Abstract
Background
Drinking in the dark (DID) procedures have recently been developed to induce high levels of ethanol drinking in C57BL/6J mice, which result in blood ethanol concentrations (BECs) reaching levels that have measurable affects on physiology and/or behavior. The present experiments determined whether the increased ethanol drinking caused by DID procedures can be attenuated by pretreatment with CP-154,526; a corticotropin releasing factor type-1 (CRF1) receptor antagonist.
Methods
In Experiment 1, male C57BL/6J mice received ethanol (20% v/v) in place of water for 4 hours, beginning with 3 hours into the dark cycle. On the fourth day, mice were given an intraperitoneal injection of one of the 4 doses of CP-154,526 (0, 1, 3, 10 mg/kg) 30 minutes before receiving their ethanol bottle. In Experiment 2, C57BL/6J mice had 2 hours of access to the 20% ethanol solution, beginning with 3 hours into the dark cycle on days 1 to 3, and 4 hours of access to the ethanol bottle on day 4 of DID procedures. Mice were given an intraperitoneal injection of one of the 4 doses of CP-154,526 (0, 1, 3, 10 mg/kg) 30 minutes before receiving their ethanol bottle on day 4. Tail blood samples were collected immediately after the 4-hour ethanol access period on the fourth day of each experiment. Additional control experiments assessed the effects of CP-154,526 on 4-hour consumption of a 10% (w/v) sucrose solution and open-field locomotor activity.
Results
In Experiment 1, the vehicle-treated group consumed approximately 4.0 g/kg/4 h of ethanol and achieved BECs of approximately 30 mg%. Furthermore, pretreatment with the CRF1 receptor antagonist did not alter ethanol consumption. On the other hand, procedures used in Experiment 2 resulted in vehicle-treated mice consuming approximately 6.0 g/kg/4 h of ethanol with BECs of about 80 mg%. Additionally, the 10 mg/kg dose of CP-154,526 significantly reduced ethanol consumption and BECs to approximately 3.0 g/kg/4 h and 27 mg%, respectively, relative to vehicle-treated mice. Importantly, the 10 mg/kg dose of the CRF1R antagonist did not significantly alter 4-hour sucrose consumption or locomotor activity.
Conclusions
These data indicate that CRF1R signaling modulates high, but not moderate, levels of ethanol drinking associated with DID procedures.
Keywords: C57BL/6J Mice; Drinking in the Dark; Corticotropin Releasing Factor; CRF1 Receptor; CP-154,526
Rodent models of alcoholism, including inbred and selectively bred strains have been useful tools for identifying the genetic and neurobiological factors that underlie this disease. However, in many cases, rodents do not consume enough alcohol to reach the point of behavioral and/or pharmacological intoxication (Spanagel, 2000). Recently, “drinking in the dark” (DID) procedures have been developed to induce excessive ethanol drinking in C57BL/6J mice, which result in blood ethanol concentrations (BECs) reaching levels that have measurable effects on physiology and/or behavior (Rhodes et al., 2005, 2007). With these procedures, C57BL/6J mice are given access to a 20% ethanol solution for 2 to 4 hours, starting with 3 hours into their dark cycle. C57BL/6J can achieve BECs of >100 mg% and exhibit signs of behavioral intoxication as measured by motor deficits on the rotarod and balance beam (Rhodes et al., 2005, 2007). It has been argued that the DID model has predictive validity for testing potential pharmacological targets aimed at treating alcohol abuse disorders as naltrexone, an opioid receptor antagonist which is currently used to treat alcoholism, dose dependently attenuates high levels of ethanol drinking induced by DID procedures (Kamdar et al., 2007).
Corticotropin releasing factor (CRF) is a 41 amino acid neuromodulator that is widely expressed throughout the central nervous system (Bloom et al., 1982; Merchenthaler et al., 1982). CRF has been shown to modulate diverse biological functions including food intake, stress and anxiety-like behaviors, and neurobiological responses to ethanol [for reviews, see (Heilig and Koob, 2007; Valdez, 2006; Zorrilla and Koob, 2004; Zorrilla et al., 2003)]. Increases in CRF immunoreactivity (Olive et al., 2002; Zorrilla et al., 2001) and levels of extracellular CRF (Funk et al., 2006) are seen in the amygdala following ethanol withdrawal. Exposure to ethanol causes robust activation of the hypothalamic-pituitary-adrenal (HPA)-axis (Rivier, 1996; Rivier et al., 1990), which is initiated by ethanol-induced increases of CRF activity within the hypothalamus (Li et al., 2005; Rivier and Lee, 1996). Recent pharmacological and genetic evidence support the hypothesis that CRF exerts its effects on ethanol consumption through activation of the CRF1 receptor (CRF1R). Blockade of the CRF1R attenuates ethanol intake in dependent, but not nondependent rodents (Funk et al., 2007; Gehlert et al., 2007). Consistently, CRF1R deficient mice failed to show increased ethanol consumption following the acquisition of ethanol dependence and a period of abstinence that was observed in wild-type mice (Chu et al., 2007). Interestingly, a genetic polymorphism at the Crhr1 locus, which encodes the CRF1R was found to be significantly linked to alcoholism (Treutlein et al., 2006).
Because CRF receptor signaling has been implicated in a wide range of neurobiological responses to ethanol, the goal of the present set of experiments was to determine whether the increased consumption of ethanol associated with DID procedures can be modulated by pretreatment with CP-154,526, a CRF1R antagonist. Specifically, because ethanol triggers HPA-axis signaling which is initiated by ethanol-induced increases of CRF activity within the hypothalamus (Li et al., 2005; Rivier and Lee, 1996), and because levels of corticosterone, a HPA-axis-associated hormone, have been shown to positively correlated with ethanol intake (Fahlke et al., 1994, 1995, 1996), we predicted that CRF1R blockade would attenuate increased ethanol drinking promoted by DID procedures.
