Abstract
The risk for substance use disorders (SUD) is transmissible between generations via both genetic and environmental mechanisms. One path that is hypothesized to mediate this transmission and include both types of mechanisms is through faster physiological maturation, leading to suboptimal self-regulation, affiliation with deviant peers, and higher risk for conduct disorder (CD). Extending prior research, this hypothesis was tested in a longitudinal study. A sample of 478 males whose fathers were affected with SUD or psychiatrically normal was assessed prospectively at ages from 9−13 to 17−20. The DSM-III-R diagnoses were obtained using standard methodology. Blood testosterone was assayed by radioimmunoassay, and Tanner staging was used to evaluate sexual maturation. Peer deviance was evaluated by the Peer Delinquency Scale. Correlation and path analysis, Cox proportional hazard regression, and growth curve modeling were used to determine the relationships between the variables. The data support the hypothesis that parental SUD liability influences the rate of physiological maturation in offspring, which in turn is related to affiliation with deviant peers and an elevated rate of the development of CD and SUD.
Keywords: family study, fathers, mothers, puberty, disruptive behavior, peer environment, addiction risk
1. Introduction
The risk for substance use disorders (SUD) is transmissible over generations – to a large degree due to its significant heritability (Kendler et al., 2003). As many other behavioral traits, SUD liability is also characterized by a complex developmental trajectory (Tarter and Vanyukov, 1994; Vanyukov and Tarter, 2000). The direction of this trajectory at each point depends on the previous value of the liability phenotype and on the factors determining availability of drugs and incentives to use them. The two categories of factors partially overlap and include both organismic biobehavioral and environmental influences.
It has been hypothesized that the rate of physiological maturation may be associated with the risk for behavioral disorders including SUD (Tarter et al., 1999). It is well known that sex hormones, particularly androgens, affect personality and behavioral characteristics related to self-regulation (e.g., Rubinow and Schmidt, 1996), which is associated with the risk for SUD (Tarter et al., 2004). Adolescence is a critical period for the formation of the brain mechanisms of self-regulation. These mechanisms are related to the ontogenetic brain maturation, involving synaptic pruning, as well as to the decrease in gray matter of the frontal lobe from its maximum at 11−12 years of age (Giedd et al., 1999), and neural plasticity in response to the increased environmental demands invoked by sexual maturation and concomitant changes in social interactions. Relatively precocious sexual maturation, progressing ahead of the development of behavioral control, may be associated with elevated impulsivity, aggression and antisociality, and promote affiliation with older and deviant peers. Such peer environment, further contributing to maladjustment and substance use, may be actively sought by the individual, manifesting homophily (tendency to associate with phenotypically similar individuals) that has deep evolutionary roots (Vanyukov, 2003). This homophily will result in phenotype-environment correlations and, insofar as the respective personality/behavioral traits are heritable, in active genotype-environment correlations (Scarr and McCartney, 1983). These correlations may also be detectable at the level of intermediate (e.g., biochemical, endocrinological, physiological) characteristics associated with the behavioral traits.
Whereas the rate of sexual maturation is related to the risk for behavioral deviance, it has also been shown to be associated with environmental adversities. Belsky et al. (1991), based on evolutionary considerations, hypothesized that the child's perceived lack of resources will lead to the development of “behavior patterns that function to reduce the age of biological maturation” (p. 650). This hypothesis has received empirical support, e.g. relating father's absence as a possible stress factor to earlier menarche (Moffitt et al., 1992), although it may not necessarily be due to resource-related stress, and the presence of the mother's new partner may be of greater relevance than the father's absence (Ellis and Graber, 2000). Research in this area has mostly focused on females, although Belsky and al.'s argumentation is also applicable to males. In turn, off-time maturation, whether precocious or delayed, may be related to an increased risk of psychopathology including SUD and its common developmental precursors, disruptive behavior disorder (Tarter et al., 1999). Indeed, both late and early maturing males have been reported to have a higher risk for substance use than “on-time maturers” (Graber et al., 2003). A previous study on a smaller subsample of the current sample of prepubescent (10−12 year-old) boys found somewhat lower testosterone levels in sons of fathers with SUD (Dawes et al., 1999). However, a later study (Kirillova et al., 2001) employing longitudinal data showed that the rate of sexual maturation, evaluated by Tanner staging and testosterone level, was positively related to SUD in parents, and might contribute to the mechanisms of the intergenerational transmissibility of SUD risk, particularly through influencing sensation seeking.
The goal of the present study was to extend this research and determine, in a longitudinal dataset, the relationships between parental SUD and the son's rate of sexual maturation, rate of conduct disorder (CD) development (indicator of antisociality and a frequent precursor to SUD) and rate of SUD development. We hypothesized that parental SUD, via genetic and/or environmental mechanisms, is related to the males' faster sexual maturation, which, in turn, leads to faster development of behavioral deviance, including SUD, in part through active phenotype-environment correlation.
