Abstract
Background
Corticotropin-releasing factor (CRF) signaling modulates neurobiological responses to stress and ethanol, and may modulate observed increases in ethanol consumption following exposure to stressful events. The current experiment was conducted to further characterize the role of CRF1 receptor (CRF1R) signaling in stress-induced increases in ethanol consumption in BALB/cJ and C57BL/6N mice.
Methods
Male BALB/cJ and C57BL/6N mice were given continuous access to 8% (v/v) ethanol and water for the duration of the experiment. When a baseline of ethanol consumption was established, animals were exposed to 5 minutes of forced swim stress on each of 5 consecutive days. Thirty minutes before each forced swim session, animals were given an intraperitoneal injection of a 10 mg/kg dose of CP-154,526, a selective CRF1R antagonist, or an equal volume of vehicle. The effect of forced swim stress exposure on consumption of a 1% (w/v) sucrose solution was also investigated in an ethanol-naïve group of BALB/cJ mice.
Results
Exposure to forced swim stress significantly increased ethanol consumption by the BALB/cJ, but not of the C57BL/6N, mice. Stress-induced increases in ethanol consumption were delayed and became evident approximately 3 weeks after the first stressor. Additionally, forced swim stress did not cause increases of food or water intake and did not promote delayed increases of sucrose consumption. Importantly, BALB/cJ mice pretreated with the CRF1R antagonist showed blunted stress-induced increases in ethanol intake, and the CRF1R antagonist did not influence the ethanol drinking of non-stressed mice.
Conclusions
The present results provide evidence that CRF1R signaling modulates the delayed increase of ethanol consumption stemming from repeated exposure to a stressful event in BALB/cJ mice.
Keywords: Corticotropin, Releasing Factor, CRF1 Receptor, Ethanol, Stress, Voluntary Consumption
Stress may be a key contributor to the development of ethanol dependence and relapse (Breese et al., 2005; Koob, 2003). Stressful life events, such as those underlying post-traumatic stress disorder, are comorbid with ethanol abuse disorders and human laboratory studies show that stress increases the self-report of craving in abstinent alcoholics (Back et al., 2006; Breslau et al., 2003; Fox et al., 2007). Clinical research implicates stress in the relapse to pathological ethanol use in formerly abstinent alcoholics, perhaps as a means to self-medicate heightened anxiety and negative affect associated with withdrawal and abstinence from alcohol (Brady and Sonne, 1999; Breese et al., 2005; Kushner et al., 1994; Sinha, 2001).
Recent investigations show that stress can also impact ethanol consumption in animal models (Chester et al., 2004; Croft et al., 2005; Le et al., 2000; Little et al., 1999; Liu and Weiss, 2002; Sillaber et al., 2002). Various stress paradigms reliably elicit stress-induced increases in ethanol consumption, especially among low ethanol consuming animals (Chester et al., 2004; Croft et al., 2005; Little et al., 1999). For example, selectively bred ethanol non-preferring NP rats exposed to 10 days of restraint stress showed significant and enduring increases in ethanol consumption beginning approximately 2 weeks following the stress procedure, while ethanol preferring P rats showed only transient stress-induced increases in ethanol drinking immediately after the stress procedure (Chester et al., 2004). Additionally, 3 weeks of stress induced by daily saline injections (Little et al., 1999) or 5 consecutive days of social defeat stress (Croft et al., 2005), significantly increased ethanol consumption approximately 2 weeks after the stress procedure among C57BL/10 mice displaying initially low preference for ethanol. An interesting commonality among many animal studies that assess the effects of stress on ethanol intake is that the effects of stress on ethanol drinking are delayed, typically occurring weeks after stress exposure (Chester et al., 2004; Croft et al., 2005; Little et al., 1999).
Both ethanol and stress activate the hypothalamic-pituitary-adrenal (HPA) axis by inducing the release of corticotropin-releasing factor (CRF), adrenocorticotropic hormone (ACTH), and glucocorticoids (Brady and Sonne, 1999). The relationship between ethanol and the HPA-axis appears to be bidirectional, as exogenous administration of CRF, ACTH, and glucocorticoids alter ethanol consumption (Bell et al., 1998; O’Callaghan et al., 2002; Thorsell et al., 2005). Given that neurobiological responses to both stress and ethanol exposure involve HPA-axis signaling, it is possible that the neurochemicals and hormones associated with the HPA-axis modulate stress-induced increases of ethanol consumption. One such candidate is CRF, a 41 amino acid polypeptide that integrates both neuroendocrine and behavioral responses to stress (Smith et al., 1998). CRF-containing neurons are expressed throughout the brain, including in regions implicated in neurobiological responses to ethanol such as the bed nucleus of the stria terminalis, the amygdala, and the lateral hypothalamus (Koob, 2003). Of the two G protein-coupled receptors, the CRF1 receptor (CRF1R) appears to be involved with the integrate emotional behavior while the CRF2 receptor (CRF2R) may modulate ingestive behaviors (Koob, 2003; Zorrilla and Koob, 2004; Zorrilla et al., 2004).
Corticotropin-releasing factor receptor signaling has been implicated in a variety of neurobiological responses to ethanol. For example, CRF receptor antagonists attenuate the anxiogenic effect of ethanol withdrawal (Breese et al., 2004; Knapp et al., 2004; Overstreet et al., 2004; Rassnick et al., 1993), prevent excessive ethanol self-administration in dependent animals (Funk et al., 2007; Valdez et al., 2002), and block foot shock-induced reinstatement of ethanol-seeking behavior (Liu and Weiss, 2002). The CRF1R also appears to be involved in stress-induced increases in ethanol consumption. Mutant mice lacking normal production of the CRF1R displayed significantly greater ethanol consumption beginning approximately 2 weeks after a social defeat stress procedure, an effect that was not evident in normal wild-type mice. Subsequent exposure to forced swim stress further augmented ethanol consumption in CRF1R knockout mice (Sillaber et al., 2002).
While the Sillaber et al. (2002) study provides genetic evidence suggesting a role for the CRF1R in modulating stress-induced increases in ethanol consumption, the goal of the present experiment was to use a pharmacological approach to determine if pretreatment with the selective CRF1R antagonist, CP-154,526, would buffer the effects of stress and thus attenuate the development of stress-induced increases in ethanol intake in BALB/cJ mice. Therefore, we predicted that (1) ethanol consumption would increase among animals with a history of stress exposure and (2) pretreatment with CP-154,526 would attenuate stress-induced increases in ethanol consumption among animals with a history of stress. BALB/cJ mice were chosen because this strain has been shown to have high sensitivity to the effects of stress on both behavioral and neurobiological measures (Crawley et al., 1997) and drinks low levels of ethanol (Belknap et al., 1993). We also assessed the effects of stress exposure on ethanol consumption by C57BL/6N mice, a strain that voluntarily consumes high amounts of ethanol (Belknap et al., 1993). Here we show that 5 consecutive days of exposure to a 5-minute forced swim stress procedure caused significant and delayed increases in voluntary ethanol consumption in BALB/cJ mice, an effect which was attenuated by pretreatments with the CRF1R antagonist before each stress session. On the other hand, stress exposure did not alter ethanol intake by C57BL/6N mice.