METHODS
Animals
Male C57BL/6J mice (Jackson Laboratory, Bar Harbor, ME) were used in all experiments. Mice were 6 to 8 weeks old, weighed between 25 to 30 g at the onset of each experiment, and were single-housed in polypropylene cages with corncob bedding. Standard rodent chow (Teklad, Madison, WI) and water were available at all times, except where noted. The vivarium rooms were maintained at an ambient temperature of 22°C with a 12 h/12 h light–dark cycle. Lights came on at 10:30 pm and went off at 10:30 am. All experimental procedures were approved by the University of North Carolina Animal Care and Use Committee (IACUC) and were in compliance with the NIH Guide for Care and Use of Laboratory Animals.
Drugs
CP-154,526 (butyl-[2,5-dimethyl-7-(2,4,6-trimethylphenyl)-7H-pyrrolo[2,3-d]pyrimidin-4-yl]-ethylamine) was donated by Pfizer (Groton, CT), and was suspended in a vehicle of 0.5% carboxymethylcellulose (CMC). CP-154,526 displays high affinity for the CRF1R (Ki < 10 nM) and blocks CRF-stimulated adenylate cyclase activity in rodent pituitary and cortical membranes (Lundkvist et al., 1996; Schulz et al., 1996). Importantly, peripheral administration of CP-154,526 has been shown to cross the blood-brain barrier and reach peak brain concentrations 20 minutes after administration with significant levels of the drug observed in the cortex, striatum, cerebellum, and hippocampus (Keller et al., 2002). Additionally, intraperitoneal (i.p.) injection of CP-154,526 in the dose range examined here appears to produce antidepressant-like and anxiolytic-like effects in rodents (Breese et al., 2004; Chen et al., 1997; Lundkvist et al., 1996; Mansbach et al., 1997), data that suggest functional central actions of this drug when it is administered peripherally. All concentrations of CP-154,526 used in the present experiment were mixed such that the final injection volume was 5 ml/kg. To habituate mice to procedures, all mice handled were given i.p. injections of CMC (5 ml/kg) daily for approximately 7 days before the initiation of the experiments. The site of injection was switched daily in an attempt to limit discomfort and tissue damage.
Experiment 1: DID After Administration of CP-154,526 With 4-Hour Training Sessions
All mice (n = 39) underwent a modified DID protocol (Rhodes et al., 2005). Briefly, all homecage water bottles were replaced with a single bottle of 20% (v/v) ethanol, 3 hours into the start of the dark phase. The 20% ethanol solution remained on the homecage for 4 hours. All mice had ad libitum access to food during this time. After the 4 hour session, the 20% ethanol bottle was replaced with a bottle containing water. On the first 3 days of this procedure, mice were given an i.p. injection of CMC, 30 minutes prior to the presentation of the ethanol bottle. Mice were then distributed into 4 groups, matched for an average ethanol consumption that occurred over the first 3 days of the experiment (that is, the mice were distributed so that the baseline level of ethanol consumption was approximately equal between the groups). On the fourth day, mice were given an i.p. injection of one of 4 doses of CP-154,526 (0, 1, 3, 10 mg/kg) mixed in CMC, 30 minutes prior to the application of the ethanol bottle. Immediately following the 4 hour test session, tail blood (6 μl) was collected from mice to determine BECs.
Experiment 2: DID After Administration of CP-154,526 With 2-Hour Training Sessions
Procedures for this experiment were similar to those used in Experiment 1 except that mice (n = 40) had access to the ethanol bottle for 2 hours (rather than 4 hours) during days 1 to 3. As above, mice were given an i.p. injection of CMC, 30 minutes before access to ethanol, and mice were distributed to 4 groups matched for average ethanol consumption that occurred over the first 3 days of testing. On the fourth day, mice were injected with one of 4 doses of CP-154,526 (0, 1, 3, 10 mg/kg) mixed in CMC, 30 minutes prior to the application of the ethanol bottle. Immediately following the 4 hour test session, tail blood (6 μl) was collected from mice to determine BECs. This alternate DID procedure was used because Rhodes et al. (2005) found that shortening the length of ethanol access during the first 3 days of training led to greater ethanol consumption and greater BECs on the fourth day of access.
Experiment 3: Open-Field Locomotor Activity After Administration of CP-154,526
To determine whether CP-154,526 could impair locomotor activity, naïve male C57BL/6J mice (n = 20) were tested in an open-field arena that automatically recorded activity via photo beam breaks (Harvard Apparatus, Inc., Holliston, MA). The open field arena measured 40.64 × 40.64 × 30.48 cm and was made of clear Plexiglass. Several cms of corncob bedding were placed into the open field chamber to aid in cleaning and to prevent the buildup of odor. C57BL/6J mice were handled and injected with CMC daily for 7 days before activity testing. CMC or CP-154,526 (10 mg/kg) was administered to mice (n = 10 per group) and then 30 minutes later, mice were placed in the center of the locomotor activity chamber. All mice were tested beginning 3 hours into the dark cycle to match DID procedures. Horizontal distance traveled (in centimeters) was recorded as an index of motor function during the 4-hour test session.
Experiment 4: Sucrose DID After Administration of CP-154,526 With 2-Hour Training Sessions
To determine if a 10 mg/kg dose of CP-154,526 had a general suppressive effect on consummatory behavior, male C57BL/6J mice (n = 20) were tested with procedures similar to those used in Experiment 2 except that the solution used for each 2-hour training session and the 4-hour test session was a 10% (w/v) sucrose solution. Mice were habituated to i.p. injections with CMC over 7 days and were also given i.p. injections of CMC on days 1 to 3. Mice were distributed to 2 groups matched for average sucrose consumption that occurred over the first 3 days, and were injected with CMC or a 10 mg/kg dose of CP-154,526 (n = 10 per group), 30-minutes prior to the 4-hour test on day 4.
