation of the neurons with α1E antisense oligonucleotide caused a significant decrease in the expression of R-type currents, compared with untreated cells or cells grown in the presence of α1E sense ON. Fig. 1A illustrates averaged Ba2+ current traces obtained from untreated cells, cells cultured in the presence of with 4 μM of α1E antisense oligonucleotide, and cells cultured with α1E sense ON. As evident from the traces and the corresponding peak I–V curves (Fig. 1B), treatment with antisense ON in culture significantly reduced the peak amplitude of R-type current. In comparison to the mean peak current value for untreated controls, −10.0 ± 0.6 pA/pF (n = 6), peak current in cells treated with α1E antisense averaged −4.8 ± 0.8 pA/pF (n = 11), a 52.5% decrease (P < 0.01). In contrast, the peak current in granule cells treated with sense ON averaged −11.3 ± 3.3 pA/pF (n = 5), not significantly different from the untreated cells (P < 0.84), in support of the specificity of the antisense effect. There is considerable variation in the decrease in current induced by the antisense treatment across the entire granule cell population (Fig. 1C), consistent with variability in the uptake of antisense ONs as confirmed by examination of the uptake of fluorescein-tagged antisense ONs (data not shown).
Figure 1.
The presence of α1E antisense ONs in the culture medium decreases the R-type current amplitude. (A) Activation of Ba2+ currents with various depolarizing pulses (Vtest = −60, −40, −20, and 0 mV from a Vhold = −80 mV) in untreated cells, cells cultured in the presence of 4 μM α1E antisense ON, and cells treated with 4 μM α1E sense ON. Data pooled from 3 to 4 cells. R-type currents were measured in the presence of toxins to block L-, N-, and P/Q-type current components (see Methods). (B) Current-voltage relationship averages for untreated cells (circles, n = 6), sense α1E ON (squares, n = 5), and antisense α1E ON (triangles, n = 11). Currents from cells treated with antisense ON were significantly smaller than the untreated (P < 0.01) and sense-treated controls (P < 0.03). (C) Peak current distribution in the untreated, antisense α1E, and sense α1E-treated cell groups.
α1E Antisense Oligonucleotide Does Not Affect the P/Q Current Component.
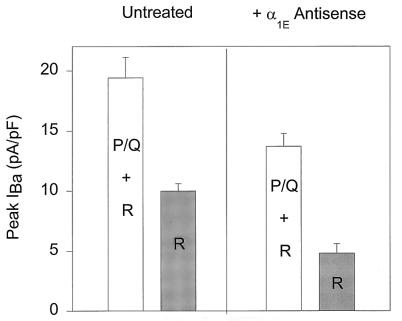
An additional test of the specificity of the α1E antisense ON for R-type currents was to investigate its effect on the P- and Q-type currents (here abbreviated P/Q because no attempt was made to distinguish between these components). R-type currents were measured in the presence of ω-CTx MVIIC, ω-CTx GVIA, and nimodipine in the bath solution to block P/Q, N-, and L-type currents present in cerebellar granule cells. P/Q + R currents were measured in the presence of ω-CTx GVIA and nimodipine in the solution. Fig. 2 compares the peak current values recorded from untreated cells (Left) and in antisense α1E-treated neurons (Right). The peak amplitude for P/Q + R current fell from 19.4 ± 1.7 pA/pF (n = 4) in untreated granule cells to a value of 13.7 ± 1.1 pA/pF (n = 8) in neurons treated with α1E antisense ON, a significant decrease (P < 0.01). The reduction was essentially the same as that found when peak R-type current was studied in isolation (decreasing from 10.0 ± 0.6 pA/pF (n = 6) in untreated cells to 4.8 ± 0.8 pA/pF (n = 11) in antisense ON-treated cells). Thus, the reduction in the aggregate P/Q + R current can be accounted for by a specific decrease in R-type current alone, without any change in the contribution P/Q current.
Figure 2.
Effect of α1E antisense ONs is consistent with specific reduction of R-type current. The presence α1E antisense ON did not change the estimated P/Q current amplitude, as shown by the peak Ba2+ currents measured in the control group (Left) and in the presence of 4 μM α1E antisense ON (Right). The P/Q + R current components recorded in the presence and the absence of α1E antisense were significantly different (P < 0.012); this reduction α1E antisense (≈30%) can be accounted for by the decrease in R current alone. R-type currents were measured in the presence of 1 μM ω-CTx MVIIC (n = 5 for the untreated group; n = 11 for antisense-treated cells). P/Q + R components were measured in the absence of the toxin (P/Q + R, n = 6 for untreated cells and n = 7 for antisense-treated group).