MATERIALS AND METHODS
Animals
Forty-seven male BALB/cJ (Jackson Laboratories, Bar Harbor, ME) and 36 male C57BL/6N (Charles River Labs, Wilmington, MA) mice approximately 8-week old and weighing 19 to 26 g were housed individually in polypropylene cages with corncob bedding upon arrival. Animals had ad libitum access to tap water and standard rodent chow throughout the experiment. All fluid was presented in 2 bottles, inserted through holes at the top of the cage. Bottle weights were recorded every 2 days, and body weights and food measurements were taken every 4 days at approximately 10:00 AM. Food intake was measured by subtracting the weight of rodent chow (grams) still present in the cage on measurement day from the initial weight when food was placed in the cage. Great care was taken to collect the remaining food in the cage on measurement day to assure accurate readings. The colony room was maintained at approximately 21°C with a 12-h/12-h light/dark cycle with lights off at 10:30 AM. All procedures in the experiments below were approved by the University of North Carolina’s Institutional Animal Care and Use Committee and follow the National Institute of Health’s guidelines.
Drug Treatment
CP-154,526 (butyl-[2,5-dimethyl-7-(2,4,6-trimethylphenyl)-7H-pyrrolo[2,3-d]pyrimidin-4-yl]-ethylamine) donated by Pfizer (Groton, CT) was suspended in a vehicle of 0.5% carboxymethylcellulose (CMC). CP-154,526 displays high affinity for the CRF1R (Ki < 10 nM) and blocks CRF-stimulated adenylate cyclase activity in rodent pituitary and cortical membranes (Lundkvist et al., 1996; Schulz et al., 1996). Peripheral administration of CP-154,526 crosses the blood–brain barrier and reaches peak brain concentrations 20 minutes after administration with significant levels of the drug observed in the cortex, striatum, cerebellum, and hippocampus (Keller et al., 2002). Importantly, previous research found that systemic administration of a 10 mg/kg dose of CP-154,526 effectively reduced anxiety-like behavior in mice (Griebel et al., 1998). Therefore, a 10 mg/kg dose of CP-154,526, or equal volume of CMC (5 ml/kg), was administered via intraperitoneal (i.p.) injection approximately 30 minutes prior to each stress or handling procedure (see below).
Forced Swim Stress
Forced swim procedures were used to induce stress in mice. Briefly, the mice were removed from their homecages and placed individually in buckets containing 4,000 ml of water maintained at approximately room temperature (21°C) for 5 minutes on each of 5 consecutive days. Mice were carefully monitored and a criteria was established that any mouse that could not keep its head above the water was removed from the procedure (however, all animals were able to swim for the entire session in each experiment). After the 5-minute session, mice were removed from the buckets and dried with a cloth towel. This forced swim stress procedure has been shown to significantly increase ethanol drinking by mice (Sillaber et al., 2002). Mice in the non-stress conditions were briefly removed and then returned to their cages.
Habituation to Environment and Voluntary Ethanol Consumption
Upon arrival, animals were allowed to habituate to their surroundings for 8 days. On day 9, 1 water bottle on each cage was replaced with an identical bottle containing a 2% (v/v) ethanol solution diluted in tap water. Every 4 days, the concentration of ethanol was increased in the following increments: 4, 6, and 8%. From this point on, animals had continuous free access to 8% ethanol and water for the duration of the experiment. The position of bottles containing ethanol were changed every 2 days to prevent the development of side preferences. Fluid loss was controlled by using dummy bottles of water and ethanol placed on an animal-free cage which was located on the same rack as cages containing mice. Daily ethanol consumption was calculated in grams of ethanol consumed/kg of body weight (g/kg).
Consumption of the 8% ethanol solution stabilized by day 13, and animals were divided into 4 groups based on ethanol consumption during the final 3 days of baseline (days 16 to 18). Mice were either pretreated with CP-154,526 (CP) or vehicle (Veh) 30 minutes before being exposed to a 5-minute forced swim stress session (Stress) or handling (No Stress). The groups were as follows: BALB/cJ Stress-CP (n = 8), BALB/cJ Stress-Veh (n = 8), BALB/cJ No Stress-CP (n = 9), BALB/cJ No Stress-Veh (n = 9), C57BL/6N Stress-CP (n = 10), C57BL/6N Stress-Veh (n = 7), C57BL/6N No Stress-CP (n = 9), and C57BL/6N No Stress-Veh (n = 10). Following the 5-forced swim days, ethanol, water, and food intake as well as body weight measures were collected over a 4-week period. The BALB/cJ mice were exposed to an additional 5 days of forced swim stress on days 56 to 60, as described above, but did not receive drug treatment prior to stress exposure.
Voluntary Sucrose Consumption and Forced Swim Stress
As a consummatory control, 20 ethanol-naïve BALB/cJ mice were given continuous access to a 1% (w/v) sucrose solution and tap water and exposed to forced swim stress or handling, as described above. Sucrose was diluted in tap water. We chose 1% sucrose because we found that this concentration produced a similar volume of consumption by the BALB/cJ mice as the 8% ethanol solution. Additionally, 1% sucrose solution has been used previously as a control for stress-induced consumption of an 8% ethanol solution (Croft et al., 2005). The position of bottles containing sucrose was changed every 2 days to prevent the development of side preferences. Fluid loss was controlled by using dummy bottles of water and sucrose placed on an animal-free cage which was located on the same rack as cages containing mice. Daily sucrose consumption was calculated in milliliters of sucrose solution consumed/kg of body weight (ml/kg). Access to food, water, and sucrose was continuously available for the duration of the experiment.
Following 7 days of access to the 1% sucrose solution, animals were divided into Stress and No Stress groups based on their sucrose consumption during the final 3 days of baseline (days 5 to 7). On days 8 through 12, animals in the Stress group (n = 10) were exposed to daily 5-minute forced swim procedures over 5 days, while animals in the No Stress group (n = 10) were handled as described above. Sucrose and water consumption were monitored every 2 days throughout the stress period, and for an additional 4 weeks thereafter.
Data Analyses
All data shown are presented as means ± SEM and were analyzed using repeated measures analyses of variance (ANOVAs). Planned comparisons were analyzed using t-tests (Winer, 1991). In accordance with a priori hypotheses, the following tests were conducted: (1) comparisons were made of the Stress-Veh and No Stress-Veh groups to determine if stress exposure significantly increased ethanol consumption, (2) comparisons were made of the Stress-CP group with No Stress-CP and No Stress-Veh groups to determine if CP-154,526 pretreatment significantly attenuated stress-induced ethanol drinking to the level of non-stressed animals, and (3) comparisons were made of the Stress-Veh and Stress-CP groups to determine if CP-154,526 pretreatment significantly blocked stress-induced increases of ethanol drinking relative to stressed animals not pretreated with the CRF1R antagonist. All reports of significance were accepted at the p < 0.05 level.
RESULTS
Figure 1 displays the effect of forced swim stress on the ethanol, water, and food consumption of BALB/cJ animals for the duration of the experiment. Because BALB/cJ mice were treated with the CRF1R antagonist during the first, but not second, 5 day stress procedure, data were collapsed across the CRF1R antagonist factor for the present analyses. As shown in Fig. 1A, forced swim stress significantly increased ethanol consumption among BALB/cJ animals in the Stress group, while handling did not alter ethanol consumption among BALB/cJ animals in the No Stress group. The results of a 2 × 11 repeated measures ANOVA revealed a significant main effect of week [F(10,340) = 4.859], a significant stress × week interaction [F(10,340) = 2.634], as well as a significant main effect of stress [F(1,34) = 8.315]. Planned comparisons revealed that stressed animals consumed significantly more ethanol than non-stressed animals at post-stress week 3 [t(34) = 2.503] and post-stress week 4 [t(34) = 2.697] following the first stressor. Additionally, stressed animals consumed significantly more ethanol during the second baseline period [t(34) = 2.271], during the second stress period [t(34) = 1.971], and at post-stress week 1 [t(34) = 2.001], post-stress week 2 [t(34) = 2.378], and post-stress week 3 [t(34) = 2.845] following the second stressor. Animals of the Stress group consumed significantly less water when compared with animals of the No Stress group for much of the experiment (see Fig. 1B). The results of a 2 × 11 repeated measures ANOVA revealed a significant main effect of week [F(10,340) = 5.750] and a significant stress × week interaction [F(10,340) = 3.342]. Planned comparisons revealed that animals of the Stress group consumed significantly less water than animals of the No Stress group at post-stress week 4 following the first stressor [t(34) = 2.423] and following the second stressor at post-stress week 1 [t(34) = 1.733], post-stress week 2 [t(34) = 2.234], and post-stress week 3 [t(34) = 1.727]. The decrease in water consumption among stressed animals is likely related to increased ethanol consumption following stress exposure. Finally, forced swim stress did not alter food consumption when compared with the handled group (see Fig. 1C), although a 2 × 11 repeated measures ANOVA revealed a significant main effect of week [F(10,320) = 7.162].