Blood Ethanol Concentrations After Administration of CP-154,526
Blood ethanol samples were analyzed with gas chromatographic methods described elsewhere (Knapp et al., 1993; Navarro et al., 2003). Tail blood (6 μl) and standards (6 μl; 0 to 300 mg/100 ml) were mixed with 375 μl of distilled water and 0.5 g of NaCl in 12 × 75 mm borosilicate glass culture tubes. The tubes were capped and then heated at 55°C for 10 minutes in a water bath, at which point 1.5 ml of headspace gas was removed with a plastic 3.0 ml syringe and injected directly into an SRI 8610C gas chromatograph (SRI Instruments, Torrance, CA) equipped with an external syringe adapter and a 1.0 ml external loading loop. The oven temperature was isothermal at 140°C and contained a Hayesep D column and a flame ionization detector. Hydrogen gas, carrier gas (also hydrogen), and internal air generator flow rates were 13.3, 25, and 250 ml/min, respectively. Peak retention time was 2 minutes, and the areas under the curve were analyzed with SRI PeakSimple software (SRI Instruments) for Windows running on a Dell (Dell, Round Rock, TX) Inspiron 3500 laptop computer.
Data Analysis
All data in this report are presented as means ± SEM. One-way analyses of variance (ANOVA) were used to analyze data from Experiments 1 and 2. When significant main effects were obtained, Tukey’s HSD post hoc tests were performed for group comparisons (Winer et al., 1991). For Experiment 3, a repeated measures ANOVA was used to analyze locomotor activity data over the 4-hour session. For Experiment 4, an independent student’s t-test was performed to assess sucrose consumption data. Significance was accepted at p < 0.05 (two-tailed).
RESULTS
Experiment 1: DID After Administration of CP-154,526 With 4-Hour Training Sessions
The volume of ethanol consumed (g/kg) and BECs achieved following 4 hours of access to ethanol on day 4 of Experiment 1 are presented in Fig. 1A and 1B, respectively. Mice pretreated with the 0, 1, 3, and 10 mg/kg doses of CP-154, 526 drank 26.13 ± 3.35, 27.11 ± 3.47, 31.31 ± 2.85, and 28.21 ± 2.92 ml/kg/4 h of ethanol, respectively. One-way ANOVAs performed on these data revealed no significant effects of pre-treatment with CP-154,526 on the amount of ethanol consumed [F(3, 35) = 0.504, p = 0.682] or BECs [F(3, 35) = 0.829, p = 0.487].
Fig. 1.
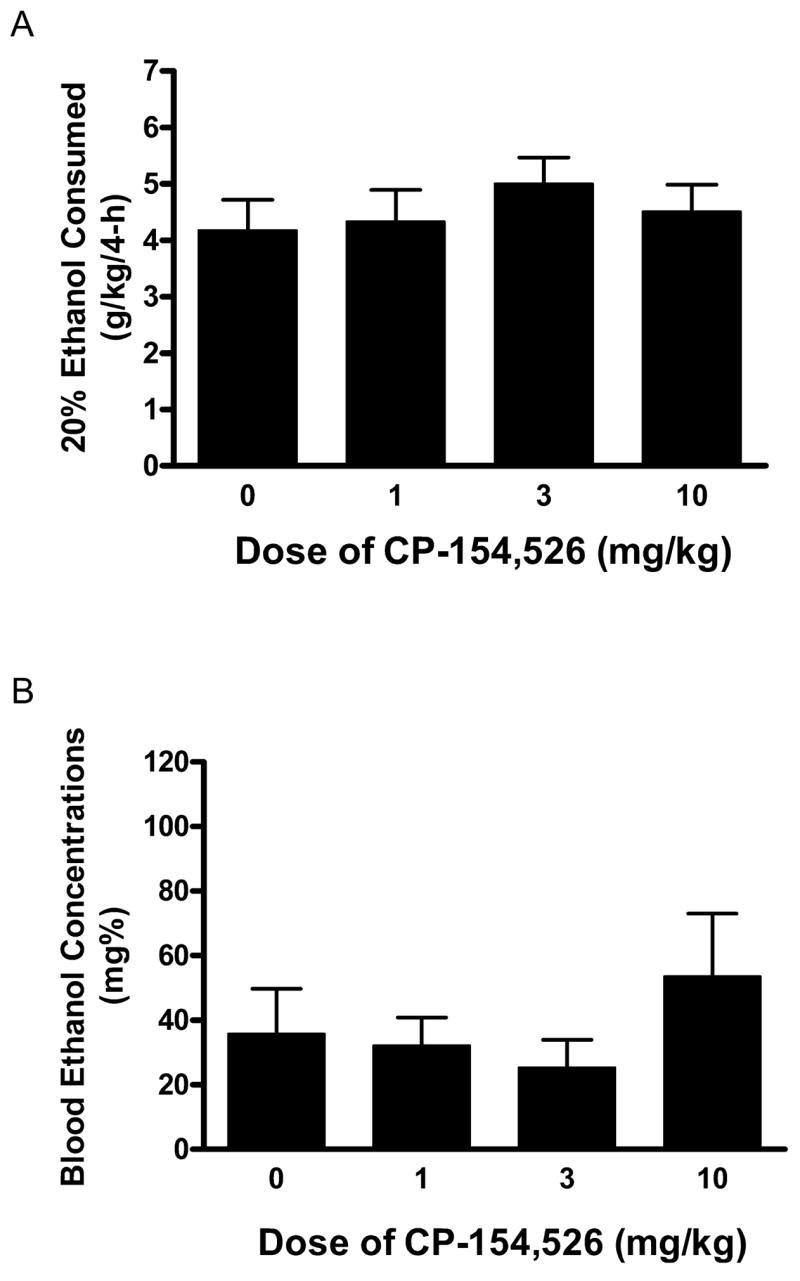
Consumption of 20% (v/v) ethanol (A) and blood ethanol concentrations (BECs) (B) following the 4-hour ethanol consumption test on day 4 of Experiment 1. Mice were given an intraperitoneal (i.p.) injection of the CRF1R antagonist CP-154,526 (0, 1, 3, 10 mg/kg) 30 minutes before access to ethanol. There were no significant differences between treatment groups. All values are means ± SEM.