α1A Antisense Does Not Affect R-Type Currents.
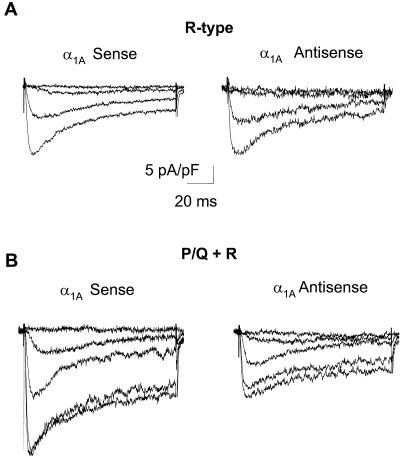
For a different kind of test of the possible relationship between α1E subunits and R-type currents, we examined the effect of a second set of ONs that targeted the α1A sequence from rat. Because the targeted region of α1A (nucleotides 145–162) lacks any appreciable homology with the α1E sequence, the ON treatment would not be expected to have an effect on the R-type current expression. Fig. 3A shows averaged R-type currents from cells cultured in the presence of 4 μM α1A sense ON or the same level of α1A antisense ON. Current traces obtained in the presence of either ON were not different from each other. Peak current amplitude was −11.5 ± 1.7 pA/pF (n = 5) in cells treated with α1A antisense ON and −11.7 ± 1.7 pA/pF in cells treated with α1A sense ON (n = 5). R-type current expression in either group of cells was not affected when compared with untreated controls (shown in Fig. 1A), nor did they change with respect to the cells treated with α1E sense ON (also shown in Fig. 1).
Figure 3.
Antisense ONs against α1A do not affect the expression of R-type currents but decrease the PQ + R components. (A) Activation of R-type Ba2+ currents with depolarizing pulses from Vhold = −80 mV to Vtest = −60, −40, −20, and 0 mV in cells cultured in the presence of either 4 μM α1A sense or α1A antisense ON. Data pooled from five cells. (B) Addition of α1A antisense ON decreased the P/Q + R component compared with cells grown in the presence of α1A sense ON. Ba2+ currents were elicited with depolarizing pulses from Vhold = −80 mV to Vtest = −60, −40, −20, 0, and +10 mV, data pooled from five cells.
We then corroborated that the lack of effect of the α1A ON in the R-type current was indeed caused by the nonspecificity of the nucleotide sequence and not because of lack of activity of the ON. The efficacy of the α1A-ON set was tested by measuring the P/Q + R current components in cells cultured in the presence of either antisense or sense α1A ONs. Average current traces obtained under these two experimental conditions are shown in Fig. 3B. Cells grown in the presence of α1A antisense showed a peak current amplitude of −13.9 ± 3.3 pA/pF (n = 5), significantly smaller than the peak current amplitude from sense-treated cells, −22.1 ± 2.1 pA/pF (n = 5) (P < 0.036). The current decrease induced by the antisense ON can be attributed to a decrease in the P/Q current component because these ON did not affect the expression of R-type currents (Fig. 3A).
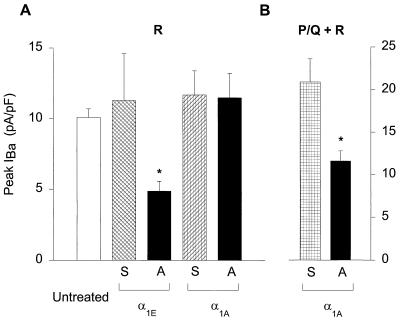
Fig. 4 summarizes the effects of the application of various oligonucleotides on Ca2+ channel currents. Only the α1E antisense ON reduced the R-type current (P < 0.01), whereas addition of α1E sense, α1A antisense and α1A sense did not alter it relative to untreated controls (Fig. 4A). On the other hand, α1A antisense ON significantly decreased the P/Q + R components (Fig. 4B), thus demonstrating that its failure to reduce the R current was not caused by ineffectiveness of the compound.
Figure 4.
(A) Comparison of R-type peak currents at various culture conditions. Incubation of cerebellar granule cells with antisense oligonucleotides against α1E (n = 11) significantly reduced the expression of R-type currents compared with untreated controls (n = 6)(P < 0.01). Addition of α1A antisense ON (n = 5), α1E sense ON (n = 5), and α1A sense ON (n = 5) did not affect the current expression. (B) Comparison of P/Q + R peak current amplitudes between cells treated with α1A antisense and sense ONs (P < 0.036). A, antisense; S, sense.