Fig. 1.
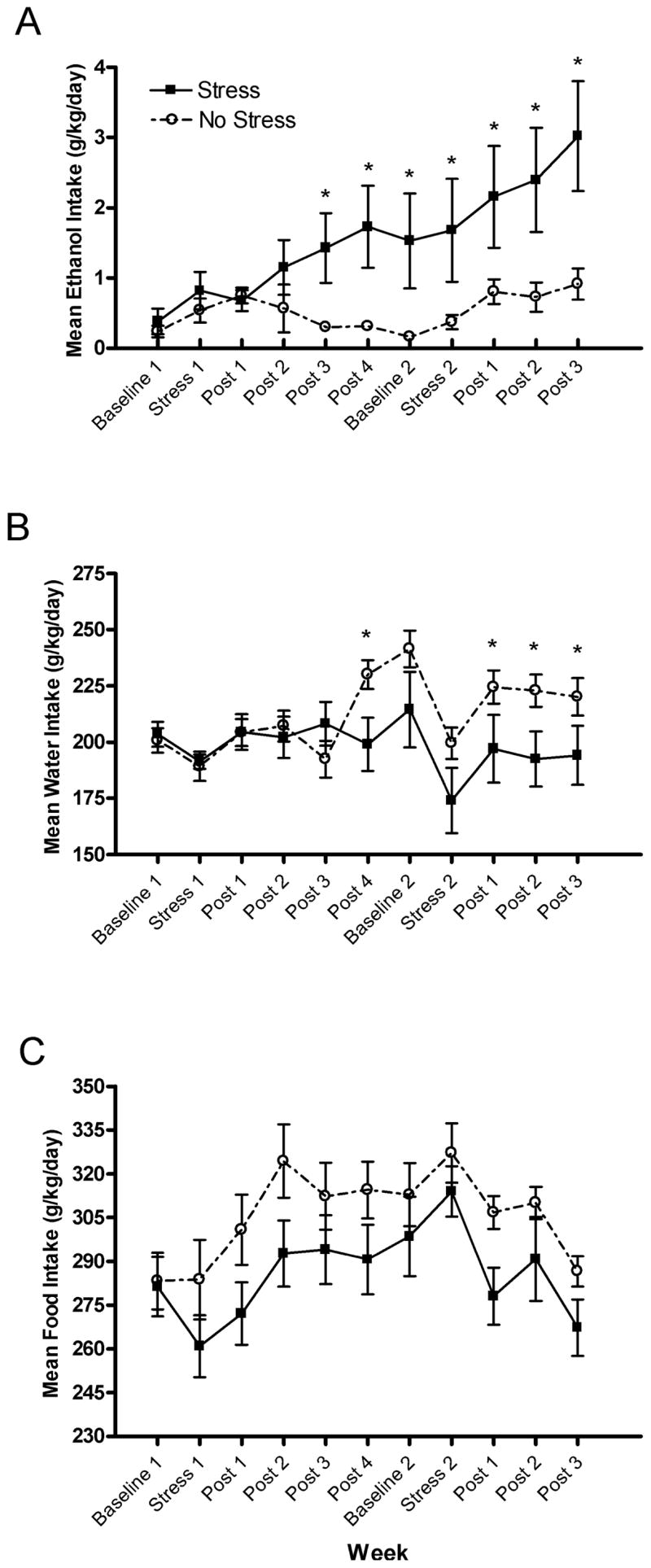
Mean consumption (g/kg/d) of (A) ethanol, (B) water, and (C) food during baselines, the first and second stressors, and post-stress periods for BALB/cJ Stress and No Stress groups. All values are means ± SEM and *denotes significant between-group differences at the p < 0.05 level.
Figure 2 shows the effect of CRF1R antagonism on ethanol, water, and food consumption of BALB/cJ animals during the first stress period. As shown in Fig. 2A, forced swim stress significantly increased ethanol consumption, an effect which was attenuated by administration of CP-154,526. The results of a 2 × 2 × 6 repeated measures ANOVA indicated a significant stress × week interaction [F(5,160) = 2.979] as well as a significant main effect of stress [F(1,32) = 17.986]. Planned comparisons revealed that animals of the Stress-Veh group consumed significantly more ethanol than animals of the No Stress-Veh groups at post-stress week 3 [t(16) = 2.046] and post-stress week 4 [t(16) = 1.963], indicating stress-induced increases of ethanol consumption. Importantly, at no time point did group Stress-CP differ significantly from the non-stressed groups.
Fig. 2.
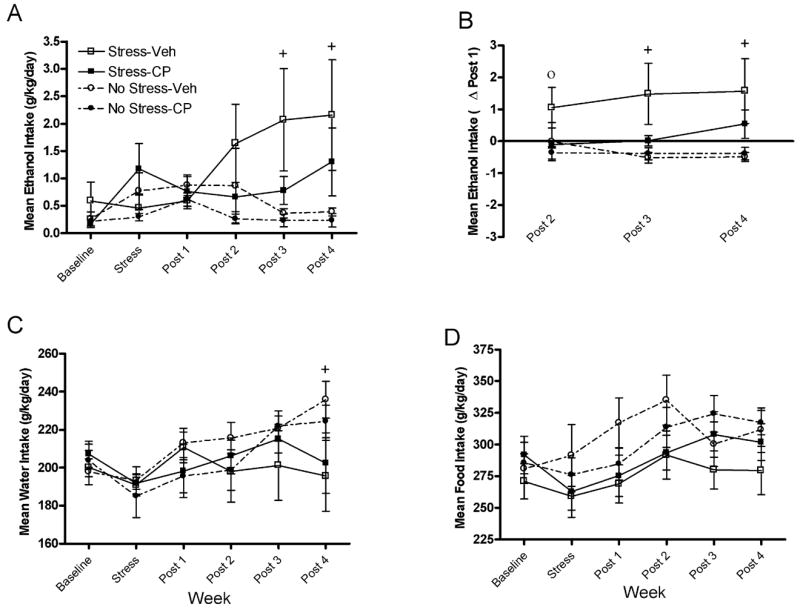
(A) Mean ethanol consumption (g/kg/d) during the first baseline, stressor, and post-stress periods for BALB/cJ mice. (B) Mean changes in ethanol consumption (g/kg/d) during post-stress weeks 2 to 4 relative to post-stress week 1 during the first post-stress period for BALB/cJ mice. (C) Mean water consumption (g/kg/d) during the first baseline, stressor, and post-stress periods for BALB/cJ mice. (D) Mean food consumption (g/kg/d) during the first baseline, stressor, and post-stress periods for BALB/cJ mice. Groups are as follows: Stress-Veh = mice pretreated with vehicle prior to forced swim exposure; Stress-CP = mice were pretreated with CP-154,526 prior to forced swim exposure; No Stress-Veh = mice were treated with vehicle and handled; No Stress-CP = mice were treated with CP-154,526 and handled. All values are means ± SEM. The high degree of variance noted in group Stress-Veh reflects an increase of random variation. Significant between group differences are as follows: ○ denotes significant differences between the Stress-Veh and Stress-CP groups and + denotes significant differences between the Stress-Veh and No Stress-Veh groups, at the p < 0.05 level.