Experiment 2: DID After Administration of CP-154,526 With 2-Hour Training Sessions
The volume of ethanol consumed (g/kg) and BECs achieved following 4 hours of access to ethanol on day 4 of Experiment 2 are presented in Fig. 2A and 2B, respectively. Mice pretreated with the 0, 1, 3, and 10 mg/kg doses of CP-154, 526 drank 37.89 ± 3.23, 40.03 ± 5.14, 32.79 ± 4.73, and 17.81 ± 4.64 ml/kg/4 h of ethanol, respectively. A one-way ANOVA performed on ethanol consumption data was significant [F(3, 36) = 4.961, p = 0.006]. Tukey’s HSD post hoc tests revealed that the 10 mg/kg dose of CP-154,526 significantly reduced ethanol consumption relative to the control group. Neither 1 nor 3 mg/kg doses of CP-154,526 significantly altered ethanol consumption relative to the CMC-treated group. A one-way ANOVA performed on BEC data was significant [F(3, 36) = 4.493, p = 0.009], and Tukey’s HSD post hoc tests showed that the group treated with the 10 mg/kg dose of CP-154,526 displayed significantly lower BECs relative to the CMC-treated group. Groups pretreated with the 1 or 3 mg/kg doses of CP-154,526 did not display BECs that were significantly different from the CMC treated group.
Fig. 2.
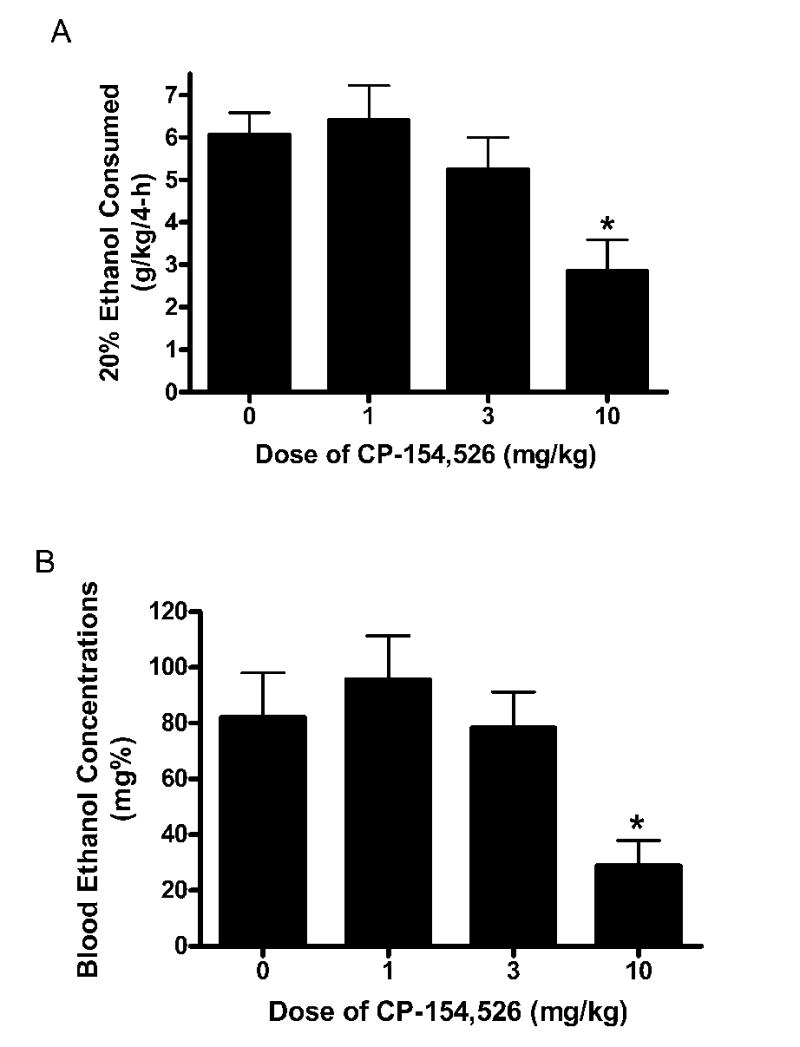
Consumption of 20% (v/v) ethanol (A) and blood ethanol concentrations (BECs) (B) following the 4-hour ethanol consumption test on day 4 of Experiment 2. Mice were given an intraperitoneal (i.p.) injection of CP-154,526 (0, 1, 3, 10 mg/kg) 30 minutes before access to ethanol. Relative to mice treated with carboxymethylcellulose (CMC), pretreatment with the 10 mg/kg dose of CP-154,526 caused a significant reduction of ethanol consumption and BECs. All values are means ± SEM. *p < 0.05 relative to the CMC treatment group.
Experiment 3: Open-Field Locomotor Activity After Administration of CP-154,526
Data representing 4 hour locomotor activity following i.p. injection of CMC or a 10 mg/kg dose of CP-154,526 are presented in Fig. 3. A 2 × 4 (dose × hours) repeated measures ANOVA run on the locomotor activity data revealed a main effect of hour [F(3, 54) = 67.614, p = 0.001]. However, neither pretreatment with CP-154,526 (dose) nor the interaction between dose and hours were statistically significant.
Fig. 3.