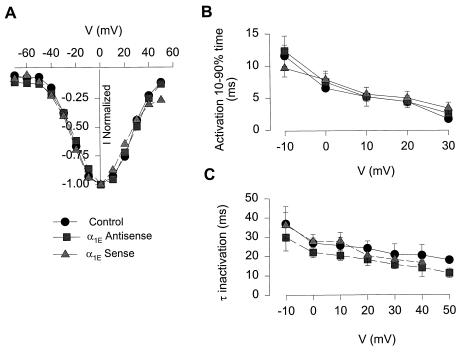
The partial nature of the antisense effect would be consistent with an incomplete turnover of the underlying α1E protein, as is often the case in antisense experiments. As an alternative explanation, we considered the possibility of some heterogeneity in the R-type current, perhaps allowing it to be subdivided by antisense treatment into two distinguishable components. This hypothesis raised the question of whether the current that was abolished by antisense oligonucleotide was somehow different than the current that remained. Accordingly, we compared the properties of currents recorded in neurons treated with antisense or sense ONs or in control cells not exposed to oligonucleotide. As illustrated in Fig. 5, no significant differences emerged in either the voltage-dependence of peak current (Fig. 5A), in the activation rate, measured as the 10–90% activation time (Fig. 5B) or in the voltage-dependent time constant of inactivation (τinactivation) (Fig. 5B). The latter measurement showed a trend toward faster values with antisense treatment, albeit not statistically significant. These results are so far consistent with the possibility that the R-type current arises from a single homogeneous population of channels under our particular culture conditions.
Figure 5.
α1E Antisense treatment does not modify the R-type current properties. (A) Voltage-dependence of peak current of untreated cells (circles, n = 6), α1E sense-treated neurones (squares, n = 5), and α1E antisense-treated neurones (triangles, n = 11). Currents were normalized by maximal peak current. (B) Time dependence of activation, depicted as time taken to rise from 10 to 90% of peak current. (C) Voltage-dependent time constant of inactivation (τinactivation) of untreated, α1E-sense, and α1E antisense-treated neurons.
DISCUSSION
There has been considerable controversy about the relationship between the α1E subunit and various types of Ca2+ channel activity (22, 30, 32, 34). This study addressed the question of whether α1E supports R-type current, a high-voltage activated current resistant to blockers of L-, N-, and P/Q-type channels (5, 32, 33, 40). Our findings with antisense oligonucleotides provided strong support for this hypothesis. The antisense oligonucleotide designed against α1E stood out in its ability to reduce R-type current. None of the control sequences tested, namely a sense α1E ON, an antisense α1A ON, and a sense α1A ON, affected the R-type current. The specific reduction of R-type current by α1E antisense is consistent with previous studies showing that biophysical and pharmacological properties of R-type currents in neurons (5, 40) are in close alignment with the characteristics of α1E expressed in cell lines (29, 32, but see ref. 34). Single channel conductances for Ba2+ (30, 31, 40) and sensitivity to the neurotoxin ω-Aga-IIIA (32) are some of the key features. Further study of unitary Ca2+ conductances of native R-type channels is needed to complete the comparisons with expressed α1E subunits (34).
We did not observe a total elimination of the residual current with the antisense treatment, which is not uncommon when using antisense strategies (21, 35). This partial effect would be expected if the kinetics of α1E turnover within the cell were slow, as often found for membrane channel proteins. Likewise, rates of oligonucleotide uptake and degradation by individual cells also can influence the availability of ON to bind its target. In addition, variability in the ON uptake by individual cells also may be important, as indicated by clear variability in the uptake of fluorescein-tagged oligonucleotide among the population of neurons.