As stress-induced increases in ethanol consumption emerged several weeks following the stress procedure, the effects of CRF1R antagonism on the development of stress-induced increases in ethanol consumption were analyzed by examining ethanol consumption at post-stress weeks 2 to 4 relative to the first week following the stress procedure (Δ post 1; see Fig. 2B). The results of a 2 × 2 × 3 repeated measures ANOVA revealed a significant main effect of stress [F(1,32) = 12.232]. Planned comparisons revealed that animals of the Stress-Veh group showed significantly greater increases of ethanol consumption compared with the No Stress-Veh group at post-stress week 3 [t(16) = 2.293] and post-stress week 4 [t(16) = 2.249], again reflecting a delayed stress-induced increase in ethanol consumption. A planned comparison revealed significant differences between the Stress-Veh and Stress-CP groups at post-stress week 2 [t(14) = 1.782], suggesting that CP-154,526 blocked stress-induced increases in ethanol consumption during this week. As above, at no time point did the Stress-CP group differ significantly from the non-stressed groups.
Exposure to forced swim stress significantly altered water consumption, as displayed in Fig. 2C. The results of a 2 × 2 × 6 repeated measures ANOVA revealed a significant main effect of week [F(5,160) = 5.514] as well as a significant stress × week interaction [F(5,160) = 2.853]. Planned comparisons revealed that the Stress-Veh group consumed significantly less water than the No Stress-Veh group at post-stress week 4 [t(16) = 2.026]. Finally, neither forced swim stress nor antagonism of the CRF1R altered food consumption (see Fig. 2D). However, a significant main effect of week was observed [F(5,160) = 7.486].
Figure 3 shows the effects of forced swim stress on consumption of the 1% sucrose solution and water by ethanol-naive BALB/cJ mice. Repeated measures ANOVA did not reveal significant effects of stress on sucrose consumption when expressed as ml/kg/d or change in consumption relative to post-stress week 1. However, planned comparisons revealed significant differences in sucrose consumption between groups. Specifically, as shown in Fig. 3A, significant differences in sucrose consumption were observed in stressed animals when compared with non-stressed animals at post-stress week 3 [t(17) = 1.884], and at post-stress week 4 [t(17) = 2.139], which appears to reflect a reduction of sucrose consumption by non-stressed mice at post-stress weeks 3 and 4 relative to prior weeks. Importantly, forced swim stress did not cause a delayed increase in sucrose consumption at post-stress weeks 2 to 4 relative to post-stress week 1 (Δ post 1). The effects of forced swim stress exposure on water consumption are shown in Fig. 3C. A 2 × 6 repeated measures ANOVA revealed a significant main effect of week [F(5,85) = 6.237], and planned comparisons revealed that the stressed animals consumed significantly less water than non-stressed animals at post-stress week 3, [t(17) = 1.829].
Fig. 3.
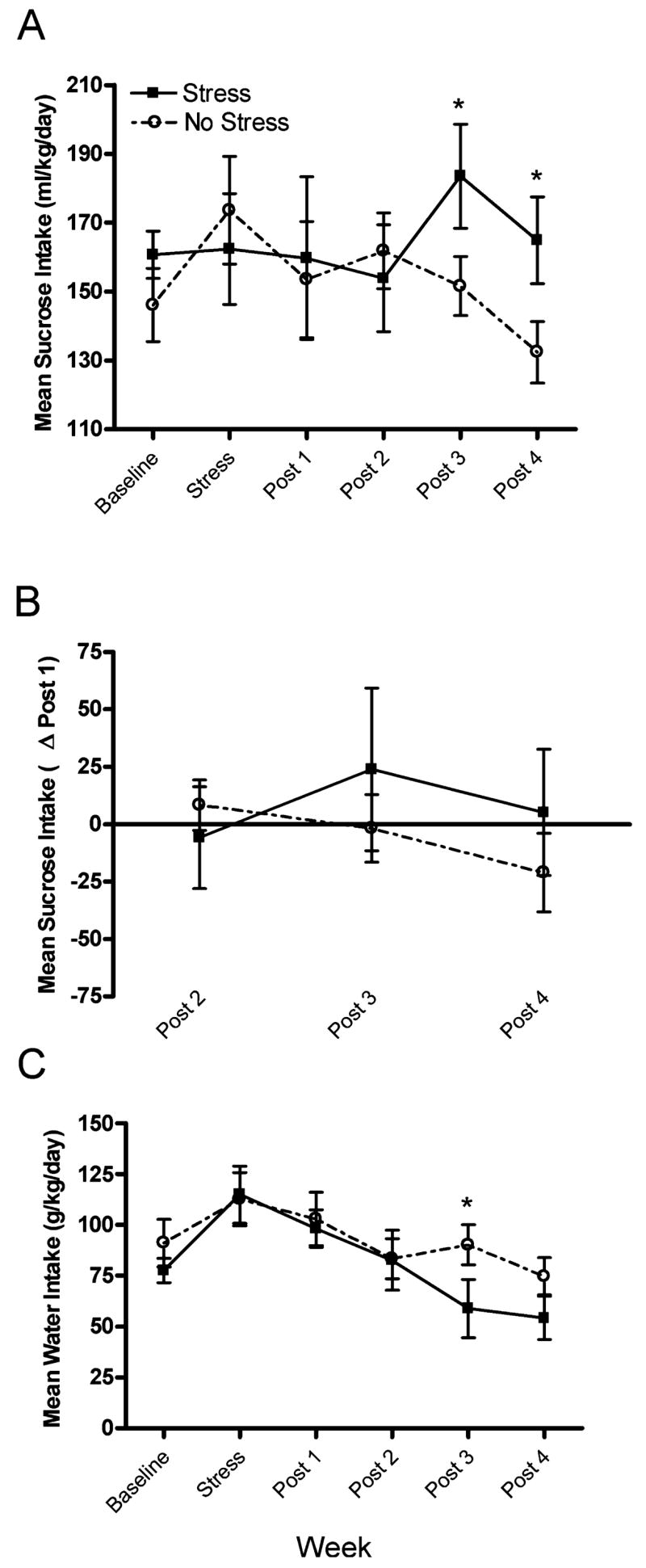
(A) Mean consumption (ml/kg/d) of a 1% (w/v) sucrose solution during the baseline, stress, and post-stress periods for BALB/cJ Stress and No Stress groups. (B) Mean change in sucrose consumption (ml/kg/d) during post-stress weeks 2 to 4 relative to post-stress week 1 for BALB/cJ Stress and No Stress groups. (C) Mean water consumption (g/kg/d) during the baseline, stress, and post-stress period for BALB/cJ Stress and No Stress groups. All values are means ± SEM, and *denotes significant differences between the Stress and No Stress groups, at the p < 0.05 level.