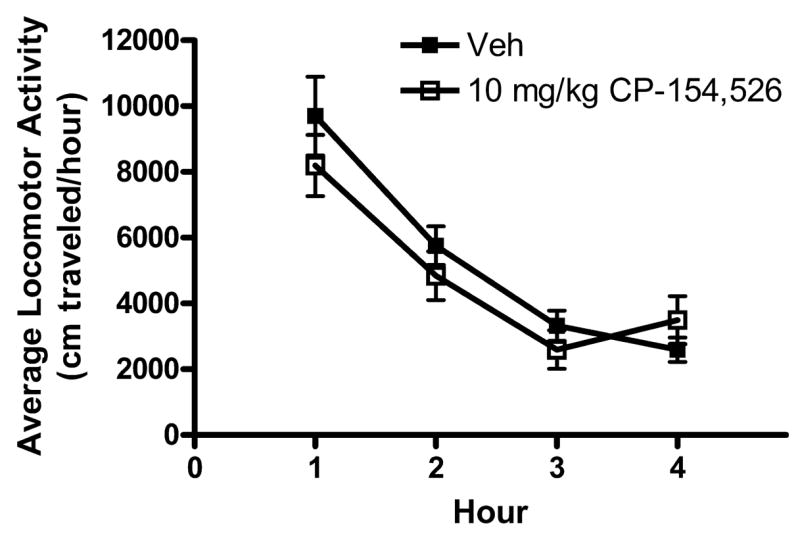
Open-field locomotor activity (cm/h) during the 4-hour test following intraperitoneal (i.p.) injection of CP-154,526 (10 mg/kg) or the vehicle (Veh) 30 minutes before testing. There were no significant differences between the drug pretreatment groups at any time point. All values are means ± SEM.
Experiment 4: Sucrose DID After Administration of CP-154,526 With 2-Hour Training Sessions
The volume of sucrose consumed (ml/kg) following 4 hours of access to ethanol on day 4 of Experiment 1 are presented in Fig. 4. An independent t-test performed on these data did not achieve statistical significance [t(18) = 1.330, p = 0.205].
Fig. 4.
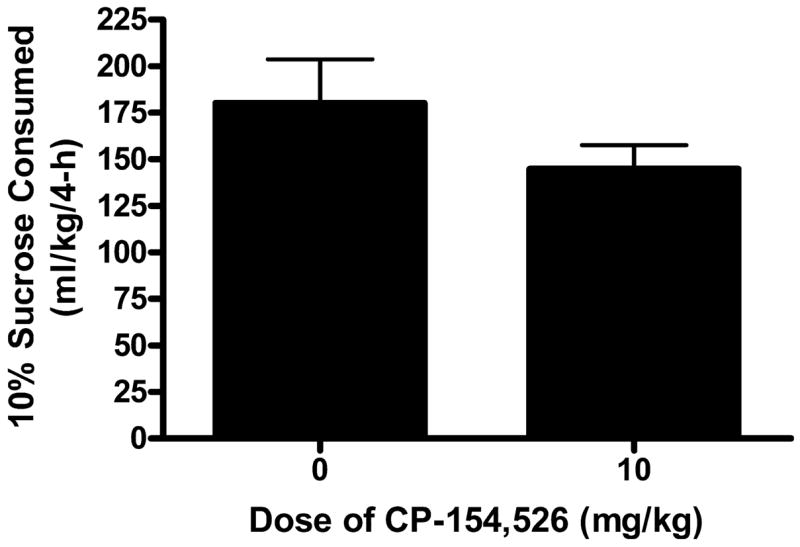
Consumption of a 10% (w/v) sucrose solution following the 4-hour sucrose consumption test on day 4 of Experiment 4. Mice were given intraperitoneal (i.p.) injection of CP-154,526 (0, 10 mg/kg), 30 minutes before access to sucrose. There was no significant difference between the two groups. All values are means ± SEM.
DISCUSSION
Here, we demonstrate that i.p. injection of a 10 mg/kg dose of CP-154,526, a selective CRF1R antagonist, significantly attenuated ethanol consumption and BECs in C57BL/6J mice when DID procedures that promoted high levels of ethanol consumption (approximately 6.0 g/kg/4 h) were employed (Experiment 2). Interestingly, CP-154,526 had no effect on ethanol consumption or BECs, when DID procedures that promoted more moderate levels ethanol consumption (approximately 4.0 g/kg/4 h) were employed (Experiment 1). These observations suggest high, but not moderate levels of ethanol consumption induced by specific DID procedures are modulated by CRF1R signaling.
It was possible that the 10 mg/kg dose of CP-154,526 reduced ethanol consumption because of non-specific effects, such as impairment of motor function or general reductions of consummatory behavior. To determine the effects of CP-154,526 on motor function, a control experiment (Experiment 3) was performed to assess the effects of the 10 mg/kg dose of CP-154,526 on locomotor activity over a 4-hour test 3 hours into the dark cycle. The 10 mg/kg dose of CP-154,526 did not significantly alter 4-hour open-field locomotor activity, thus the ability of this dose of CP-154,526 to reduce ethanol drinking in Experiment 2 was probably not related to effects of this drug on motor function. Importantly, pre-treatment with the 10 mg/kg dose of CP-154,526 did not influence high level of consumption of 10% sucrose over 4 hours when access began 3 hours into the dark cycle (Experiment 4), and this dose did not alter moderate ethanol consumption in Experiment 1. Both observations suggest that reduced ethanol drinking induced by pretreatment with CP-154,526 in Experiment 2 is probably not related to nonspecific effects of this drug on consummatory behavior. Rather, it appears that CP-154,526 specifically modulates ethanol drinking when consumption levels are elevated.