An alternative explanation for the partial effect of the antisense would be the presence of more than one component of R-type current, perhaps including one not supported by α1E. Pietrobon and coworkers (40, 41) have provided evidence for two forms of unitary R-type channel activity, designated G2 and G3, differing by ≈15 mV in their voltage-dependence of activation. We considered the possibility that our antisense sequence affects only one of these subtypes, but we did not observe the expected changes in the voltage-dependence of peak current or in the rate of inactivation. Rather than invoking additional α1 subunits, we preferred to hypothesize that multiple forms of R-type Ca2+ may arise from splice variations in α1E (42) or from association of α1E with diverse ancillary subunits. This kind of explanation also may apply to pharmacological studies with SNX-482, a new peptide neurotoxin that blocks α1E currents in mammalian cell lines and R-type currents in nerve terminals of rat neurohypophysis but fails to inhibit R-type current in rat cerebellar granule cells (43). Interestingly, in cerebellar granule cells cultured under the conditions used by Tottene et al. (40), SNX-482 appears able to block a subfraction of R-type current (D. Pietrobon, personal communication). The α1E subunit has been considered for some time as a possible basis for low voltage-activated T-type currents (28, 34–37). The recent cloning and expression of novel subunits labeled α1G and α1H provides a convincing underpinning for T-type channel activity (44). Nonetheless, the possibility remains open that the α1E subunit also may support some form of LVA channel activity (35, 37).
Our study also provides strong confirmation of the generally accepted notion that α1A subunits underlie P/Q-type currents. The most abundant voltage-gated Ca2+ channel currents in cerebellar granule cells, P/Q-type currents, are blocked by ω-Aga IVA and ω-CTx MVIIC, like currents generated by α1A cRNA in oocytes and cell lines (16, 20, 45, 46). We found that the antisense oligonucleotide designed against α1A specifically reduced the peak amplitude of the P/Q-type components while leaving the R-type current unaffected. Again, none of the control ONs (α1A sense, α1E antisense, and α1E sense) had any effect on the P/Q components. Based on comparison of pooled data from sense and from antisense-treated neurons (Fig. 4), the component suppressed by the α1A antisense had a prominently decaying time course, as expected if Q-type current were predominant. These results may be compared with α1A antisense experiments in cerebellar Purkinje cells (21), in which P-type currents are strongly predominant (47, 48). α1A antisense reduced P-type current in Purkinje neurons, consistent with previous findings of α1A transcripts and immunoreactivity in these cells. Taken together, these studies leave little doubt that α1A can support both Q- and P-type currents, whatever the explanation for how they differ in pharmacology and inactivation kinetics (see ref. 20).
In summary, treatment of cerebellar granule cells with antisense α1E oligonucleotides induced a specific decrease in R-type current amplitude, consistent with the idea that members of the E class of α1 subunits engender this high voltage-activated current.
Acknowledgments
We are grateful to Drs. X.-H. Chen, E. T. Kavalali, P. G. Mermelstein, D. Pietrobon, and D. B. Wheeler for critically reading the manuscript and to all members of the Tsien laboratory for helpful discussions. Supported by National Institutes of Health (R.W.T.) and American Heart Association, Western States Affiliate postdoctoral fellowship (E.S.P.-R).
ABBREVIATIONS
- pA/pF
picoampere/picofarad
- FBS
fetal bovine serum
- ON
oligonucleotides
- P/Q
P- and Q-type currents
References
- 1.Carbone E, Lux H D. Nature (London) 1984;310:501–502. doi: 10.1038/310501a0. [DOI] [PubMed] [Google Scholar]