Figure 4 displays the effects of forced swim stress and CRF1R antagonism on the ethanol and water consumption of C57BL/6N animals. As shown in Fig. 4A, neither forced swim stress nor CRF1R antagonism significantly altered ethanol consumption by C57BL/6N animals. A 2 × 2 × 6 repeated measures ANOVA revealed a significant main effect of week [F(5,160) = 20.425]. Planned comparisons revealed no group differences. Figure 4B shows water consumption by C57BL/6N mice. The results of a 2 × 2 × 6 repeated measures ANOVA revealed a significant main effect of week [F(5,160) = 7.087], as well as a significant week × stress × drug interaction [F(5,160) = 2.561]. Planned comparisons revealed that animals of the Stress-Veh group consumed significantly more water than animals of the No Stress-Veh group at post-stress week 1 [t(17) = 1.789].
Fig. 4.
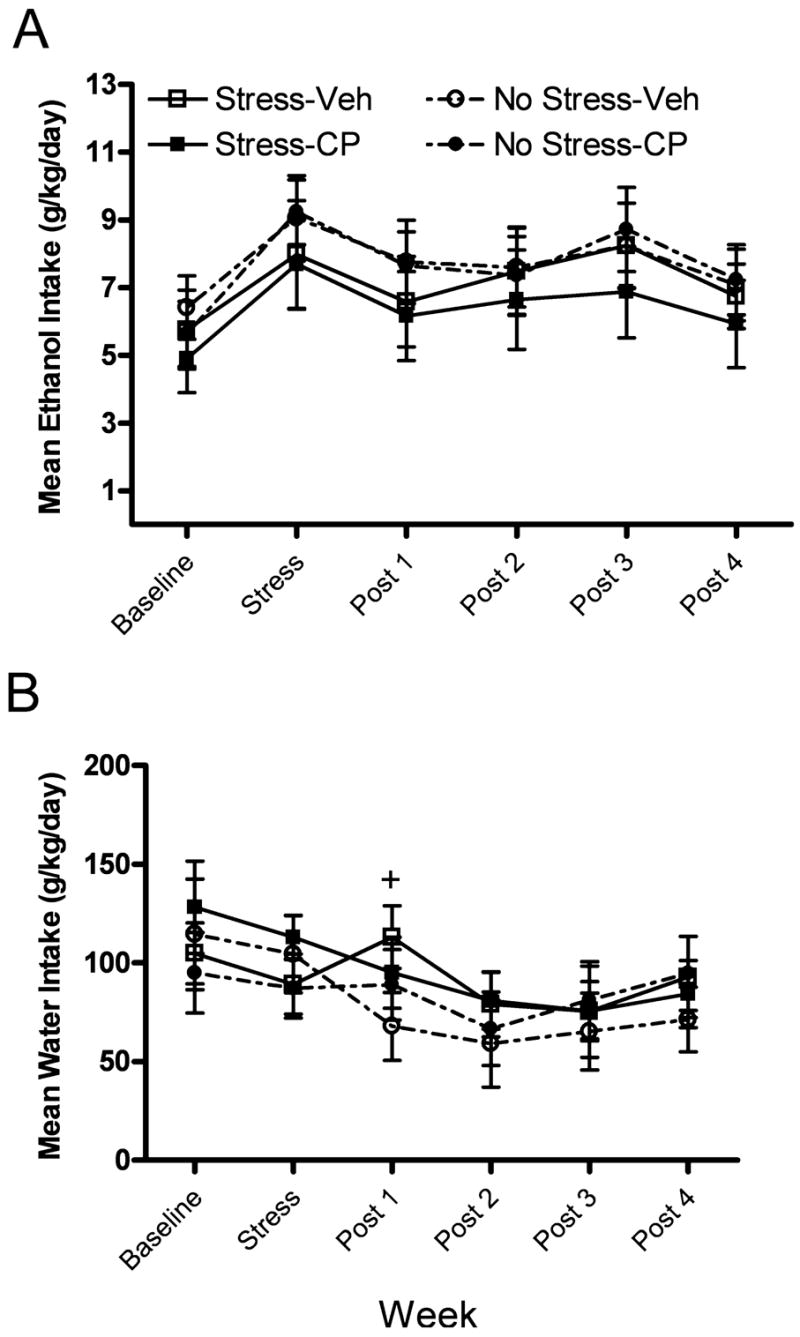
(A) Mean consumption (g/kg/d) of ethanol during the baseline, stress, and post-stress periods for C57BL/6N mice. (B) Mean water consumption (g/kg/d) during the baseline, stress, and post-stress periods for C57BL/6N mice. Groups are as follows: Stress-Veh = mice pretreated with vehicle prior to forced swim exposure; Stress-CP = mice were pretreated with CP-154,526 prior to forced swim exposure; No Stress-Veh = mice were treated with vehicle and handled; No Stress-CP = mice were treated with CP-154,526 and handled. All values are means ± SEM, and + denotes significant differences between the Stress-Veh and No Stress-Veh groups at the p < 0.05 level.
DISCUSSION
The results of the current experiment show that forced swim stress induced a delayed increase in ethanol consumption by initially low ethanol consuming BALB/cJ mice, but did not affect ethanol consumption in the initially high ethanol consuming C57BL/6N mice. The lack of effect of stress exposure on ethanol consumption by the C57BL/6N mice is unlikely due to the high baseline ethanol consumption observed in these animals (e.g., a ceiling effect) as experimental manipulations, such as procedures that promote the alcohol deprivation effect, have been shown to reliably increase ethanol consumption significantly above baseline levels which are similar to consumption levels observed in the present experiment (Melendez et al., 2006). These results are consistent with the literature suggesting that a variety of stressors can have delayed effects on ethanol consumption in rodents (Chester et al., 2004; Croft et al., 2005; Little et al., 1999; Sillaber et al., 2002), and that the effects of stress on ethanol consumption may depend on initial preference for ethanol (Chester et al., 2004; Little et al., 1999; Rockman et al., 1987). The results of the current experiment also provide additional support for research suggesting that CRF1R signaling is involved in stress-related ethanol consumption as pretreatment before each stress episode with CP-154,526, a CRF1R antagonist, attenuated the observed stress-induced increases in ethanol consumption among BALB/cJ mice. This conclusion is supported by the observation that stress-treated BALB/cJ mice that were pretreated with CP-154,526 never differed significantly in ethanol consumption from non-stressed groups, while stress-treated mice pretreated with the vehicle showed significantly higher levels of ethanol consumption than the non-stressed groups at multiple time points.
Although there were group differences in sucrose consumption, such differences appear to be related, in part, to a reduction of sucrose intake by non-stressed mice at post-stress weeks 3 and 4 relative to prior weeks. Furthermore, there were no group differences in sucrose consumption at post-stress weeks 2 through 4 relative to post-stress week 1, indicating that stress did not promote a delayed increase of sucrose consumption, a delayed effect of stress that was noted when mice drank ethanol. This observation, and the fact that stress did not significantly alter food intake, suggests that the delayed effect of stress to increase consumption over weeks is specific to ethanol. The observed decrease in water consumption among animals exposed to stress is likely related to the observed increase in ethanol solution intake among these animals, as a portion of the animal’s water intake was obtained from the ethanol solution.
Although the literature on stress and ethanol consumption has been mixed, recent reports indicate that the effects of stress on ethanol consumption may differ depending on the length of time that has elapsed since termination of the stressor. For example, some studies investigating the immediate effects of stress on ethanol consumption suggest that ethanol consumption is transiently reduced (van Erp and Miczek, 2001), and some studies investigating the long-term effects of stress on ethanol consumption reveal delayed increases in ethanol consumption (Chester et al., 2004; Croft et al., 2005; Sillaber et al., 2002), though other studies have failed to find a stress effect on ethanol consumption at any experimental time point (Bowers et al., 1997; Boyce-Rustay et al., 2007). Indeed, direct comparison of the results of these studies is difficult due to use of a wide variety of stressors and rodent strains, as well as varying experimental time points and ethanol access periods. Nonetheless, our work and the work of others indicate that stress can increase ethanol consumption by rodents under certain conditions.