These data present novel evidence suggesting that CRF1R signaling is involved with modulating high or excessive binge-like ethanol consumption in C57BL/6J mice that are induced by specific DID procedures. Interestingly, these observations parallel previous data, where antagonism of CRF receptors attenuated increased ethanol drinking in rodents made dependent to ethanol by exposure to ethanol diet or ethanol vapor, but had no effect on moderate levels of ethanol consumption in nondependent rodents (Finn et al., 2007; Sabino et al., 2006; Valdez et al., 2002). While ethanol drinking associated with DID procedures is unlikely to promote ethanol dependence to the degree achieved by exposure to ethanol vapor or ethanol-containing diets, the present findings, in tandem with previous work, suggest that CRF1R signaling modulates increased ethanol drinking induced by a variety of rodent models. Ethanol exposure induces rapid activation (within 15 minutes) of HPA-axis signaling (Rivier, 1996; Rivier et al., 1990), an effect which is attenuated by pretreatment with CRF receptor antagonists (Rivier and Lee, 1996). Because CP-154,526 has been shown to attenuate stress-induced activation of HPA-axis activity (Arborelius et al., 2000; Xu et al., 2005), it is tempting to speculate that increased ethanol drinking associated with DID procedures is mediated, in part, by an up-regulation of HPA-axis activity, an effect which may be prevented by pretreatment with the CRF1R antagonist. Consistently, treatment with corticosterone (a hormone that is secreted with HPA-axis activation) increases daily ethanol drinking by rodents, while inhibition of endogenous corticosterone synthesis or adrenalectomy suppress ethanol consumption (Fahlke et al., 1994, 1995, 1996). However, it should be noted that corticosterone pretreatment blocked the acquisition ethanol-induced conditioned place preference (CPP) in the TO strain of mice (Brooks et al., 2004), while a corticosterone synthesis inhibitor did not alter the expression or acquisition of CPP in DBA/2J mice (Chester and Cunningham, 1998), suggesting that corticosterone may not modulate ethanol’s reinforcing properties. The possible role of HPA-axis activity in the modulation of increased ethanol drinking with DID procedures, or extrahypothalamic CRF signaling if involved, will be the topic of future research.
Consistent with Rhodes et al. (2005), we show here that the level of ethanol consumption is sensitive to the specific DID procedures. Thus, the highest levels of ethanol consumption occurred when mice had 2 hours of access to ethanol during the first 3 days of the procedure and 4 hours of ethanol access on the final test day when BECs were assessed (Experiment 2). With this procedure, mice achieved BECs of approximately 80 mg%. On the other hand, when mice had access to ethanol solution for 4 hours on each of the 4 days of the experiment, mice achieved BECs of approximately 30 mg% (Experiment 1). However, despite higher levels of ethanol consumption with procedures used in Experiment 2, the level of ethanol consumption and the associated BECs were lower than those reported by Rhodes et al. (2005) using identical procedures and the same strain of mice. It is likely that subtle environmental differences between laboratories are the bases of differences in the level of ethanol consumption between the present observations and those previously reported (Rhodes et al., 2005), as environmental factors have been demonstrated to have significant impact on behavioral measures (Crabbe et al., 1999; Wahlsten et al., 2003).
In conclusion, this study demonstrates that i.p. administration of the systemically bioavailable and selective CRF1R antagonist, CP-154,526, reduces excessive ethanol consumption caused by specific DID procedures. These results are consistent with research showing that the CRF system modulates a spectrum of neurobiological responses to ethanol. A recent report found that both naltrexone and the dopamine re-uptake inhibitor GBR 12909 can attenuate increased ethanol consumption associated with DID procedures, suggesting a role for opioid and dopamine receptor signaling (Kamdar et al., 2007). The present observations add to this small but growing literature by demonstrating that CRF1R signaling selectively modulates high ethanol drinking without altering moderate levels of ethanol consumption or sucrose drinking. Future research is needed to determine the brain regions in which CRF1R signaling modulates increased ethanol drinking associated with DID procedures and if the CRF2 receptor plays a role.
Acknowledgments
This work was supported by National Institute of Health grants AA013573, AA015148, AA015875, AA015878, and the Department of Defense grant W81XWH-06-1-0158. We thank Pfizer Inc. for their generous donation of CP-154,526.
References
- Arborelius L, Skelton KH, Thrivikraman KV, Plotsky PM, Schulz DW, Owens MJ. Chronic administration of the selective corticotropin-releasing factor 1 receptor antagonist CP-154,526: behavioral, endocrine and neurochemical effects in the rat. J Pharm Exp Ther. 2000;294:588–597. [PubMed] [Google Scholar]
- Bloom FE, Battenberg EL, Rivier J, Vale W. Corticotropin releasing factor (CRF): immunoreactive neurones and fibers in rat hypothalamus. Regul Pept. 1982;4:43–48. doi: 10.1016/0167-0115(82)90107-0. [DOI] [PubMed] [Google Scholar]