- 2.Armstrong C M, Matteson D R. Science. 1985;227:65–67. doi: 10.1126/science.2578071. [DOI] [PubMed] [Google Scholar]
- 3.Nowycky M C, Fox A P, Tsien R W. Nature (London) 1985;316:440–443. doi: 10.1038/316440a0. [DOI] [PubMed] [Google Scholar]
- 4.Fedulova S A, Kostyuk P G, Veselovsky N S. J Physiol (London) 1985;359:431–446. doi: 10.1113/jphysiol.1985.sp015594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Randall A, Tsien R W. J Neurosci. 1995;15:2995–3012. doi: 10.1523/JNEUROSCI.15-04-02995.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsien R W, Wheeler D B. In: Regulation of Intracellular Calcium. Klee C B, Carafoli E, editors. New York: Oxford Univ. Press; 1998. , in press. [Google Scholar]
- 7.Hille B. Ionic Channels of Excitable Membranes. Sunderland, MA: Sinauer Associates; 1992. [Google Scholar]
- 8.Catterall W A. Annu Rev Biochem. 1995;64:493–531. doi: 10.1146/annurev.bi.64.070195.002425. [DOI] [PubMed] [Google Scholar]
- 9.Campbell K P, Leung A T, Sharp A H. Trends Neurosci. 1988;11:425–430. doi: 10.1016/0166-2236(88)90193-2. [DOI] [PubMed] [Google Scholar]
- 10.Hofmann F, Biel M, Flockerzi V. Annu Rev Neurosci. 1994;17:399–418. doi: 10.1146/annurev.ne.17.030194.002151. [DOI] [PubMed] [Google Scholar]
- 11.Snutch T P, Reiner P B. Curr Opin Neurobiol. 1992;2:247–253. doi: 10.1016/0959-4388(92)90111-w. [DOI] [PubMed] [Google Scholar]
- 12.Diriong S, Lory P, Williams M E, Ellis S B, Harpold M M, Taviaux S. Genomics. 1995;30:605–609. doi: 10.1006/geno.1995.1284. [DOI] [PubMed] [Google Scholar]
- 13.Mikami A, Imoto K, Tanabe T, Niidome T, Mori Y, Takeshima H, Narumiya S, Numa S. Nature (London) 1989;340:230–233. doi: 10.1038/340230a0. [DOI] [PubMed] [Google Scholar]
- 14.Williams M E, Feldman D H, McCue A F, Brenner R, Veliçelebi G, Ellis S B, Harpold M M. Neuron. 1992;8:71–84. doi: 10.1016/0896-6273(92)90109-q. [DOI] [PubMed] [Google Scholar]
- 15.Williams M E, Brust P F, Feldman D H, Patthi S, Simerson S, Maroufi A, McCue A F, Veliçelebi G, Ellis S B, Harpold M M. Science. 1992;257:389–395. doi: 10.1126/science.1321501. [DOI] [PubMed] [Google Scholar]
- 16.Sather W A, Tanabe T, Zhang J-F, Mori Y, Adams M E, Tsien R W. Neuron. 1993;11:291–303. doi: 10.1016/0896-6273(93)90185-t. [DOI] [PubMed] [Google Scholar]
- 17.Mori Y, Friedrich T, Kim M S, Mikami A, Nakai J, Ruth P, Bosse E, Hofmann F, Flockerzi V, Furuichi T, et al. Nature (London) 1991;350:398–402. doi: 10.1038/350398a0. [DOI] [PubMed] [Google Scholar]
- 18.Llinas R, Sugimori M. J Physiol (London) 1980;305:171–195. doi: 10.1113/jphysiol.1980.sp013357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mintz I M, Venema V J, Swiderek K M, Lee T D, Bean B P, Adams M E. Nature (London) 1992;355:827–829. doi: 10.1038/355827a0. [DOI] [PubMed] [Google Scholar]
- 20.Moreno H, Rudy B, Llinas R. Proc Natl Acad Sci USA. 1997;94:14042–14047. doi: 10.1073/pnas.94.25.14042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gillard S E, Volsen S G, Smith W, Beattie R E, Bleakman D, Lodge D. Neuropharmacology. 1997;36:405–409. doi: 10.1016/s0028-3908(97)00046-4. [DOI] [PubMed] [Google Scholar]
- 22.Ellinor P T, Zhang J-F, Randall A D, Zhou M, Schwarz T L, Tsien R W, Horne W A. Nature (London) 1993;363:455–458. doi: 10.1038/363455a0. [DOI] [PubMed] [Google Scholar]
- 23.Zhang J-F, Randall A D, Ellinor P T, Horne W A, Sather W A, Tanabe T, Schwarz T L, Tsien R W. Neuropharmacology. 1993;32:1075–1088. doi: 10.1016/0028-3908(93)90003-l. [DOI] [PubMed] [Google Scholar]
- 24.Kavalali E T, Zhuo M, Bito H, Tsien R W. Neuron. 1997;18:651–663. doi: 10.1016/s0896-6273(00)80305-0. [DOI] [PubMed] [Google Scholar]