The results of the current experiment coincide with an increasing number of reports suggesting that the pattern of ethanol consumption following stress may be dependent on predisposed ethanol preference (Chester et al., 2004; Little et al., 1999; Rockman et al., 1987), as increases in ethanol consumption were observed in initially low ethanol consuming BALB/cJ mice approximately 3 weeks after exposure to forced swim stress, but not in initially high ethanol consuming C57BL/6N mice. Prior research suggests that animals genetically predisposed, or phenotypically selected, for high ethanol consumption, such as the C57BL/6 strain of mice, reduce ethanol consumption during stress exposure and gradually return to baseline levels of consumption after termination of the stressor (Chester et al., 2004; Rockman et al., 1987). For example, ethanol preferring P rats displayed significantly reduced ethanol consumption during the first 5 days of exposure to 10 days of unpredictable restraint stress, an increase in ethanol consumption during the 5 days immediately following the termination of the restraint stress, and a subsequent return to baseline levels of ethanol consumption (Chester et al., 2004). Similarly, Wistar rats screened for high ethanol preference and exposed to unpredictable restraint stress at cold temperatures significantly reduced their ethanol consumption during the first 12 days of an 18-day stress period, after which consumption returned to baseline levels (Rockman et al., 1987).
Conversely, a variety of observations reveal that animals showing initial low ethanol preference, such as the BALB/c strain of mice, continue consuming baseline levels of ethanol during, and immediately following stress exposure, but increase levels of ethanol consumption approximately 2 to 3 weeks following termination of the stressor (Chester et al., 2004; Croft et al., 2005; Rockman et al., 1987). Consistently, ethanol non-preferring NP rats exposed to 10 days of unpredictable restraint stress maintained baseline levels of ethanol consumption throughout the stress period and immediately thereafter, and significantly increased ethanol consumption approximately 2 weeks following stress exposure (Chester et al., 2004). Wistar rats screened for low ethanol preference and exposed to 18 days of unpredictable restraint stress at cold temperatures displayed gradual increases in ethanol consumption beginning in the final 12 days of the stress period and continuing several weeks after the stress exposure (Rockman et al., 1987). Similar delayed increases in ethanol consumption have been observed in C57BL/10 mice screened for low ethanol preference and exposed to social defeat stress (Croft et al., 2005), and stress caused by repeated saline injections (Little et al., 1999; O’Callaghan et al., 2002). Thus, an emerging literature provides converging evidence that a variety of stressors induce delayed increases in ethanol consumption in initially low ethanol consuming animals. While the present observations provide additional evidence that stress-induced increases in ethanol drinking are evident in low (BALB/cJ), but not high (C57BL/6N), ethanol preferring strains, an alternative explanation for the present data is that the BALB/cJ mice were more stress-responsive than the C57BL/6N mice. Indeed, a well-established literature suggests that the BALB/c strain of mice display higher levels of anxiety and are more stress-responsive on certain behavioral measures than the C57BL/6 strain of mice (Anisman et al., 2007; Carola et al., 2002; Crawley et al., 1997; Depino and Gross, 2007; Ducottet and Belzung, 2004; Griebel et al., 2000). As such, it may be stress sensitivity, rather than initial ethanol preference, that predicts the effects of stress on subsequent ethanol intake.
The HPA-axis has been implicated in neurobiological responses to stress and ethanol consumption, and the involvement of neurochemicals and hormones associated with the HPA-axis in stress-induced ethanol consumption has been demonstrated. For example, Sprague–Dawley rats with intact HPA-axis function displayed increases in ethanol consumption following 11 days of unpredictable exposure to either isolation or immobilization stress, while the post-stress ethanol consumption of hypophysectomized rats did not change (Nash and Maickel, 1988). Pharmacological manipulations also provide evidence for a role of HPA-axis signaling. ACTH administered via unpredictable, i.v. injections for 11 days in intact rats produced increases in ethanol consumption similar to those observed following stress exposure (Nash and Maickel, 1988). Mice screened for low ethanol preference and given 3 weeks of daily i.p. injections of the corticosterone synthesis inhibitor metyrapone did not display stress-induced increases in ethanol preference caused by repeated i.p. injection, while mice injected with vehicle over 3 weeks did display increases in ethanol preference (O’Callaghan et al., 2002). The Type II glucocorticoid receptor appears to modulate the effects of corticosterone on stress-induced increases in ethanol consumption as mice screened for low ethanol preference and given daily i.p. injections of the glucocorticoid Type II receptor antagonist RU38486 did not display stress-induced increases in ethanol preference, an effect observed in mice with low ethanol preference and given daily i.p. injections of vehicle (O’Callaghan et al., 2002).
The results of the current experiment, as well as those of Sillaber et al. (2002), indicate that CRF signaling, via the CRF1R, is another HPA-axis-associated neurochemical that modulates stress-induced ethanol consumption. In the current experiment, the role of the CRF1R was investigated pharmacologically through the administration of the CRF1R antagonist CP-154,526 prior to each exposure to forced swim stress. While only 1 dose of the CRF1R antagonist was used in the present study, this 10 mg/kg dose of CP-154,526 has been previously shown to reduce anxiety-like behavior in BALB/cJ mice (Griebel et al., 1998). Importantly our results indicate that pharmacological antagonism of the CRF1R with a 10 mg/kg dose of CP-154,526 attenuates the delayed stress-induced increases in ethanol consumption observed in vehicle and stress treated animals. On the other hand, Sillaber et al. (2002) found that disruption of CRF1R signaling by genetic mutation augmented the delayed stress-induced increases of ethanol consumption relative to wild-type mice. While the factors that contribute to the inconsistencies between pharmacological and genetic manipulation of CRF1R signaling are not completely clear, Sillaber et al. (2002) suggest that the observed increases in ethanol consumption among CRF1R knockout mice following stress exposure may result from developmental compensation associated with mutation of the CRF1R gene. It should be noted that although the results of the current experiment suggest that the CRF1R modulates stress-related ethanol consumption, it remains unclear if CRF1R signaling within the HPA-axis and/or within extrahypothalamic brain regions are involved. In fact, a recent report found that pretreatment with the CRF1R antagonist antalarmin attenuated yohimbine-induced increases in ethanol self-administration in rats without altering yohimbine-induced increases of corticosterone levels, suggesting that extrahypothalamic CRF1R signaling was involved (Marinelli et al., 2007).
In summary, the current experiment indicates that exposure to stress is associated with delayed increases in ethanol consumption among initially low consuming BALB/cJ mice, but not initially high consuming C57BL/6N mice. Importantly, stress did not alter the consumption of food or cause delayed increases of sucrose intake in BALB/cJ mice. Pretreatment before each stress episode with the CRF1R antagonist CP-154,526 attenuated the delayed increases in ethanol consumption observed in stressed BALB/cJ mice, but did not alter the consumption of ethanol by non-stressed mice. Current research indicates that CRF signaling, via the CRF1R, is intricately involved in the development of ethanol dependence and relapse to ethanol seeking during abstinence (Heilig and Koob, 2007), perhaps due to the role CRF plays in mediating increased anxiety during withdrawal from ethanol (Breese et al., 2004). The current experiment supports the hypothesis that CRF, and more specifically the CRF1R, is also involved in delayed and long-lasting stress-induced increases in ethanol drinking. Thus targets aimed at the CRF1R may be useful compounds for treating and/or preventing the lasting effects of stress exposure to induce excessive and uncontrolled ethanol consumption in the human population. Finally, future research will extend the current findings by investigating the role of CRF1R signaling in targeted brain areas, as well as the role of CRF in stress-induced ethanol drinking by ethanol dependent animals.