- Breese GR, Knapp DJ, Overstreet DH. Stress sensitization of ethanol withdrawal-induced reduction in social interaction: inhibition by CRF-1 and benzodiazepine receptor antagonists and a 5-HT1A-receptor agonist. Neuropsychopharmacology. 2004;29:470–482. doi: 10.1038/sj.npp.1300282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks SP, Hennebry G, Croft AP, Thomas A, Little HJ. Effects of corticosterone on place conditioning to ethanol. Psychopharmacology (Berl) 2004;174:291–299. doi: 10.1007/s00213-003-1745-y. [DOI] [PubMed] [Google Scholar]
- Chen YL, Mansbach RS, Winter SM, Brooks E, Collins J, Corman ML, Dunaiskis AR, Faraci WS, Gallaschun RJ, Schmidt A, Schulz DW. Synthesis and oral efficacy of a 4-(butylethylamino)pyrrolo[2,3-d]pyrimidine: a centrally active corticotropin-releasing factor1 receptor antagonist. J Med Chem. 1997;40:1749–1754. doi: 10.1021/jm960861b. [DOI] [PubMed] [Google Scholar]
- Chester JA, Cunningham CL. Modulation of corticosterone does not affect the acquisition or expression of ethanol-induced conditioned place preference in DBA/2J mice. Pharmacol Biochem Behav. 1998;59:67–75. doi: 10.1016/s0091-3057(97)00320-1. [DOI] [PubMed] [Google Scholar]
- Chu K, Koob GF, Cole M, Zorrilla EP, Roberts AJ. Dependence-induced increases in ethanol self-administration in mice are blocked by the CRF1 receptor antagonist antalarmin and by CRF1 receptor knockout. Pharmacol Biochem Behav. 2007;86:813–821. doi: 10.1016/j.pbb.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabbe JC, Wahlsten D, Dudek BC. Genetics of mouse behavior: interactions with laboratory environment. Science. 1999;284:1670–1672. doi: 10.1126/science.284.5420.1670. [DOI] [PubMed] [Google Scholar]
- Fahlke C, Hard E, Eriksson CJ, Engel JA, Hansen S. Consequence of long-term exposure to corticosterone or dexamethasone on ethanol consumption in the adrenalectomized rat, and the effect of type I and type II corticosteroid receptor antagonists. Psychopharmacology (Berl) 1995;117:216–224. doi: 10.1007/BF02245190. [DOI] [PubMed] [Google Scholar]
- Fahlke C, Hard E, Hansen S. Facilitation of ethanol consumption by intracerebroventricular infusions of corticosterone. Psychopharmacology (Berl) 1996;127:133–139. doi: 10.1007/BF02805986. [DOI] [PubMed] [Google Scholar]
- Fahlke C, Hard E, Thomasson R, Engel JA, Hansen S. Metyrapone-induced suppression of corticosterone synthesis reduces ethanol consumption in high-preferring rats. Pharmacol Biochem Behav. 1994;48:977–981. doi: 10.1016/0091-3057(94)90208-9. [DOI] [PubMed] [Google Scholar]
- Finn DA, Snelling C, Fretwell AM, Tanchuck MA, Underwood L, Cole M, Crabbe JC, Roberts AJ. Increased drinking during withdrawal from intermittent ethanol exposure is blocked by the CRF receptor antagonist D-Phe-CRF(12–41) Alcohol Clin Exp Res. 2007;31:939–949. doi: 10.1111/j.1530-0277.2007.00379.x. [DOI] [PubMed] [Google Scholar]
- Funk CK, O’Dell LE, Crawford EF, Koob GF. Corticotropin-releasing factor within the central nucleus of the amygdala mediates enhanced ethanol self-administration in withdrawn, ethanol-dependent rats. J Neurosci. 2006;26:11324–11332. doi: 10.1523/JNEUROSCI.3096-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk CK, Zorrilla EP, Lee MJ, Rice KC, Koob GF. Corticotropin-releasing factor 1 antagonists selectively reduce ethanol self-administration in ethanol-dependent rats. Biol Psychiatry. 2007;61:78–86. doi: 10.1016/j.biopsych.2006.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehlert DR, Cippitelli A, Thorsell A, Le AD, Hipskind PA, Hamdouchi C, Lu J, Hembre EJ, Cramer J, Song M, McKinzie D, Morin M, Ciccocioppo R, Heilig M. 3-(4-Chloro-2-morpholin-4-yl-thiazol-5-yl)-8-(1-ethyl-propyl)-2,6-dimethyl- imidazo[1,2-b]pyridazine: a novel brain-penetrant, orally available corticotropin-releasing factor receptor 1 antagonist with efficacy in animal models of alcoholism. J Neurosci. 2007;27:2718–2726. doi: 10.1523/JNEUROSCI.4985-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilig M, Koob GF. A key role for corticotropin-releasing factor in alcohol dependence. Trends Neurosci. 2007;30:399–406. doi: 10.1016/j.tins.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamdar NK, Miller SA, Syed YM, Bhayana R, Gupta T, Rhodes JS. Acute effects of naltrexone and GBR 12909 on ethanol drinking-in-the-dark in C57BL/6J mice. Psychopharmacology (Berl) 2007;192:207–217. doi: 10.1007/s00213-007-0711-5. [DOI] [PubMed] [Google Scholar]
- Keller C, Bruelisauer A, Lemaire M, Enz A. Brain pharmacokinetics of a nonpeptidic corticotropin-releasing factor receptor antagonist. Drug Metab Dispos. 2002;30:173–176. doi: 10.1124/dmd.30.2.173. [DOI] [PubMed] [Google Scholar]
- Knapp DJ, Saiers JA, Pohorecky LA. In: Observations of novel behaviors as indices of ethanol withdrawal-induced anxiety, in Advances in Biomedical Alcohol Research. Taberner PV, Badaway AA, editors. Pergamon; New York: 1993. pp. 489–493. [PubMed] [Google Scholar]
- Li Z, Kang SS, Lee S, Rivier C. Effect of ethanol on the regulation of corticotropin-releasing factor (CRF) gene expression. Mol Cell Neurosci. 2005;29:345–354. doi: 10.1016/j.mcn.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Lundkvist J, Chai Z, Teheranian R, Hasanvan H, Bartfai T, Jenck F, Widmer U, Moreau JL. A non peptidic corticotropin releasing factor receptor antagonist attenuates fever and exhibits anxiolytic-like activity. Eur J Pharmacol. 1996;309:195–200. doi: 10.1016/0014-2999(96)00337-8. [DOI] [PubMed] [Google Scholar]