- 25.Wu L G, Saggau P. J Neurosci. 1994;14:5613–5622. doi: 10.1523/JNEUROSCI.14-09-05613.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang G, Dayanithi G, Kim S, Hom D, Nadasdi L, Kristipati R, Ramachandran J, Stuenkel E L, Nordmann J J, Newcomb R, et al. J Physiol (London) 1997;502:351–363. doi: 10.1111/j.1469-7793.1997.351bk.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu L G, Borst J G, Sakmann B. Proc Natl Acad Sci USA. 1998;95:4720–4725. doi: 10.1073/pnas.95.8.4720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Soong T W, Stea A, Hodson C D, Dubel S J, Vincent S R, Snutch T P. Science. 1993;260:1133–1136. doi: 10.1126/science.8388125. [DOI] [PubMed] [Google Scholar]
- 29.Williams M E, Marubio L M, Deal C R, Hans M, Brust P F, Philipson L H, Miller R J, Johnson E C, Harpold M M, Ellis S B. J Biol Chem. 1994;269:22347–22357. [PubMed] [Google Scholar]
- 30.Schneider T, Wei X, Olcese R, Costantin J L, Neely A, Palade P, Perez-Reyes E, Qin N, Zhou J, Crawford G D, et al. Recept Channels. 1994;2:255–270. [PubMed] [Google Scholar]
- 31.Wakamori M, Niidome T, Furutama D, Furuichi T, Mikoshiba K, Fujita Y, Tanaka I, Katayama K, Yatani A, Schwartz A. Recept Channels. 1994;2:303–314. [PubMed] [Google Scholar]
- 32.Rock D M, Horne W A, Stoehr S J, Hashimoto C, Cong R Z, M, Palma A, Hidayetoglu D, Offord J. In: T-Type Calcium Channels. Nargeot J, Clozel J P, Tsien R W, editors. Chester, England: Aidis; 1998. , in press. [Google Scholar]
- 33.Randall A D, Tsien R W. Neuropharmacology. 1997;36:879–893. doi: 10.1016/s0028-3908(97)00086-5. [DOI] [PubMed] [Google Scholar]
- 34.Bourinet E, Zamponi G W, Stea A, Soong T W, Lewis B A, Jones L P, Yue D T, Snutch T P. J Neurosci. 1996;16:4983–4993. doi: 10.1523/JNEUROSCI.16-16-04983.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Piedras-Rentería E S, Chen C C, Best P M. Proc Natl Acad Sci USA. 1997;94:14936–14941. doi: 10.1073/pnas.94.26.14936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stephens G J, Page K M, Burley J R, Berrow N S, Dolphin A C. Pflügers Arch. 1997;433:523–532. doi: 10.1007/s004240050308. [DOI] [PubMed] [Google Scholar]
- 37.Meir A, Dolphin A C. Neuron. 1998;20:341–351. doi: 10.1016/s0896-6273(00)80461-4. [DOI] [PubMed] [Google Scholar]
- 38.Malgaroli A, Tsien R W. Neuron. 1992;8:1109–1125. doi: 10.1016/0896-6273(92)90132-w. [DOI] [PubMed] [Google Scholar]
- 39.Stea A, Tomlinson W J, Soong T W, Bourinet E, Dubel S J, Vincent S R, Snutch T P. Proc Natl Acad Sci USA. 1994;91:10576–10580. doi: 10.1073/pnas.91.22.10576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tottene A, Moretti A, Pietrobon D. J Neurosci. 1996;16:6353–6363. doi: 10.1523/JNEUROSCI.16-20-06353.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Forti L, Tottene A, Moretti A, Pietrobon D. J Neurosci. 1994;14:5243–5256. doi: 10.1523/JNEUROSCI.14-09-05243.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schneider T, Vajna R, Pereverzev A, Schramm M, Grabsch H, Klockner U, Hescheler J. Biophys J. 1998;72:119. doi: 10.1046/j.1432-1327.1998.2570274.x. (abstr.). [DOI] [PubMed] [Google Scholar]
- 43.Newcomb R, Szoke B, Palma A, Long R, Tarczy-Hornoch K, Loo J A, Dooley D J, Hopkins W, Crea R, Miljanich J, et al. Soc Neurosci Abstr. 1997;23:856. [Google Scholar]
- 44.Perez-Reyes E, Cribbs L L, Daud A, Lacerda A E, Barclay J, Williamson M P, Fox M, Rees M, Lee J H. Nature (London) 1998;391:896–900. doi: 10.1038/36110. [DOI] [PubMed] [Google Scholar]
- 45.De Waard M, Campbell K P. J Physiol (London) 1995;485:619–634. doi: 10.1113/jphysiol.1995.sp020757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Berrow N S, Brice N L, Tedder I, Page K M, Dolphin A C. Eur J Neurosci. 1997;9:739–748. doi: 10.1111/j.1460-9568.1997.tb01422.x. [DOI] [PubMed] [Google Scholar]
- 47.Llinas R, Sugimori M, Hillman D E, Cherksey B. Trends Neurosci. 1992;15:351–355. doi: 10.1016/0166-2236(92)90053-b. [DOI] [PubMed] [Google Scholar]
- 48.Regan L J, Sah D W, Bean B P. Neuron. 1991;6:269–280. doi: 10.1016/0896-6273(91)90362-4. [DOI] [PubMed] [Google Scholar]