Acknowledgments
This work was supported by NIH Grants AA013573, AA015148, AA011605, AA014949, and Department of Defense Grant W81XWH-06-1-0158.
References
- Anisman H, Prakash P, Merali Z, Poulter MO. Corticotropin releasing hormone receptor alterations elicited by acute and chronic unpredictable stressor challenges in stressor-susceptible and resilient strains of mice. Behav Brain Res. 2007;181:180–190. doi: 10.1016/j.bbr.2007.04.002. [DOI] [PubMed] [Google Scholar]
- Back SE, Brady KT, Sonne SC, Verduin ML. Symptom improvement in co-occurring PTSD and alcohol dependence. J Nerv Ment Dis. 2006;194:690–696. doi: 10.1097/01.nmd.0000235794.12794.8a. [DOI] [PubMed] [Google Scholar]
- Belknap JK, Crabbe JC, Young ER. Voluntary consumption of ethanol in 15 inbred mouse strains. Psychopharmacology. 1993;112:503–510. doi: 10.1007/BF02244901. [DOI] [PubMed] [Google Scholar]
- Bell SM, Reynolds JG, Thiele TE, Gan J, Figlewicz DP, Woods SC. Effects of third intracerebroventricular injections of corticotropin-releasing factor (CRF) on ethanol drinking and food intake. Psychopharmacology. 1998;139:128–135. doi: 10.1007/s002130050697. [DOI] [PubMed] [Google Scholar]
- Bowers WJ, Sabongui AG, Amit Z. The role of ethanol availability on stress-induced increases in ethanol consumption. Alcohol. 1997;14:551–556. doi: 10.1016/s0741-8329(96)00046-8. [DOI] [PubMed] [Google Scholar]
- Boyce-Rustay JM, Cameron HA, Holmes A. Chronic swim stress alters sensitivity to acute behavioral effects of ethanol in mice. Physiol Behav. 2007;91:77–86. doi: 10.1016/j.physbeh.2007.01.024. [DOI] [PubMed] [Google Scholar]
- Brady KT, Sonne SC. The role of stress in alcohol use, alcoholism treatment, and relapse. Alcohol Res Health. 1999;23:263–271. [PMC free article] [PubMed] [Google Scholar]
- Breese GR, Chu K, Dayas CV, Funk D, Knapp DJ, Koob GF, Le AD, O’Dell L, Overstreet DH, Roberts AJ, Sinha R, Valdez GR, Weiss F. Stress enhancement of craving during sobriety: A risk for relapse. Alcohol Clin Exp Res. 2005;29:185–195. doi: 10.1097/01.alc.0000153544.83656.3c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breese GR, Knapp DJ, Overstreet DH. Stress sensitization of ethanol withdrawal-induced reduction in social interaction: inhibition by CRF-1 and benzodiazepine receptor antagonists and a 5-HT1A-receptor agonist. Neuropsychopharmacology. 2004;29:470–482. doi: 10.1038/sj.npp.1300282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslau N, Davis GC, Schultz LR. Posttraumatic stress disorder and the incidence of nicotine, alcohol, and other drug disorders in persons who have experienced trauma. Arch Gen Psychiatry. 2003;60:289–294. doi: 10.1001/archpsyc.60.3.289. [DOI] [PubMed] [Google Scholar]
- Carola V, D’Olimpio F, Brunamonti E, Mangia F, Renzi P. Evaluation of the elevated plus-maze and open-field tests for the assessment of anxiety-related behaviour in inbred mice. Behav Brain Res. 2002;134:49–57. doi: 10.1016/s0166-4328(01)00452-1. [DOI] [PubMed] [Google Scholar]
- Chester JA, Blose AM, Zweifel M, Froehlich JC. Effects of stress on alcohol consumption in rats selectively bred for high or low alcohol drinking. Alcohol Clin Exp Res. 2004;28:385–393. doi: 10.1097/01.alc.0000117830.54371.7a. [DOI] [PubMed] [Google Scholar]
- Crawley JN, Belknap JK, Collins A, Crabbe JC, Frankel W, Henderson N, Hitzemann RJ, Maxson SC, Miner LL, Silva AJ, Wehner JM, Wynshaw-Boris A, Paylor R. Behavioral phenotypes of inbred mouse strains: implications and recommendations for molecular studies. Psychopharmacology. 1997;132:107–124. doi: 10.1007/s002130050327. [DOI] [PubMed] [Google Scholar]
- Croft AP, Brooks SP, Cole J, Little HJ. Social defeat increases alcohol preference of C57BL/10 strain mice; effect prevented by a CCKB antagonist. Psychopharmacology (Berl) 2005;183:163–170. doi: 10.1007/s00213-005-0165-6. [DOI] [PubMed] [Google Scholar]
- Depino AM, Gross C. Simultaneous assessment of autonomic function and anxiety-related behavior in BALB/c and C57BL/6 mice. Behav Brain Res. 2007;177:254–260. doi: 10.1016/j.bbr.2006.11.025. [DOI] [PubMed] [Google Scholar]
- Ducottet C, Belzung C. Behaviour in the elevated plus-maze predicts coping after subchronic mild stress in mice. Physiol Behav. 2004;81:417–426. doi: 10.1016/j.physbeh.2004.01.013. [DOI] [PubMed] [Google Scholar]
- van Erp AM, Miczek KA. Persistent suppression of ethanol self-administration by brief social stress in rats and increased startle response as index of withdrawal. Physiol Behav. 2001;73:301–311. doi: 10.1016/s0031-9384(01)00458-9. [DOI] [PubMed] [Google Scholar]
- Fox HC, Bergquist KL, Hong KI, Sinha R. Stress-induced and alcohol cue-induced craving in recently abstinent alcohol-dependent individuals. Alcohol Clin Exp Res. 2007;31:395–403. doi: 10.1111/j.1530-0277.2006.00320.x. [DOI] [PubMed] [Google Scholar]
- Funk CK, Zorrilla EP, Lee MJ, Rice KC, Koob GF. Corticotropin-releasing factor 1 antagonists selectively reduce ethanol self-administration in ethanol-dependent rats. Biol Psychiatry. 2007;61:78–86. doi: 10.1016/j.biopsych.2006.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griebel G, Belzung C, Perrault G, Sanger DJ. Differences in anxiety-related behaviours and in sensitivity to diazepam in inbred and outbred strains of mice. Psychopharmacology. 2000;148:164–170. doi: 10.1007/s002130050038. [DOI] [PubMed] [Google Scholar]
- Griebel G, Perrault G, Sanger DJ. Characterization of the behavioral profile of the non-peptide CRF receptor antagonist CP-154,526 in anxiety models in rodents. Comparison with diazepam and buspirone. Psychopharmacology (Berl) 1998;138:55–66. doi: 10.1007/s002130050645. [DOI] [PubMed] [Google Scholar]
- Heilig M, Koob GF. A key role for corticotropin-releasing factor in alcohol dependence. Trends Neurosci. 2007;30:399–406. doi: 10.1016/j.tins.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller C, Bruelisauer A, Lemaire M, Enz A. Brain pharmacokinetics of a nonpeptidic corticotropin-releasing factor receptor antagonist. Drug Metab Dispos. 2002;30:173–176. doi: 10.1124/dmd.30.2.173. [DOI] [PubMed] [Google Scholar]