- Mansbach RS, Brooks EN, Chen YL. Antidepressant-like effects of CP-154,526, a selective CRF1 receptor antagonist. Eur J Pharmacol. 1997;323:21–26. doi: 10.1016/s0014-2999(97)00025-3. [DOI] [PubMed] [Google Scholar]
- Merchenthaler I, Vigh S, Petrusz P, Schally AV. Immunocytochemical localization of corticotropin-releasing factor (CRF) in the rat brain. Am J Anat. 1982;165:385–396. doi: 10.1002/aja.1001650404. [DOI] [PubMed] [Google Scholar]
- Navarro M, Cubero I, Knapp DJ, Thiele TE. MTII-induced reduction of voluntary ethanol drinking is blocked by pretreatment with AgRP-(83-132) Neuropeptides. 2003;37:338–344. doi: 10.1016/j.npep.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Olive MF, Koenig HN, Nannini MA, Hodge CW. Elevated extracellular CRF levels in the bed nucleus of the stria terminalis during ethanol withdrawal and reduction by subsequent ethanol intake. Pharmacol Biochem Behav. 2002;72:213–220. doi: 10.1016/s0091-3057(01)00748-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes JS, Best K, Belknap JK, Finn DA, Crabbe JC. Evaluation of a simple model of ethanol drinking to intoxication in C57BL/6J mice. Physiol Behav. 2005;84:53–63. doi: 10.1016/j.physbeh.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, Ford MM, Yu CH, Brown LL, Finn DA, Garland T, Jr, Crabbe JC. Mouse inbred strain differences in ethanol drinking to intoxication. Genes Brain Behav. 2007;6:1–18. doi: 10.1111/j.1601-183X.2006.00210.x. [DOI] [PubMed] [Google Scholar]
- Rivier C. Alcohol stimulates ACTH secretion in the rat: mechanisms of action and interactions with other stimuli. Alcohol Clin Exp Res. 1996;20:240–254. doi: 10.1111/j.1530-0277.1996.tb01636.x. [DOI] [PubMed] [Google Scholar]
- Rivier C, Imaki T, Vale W. Prolonged exposure to alcohol: effect on CRF mRNA levels, and CRF- and stress-induced ACTH secretion in the rat. Brain Res. 1990;520:1–5. doi: 10.1016/0006-8993(90)91685-a. [DOI] [PubMed] [Google Scholar]
- Rivier C, Lee S. Acute alcohol administration stimulates the activity of hypothalamic neurons that express corticotropin-releasing factor and vasopressin. Brain Res. 1996;726:1–10. [PubMed] [Google Scholar]
- Sabino V, Cottone P, Koob GF, Steardo L, Lee MJ, Rice KC, Zorrilla EP. Dissociation between opioid and CRF1 antagonist sensitive drinking in Sardinian alcohol-preferring rats. Psychopharmacology (Berl) 2006;189:175–186. doi: 10.1007/s00213-006-0546-5. [DOI] [PubMed] [Google Scholar]
- Schulz DW, Mansbach RS, Sprouse J, Braselton JP, Collins J, Corman M, Dunaiskis A, Faraci S, Schmidt AW, Seeger T, Seymour P, Tingley FD, III, Winston EN, Chen YL, Heym J. CP-154,526: a potent and selective nonpeptide antagonist of corticotropin releasing factor receptors. Proc Natl Acad Sci U S A. 1996;93:10477–10482. doi: 10.1073/pnas.93.19.10477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanagel R. Recent animal models of alcoholism. Alcohol Res Health. 2000;24:124–131. [PMC free article] [PubMed] [Google Scholar]
- Treutlein J, Kissling C, Frank J, Wiemann S, Dong L, Depner M, Saam C, Lascorz J, Soyka M, Preuss UW, Rujescu D, Skowronek MH, Rietschel M, Spanagel R, Heinz A, Laucht M, Mann K, Schumann G. Genetic association of the human corticotropin releasing hormone receptor 1 (CRHR1) with binge drinking and alcohol intake patterns in two independent samples. Mol Psychiatry. 2006;11:594–602. doi: 10.1038/sj.mp.4001813. [DOI] [PubMed] [Google Scholar]
- Valdez GR. Development of CRF1 receptor antagonists as antidepressants and anxiolytics: progress to date. CNS Drugs. 2006;20:887–896. doi: 10.2165/00023210-200620110-00002. [DOI] [PubMed] [Google Scholar]
- Valdez GR, Roberts AJ, Chan K, Davis H, Brennan M, Zorrilla EP, Koob GF. Increased ethanol self-administration and anxiety-like behavior during acute ethanol withdrawal and protracted abstinence: regulation by corticotropin-releasing factor. Alcohol Clin Exp Res. 2002;26:1494–1501. doi: 10.1097/01.ALC.0000033120.51856.F0. [DOI] [PubMed] [Google Scholar]
- Wahlsten D, Metten P, Phillips TJ, Boehm SL, II, Burkhart-Kasch S, Dorow J, Doerksen S, Downing C, Fogarty J, Rodd-Henricks K, Hen R, McKinnon CS, Merrill CM, Nolte C, Schalomon M, Schlumbohm JP, Sibert JR, Wenger CD, Dudek BC, Crabbe JC. Different data from different labs: lessons from studies of gene-environment interaction. J Neurobiol. 2003;54:283–311. doi: 10.1002/neu.10173. [DOI] [PubMed] [Google Scholar]
- Winer BJ, Brown DR, Michels KM. Statistical Principles in Experimental Design. 3. New York: McGraw-Hill Inc; 1991. [Google Scholar]
- Xu JF, Chen XQ, Du JZ, Wang TY. CRF receptor type 1 mediates continual hypoxia-induced CRF peptide and CRF mRNA expression increase in hypothalamic PVN of rats. Peptides. 2005;26:639–646. doi: 10.1016/j.peptides.2004.11.014. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP, Koob GF. The therapeutic potential of CRF1 antagonists for anxiety. Expert Opin Investig Drugs. 2004;13:799–828. doi: 10.1517/13543784.13.7.799. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP, Tache Y, Koob GF. Nibbling at CRF receptor control of feeding and gastrocolonic motility. Trends Pharmacol Sci. 2003;24:421–427. doi: 10.1016/S0165-6147(03)00177-9. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP, Valdez GR, Weiss F. Changes in levels of regional CRF-like-immunoreactivity and plasma corticosterone during protracted drug withdrawal in dependent rats. Psychopharmacology (Berl) 2001;158:374–381. doi: 10.1007/s002130100773. [DOI] [PubMed] [Google Scholar]