- Knapp DJ, Overstreet DH, Moy SS, Breese GR. SB242084, flumazenil, and CRA1000 block ethanol withdrawal-induced anxiety in rats. Alcohol. 2004;32:101–111. doi: 10.1016/j.alcohol.2003.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. Alcoholism: allostasis and beyond. Alcohol Clin Exp Res. 2003;27:232–243. doi: 10.1097/01.ALC.0000057122.36127.C2. [DOI] [PubMed] [Google Scholar]
- Kushner MG, Sher KJ, Wood MD, Wood PK. Anxiety and drinking behavior: moderating effects of tension-reduction alcohol outcome expectancies. Alcohol Clin Exp Res. 1994;18:852–860. doi: 10.1111/j.1530-0277.1994.tb00050.x. [DOI] [PubMed] [Google Scholar]
- Le AD, Harding S, Juzytsch W, Watchus J, Shalev U, Shaham Y. The role of corticotropin-releasing factor in stress-induced relapse to alcohol-seeking behavior in rats. Psychopharmacology. 2000;150:317–324. doi: 10.1007/s002130000411. [DOI] [PubMed] [Google Scholar]
- Little HJ, O’Callaghan MJ, Butterworth AR, Wilson J, Cole J, Watson WP. Low alcohol preference among the “high alcohol preference” C57 strain of mice; preference increased by saline injections. Psychopharmacology (Berl) 1999;147:182–189. doi: 10.1007/s002130051159. [DOI] [PubMed] [Google Scholar]
- Liu X, Weiss F. Additive effect of stress and drug cues on reinstatement of ethanol seeking: exacerbation by history of dependence and role of concurrent activation of corticotropin-releasing factor and opioid mechanisms. J Neurosci. 2002;22:7856–7861. doi: 10.1523/JNEUROSCI.22-18-07856.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundkvist J, Chai Z, Teheranian R, Hasanvan H, Bartfai T, Jenck F, Widmer U, Moreau JL. A non peptidic corticotropin releasing factor receptor antagonist attenuates fever and exhibits anxiolytic-like activity. Eur J Pharmacol. 1996;309:195–200. doi: 10.1016/0014-2999(96)00337-8. [DOI] [PubMed] [Google Scholar]
- Marinelli PW, Funk D, Juzytsch W, Harding S, Rice KC, Shaham Y, Le AD. The CRF(1) receptor antagonist antalarmin attenuates yohimbine-induced increases in operant alcohol self-administration and reinstatement of alcohol seeking in rats. Psychopharmacology (Berl) 2007;195:345–355. doi: 10.1007/s00213-007-0905-x. [DOI] [PubMed] [Google Scholar]
- Melendez RI, Middaugh LD, Kalivas PW. Development of an alcohol deprivation and escalation effect in C57BL/6J mice. Alcohol Clin Exp Res. 2006;30:2017–2025. doi: 10.1111/j.1530-0277.2006.00248.x. [DOI] [PubMed] [Google Scholar]
- Nash JF, Jr, Maickel RP. The role of the hypothalamic-pituitary-adrenocortical axis in post-stress induced ethanol consumption by rats. Prog Neuropsychopharmacol Biol Psychiatry. 1988;12:653–671. doi: 10.1016/0278-5846(88)90010-3. [DOI] [PubMed] [Google Scholar]
- O’Callaghan MJ, Croft AP, Watson WP, Brooks SP, Little HJ. Low alcohol preference among the “high alcohol preference” C57/BL10 mice; factors affecting such preference. Pharmacol Biochem Behav. 2002;72:475–481. doi: 10.1016/s0091-3057(02)00717-7. [DOI] [PubMed] [Google Scholar]
- Overstreet DH, Knapp DJ, Breese GR. Modulation of multiple ethanol withdrawal-induced anxiety-like behavior by CRF and CRF1 receptors. Pharmacol Biochem Behav. 2004;77:405–413. doi: 10.1016/j.pbb.2003.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rassnick S, Heinrichs SC, Britton KT, Koob GF. Microinjection of a corticotropin-releasing factor antagonist into the central nucleus of the amygdala reverses anxiogenic-like effects of ethanol withdrawal. Brain Res. 1993;605:25–32. doi: 10.1016/0006-8993(93)91352-s. [DOI] [PubMed] [Google Scholar]
- Rockman GE, Hall A, Hong J, Glavin GB. Unpredictable cold-immobilization stress effects on voluntary ethanol consumption in rats. Life Sci. 1987;40:1245–1251. doi: 10.1016/0024-3205(87)90580-7. [DOI] [PubMed] [Google Scholar]
- Schulz DW, Mansbach RS, Sprouse J, Braselton JP, Collins J, Corman M, Dunaiskis A, Faraci S, Schmidt AW, Seeger T, Seymour P, Tingley FD, 3rd, Winston EN, Chen YL, Heym J. CP-154,526: a potent and selective nonpeptide antagonist of corticotropin releasing factor receptors. PNAS. 1996;93:10477–10482. doi: 10.1073/pnas.93.19.10477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sillaber I, Rammes G, Zimmermann S, Mahal B, Zieglgansberger W, Wurst W, Holsboer F, Spanagel R. Enhanced and delayed stress-induced alcohol drinking in mice lacking functional CRH1 receptors. Science. 2002;296:931–933. doi: 10.1126/science.1069836. [DOI] [PubMed] [Google Scholar]
- Sinha R. How does stress increase risk of drug abuse and relapse? Psychopharmacology (Berl) 2001;158:343–359. doi: 10.1007/s002130100917. [DOI] [PubMed] [Google Scholar]
- Smith GW, Aubry JM, Dellu F, Contarino A, Bilezikjian LM, Gold LH, Chen R, Marchuk Y, Hauser C, Bentley CA, Sawchenko PE, Koob GF, Vale W, Lee KF. Corticotropin releasing factor receptor 1-deficient mice display decreased anxiety, impaired stress response, and aberrant neuroendocrine development. Neuron. 1998;20:1093–1102. doi: 10.1016/s0896-6273(00)80491-2. [DOI] [PubMed] [Google Scholar]
- Thorsell A, Slawecki CJ, Ehlers CL. Effects of neuropeptide Y and corticotropin-releasing factor on ethanol intake in Wistar rats: interaction with chronic ethanol exposure. Behav Brain Res. 2005;161:133–140. doi: 10.1016/j.bbr.2005.01.016. [DOI] [PubMed] [Google Scholar]
- Valdez GR, Roberts AJ, Chan K, Davis H, Brennan M, Zorrilla EP, Koob GF. Increased ethanol self-administration and anxiety-like behavior during acute ethanol withdrawal and protracted abstinence: regulation by corticotropin-releasing factor. Alcohol Clin Exp Res. 2002;26:1494–1501. doi: 10.1097/01.ALC.0000033120.51856.F0. [DOI] [PubMed] [Google Scholar]
- Winer BJ, Brown DR, Michels KM. Statistical Principles in Experimental Design. 3. New York: McGraw-Hill; 1991. [Google Scholar]
- Zorrilla EP, Koob GF. The therapeutic potential of CRF1 antagonists for anxiety. Expert Opin Investig Drugs. 2004;13:799–828. doi: 10.1517/13543784.13.7.799. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP, Reinhardt LE, Valdez GR, Inoue K, Rivier JE, Vale WW, Koob GF. Human urocortin 2, a corticotropin-releasing factor (CRF)2 agonist, and ovine CRF, a CRF1 agonist, differentially alter feeding and motor activity. J Pharmacol Exp Ther. 2004;310:1027–1034. doi: 10.1124/jpet.104.068676. [DOI] [PubMed] [Google Scholar]