2. Methods
2.1. Subjects
The sample of nuclear families of adult male probands was ascertained by the Center for Education and Drug Abuse Research (CEDAR), a longitudinal family/high-risk study of SUD (Tarter and Vanyukov, 2001). The study was approved by the University of Pittsburgh Institutional Review Board. Written informed consent was obtained from the subjects prior to implementing the research protocol. Recruitment was via newspaper advertisements, social service agencies, substance abuse treatment programs and various other media. Targeting etiological mechanisms that are shared in common by drug use disorders (Vanyukov et al., 2003a,b), the probands were required to have a DSM-III-R diagnosis (DSM-IV was introduced after this study started) of substance abuse or dependence disorder (substance use disorder, SUD+) related to the use of any illicit drug, or had no Axis I or II psychiatric disorder (SUD-), and to have a preadolescent child at the time of ascertainment and first assessment, and the biological mother of the child available for the study. The sample analyzed herein consists of sons (one per family) of 478 European-American and African-American probands (78 and 22%, respectively). The sample was limited to boys because the female sample's accrual started later than the males', and it is insufficient for longitudinal analyses. These individuals were prospectively assessed at four consecutive visits, at ages 9−13 (mean age±SD, 10.9±0.9, n=478), 11−15 (12.9±1.0, n=388), 15−17 (15.5±0.6, n=331), and 17−20 (18.2±0.6, n=221). The progressive decrease in the sample size is largely related to the protracted accrual of the baseline sample. The recent analyses of the sample indicate no attrition bias (Kirisci et al., 2006; Tarter et al., 2006 in press).
2.2. Psychiatric diagnosis
An expanded version of the Structured Clinical Interview for DSM-III-R-outpatient version (SCID-OP) (Spitzer et al., 1987) was administered by experienced research associates to obtain psychiatric diagnoses for adults. The expanded SCID evaluates current episode (past 6 months) and worst past episode of psychopathology (before the past 6 months). The children were assessed for CD and SUD with the Kiddie-Schedule for Affective Disorders and Schizophrenia for School Age Children-Epidemiological Version (K-SADS-E) (Orvaschel and Puig-Antich, 1987), a widely used semistructured diagnostic interview for children and adolescents aged 6−17, covering current and lifetime psychopathology. The standard procedure is to first interview the mother about the psychiatric status of the child. During the subsequent interview of the child, the interviewer attempts to resolve any discrepancies between parent and child in case of disagreement. A summary score is obtained based on positive ratings of either informant. The diagnoses were finalized at a consensus conference according to the best estimate procedure (Kosten and Rounsaville, 1992).
To analyze the relationship between parental liability to SUD and the rate of phenotypic development (physiological maturation, rate of disorder development) in offspring, we used the number of affected parents (NAP), from 0 to 2 as the indicator of familial load (Kirillova et al., 2001).
2.3. Peer deviancxe
Peer deviance was evaluated at visits 1−3 using Peer Delinquency Scale (Loeber, 1989), a Lykert-type instrument reflecting the child's assessment of his friends' involvement in activities of various severity, from skipping school to armed robbery. The skewed score distribution was log-transformed for analysis.
2.4. Laboratory methods
A blood sample was collected at 8:30 a.m. and assayed in triplicate to determine testosterone (T) concentrations using DSL-4000 Active kits (Diagnostic Systems Laboratories, Webster, TX) at visits 1−3. The intra-assay coefficient of variation (CV) was 6.9%; the inter-assay CV was 8.1%. The detection limit was 0.08 ng/mL. The T concentrations were log-transformed for analyses. Sexual maturation was assessed at visits 1−4 by a trained registered nurse using Tanner staging (TS; Marshall and Tanner, 1970), based on pubic hair and genital development (stages 1−5).
2.5 Statistical methods
Correlation analysis (Pearson's correlations for continuous variables and Spearman's rank correlations, ρ, where NAP or diagnoses were involved) and path analysis were used to examine relationships between the variables. All variables were standardized for path analysis. Indirect effects (mediation) were evaluated using Sobel test (Baron and Kenny, 1986; Sobel, 1982; Aroian, 1944; MacKinnon et al., 2002; Preacher and Hayes, 2004). The relationships of independent variables with the rates of development were examined using survival analysis (Cox proportional hazard regression). The target event for the survival analysis of sexual maturation was reaching Tanner stage 5 (maturity) by pubic hair or genital development. Time was indicated by age in years. For all terms, two-tailed probability levels are presented. The relationships between patterns of developmental changes were assessed using path analysis and growth curve modeling. The χ2, root mean square error of approximation (RMSEA) and Akaike Information Criterion (AIC) were used as fit indices. An insignificant χ2 value and an RMSEA < 0.05 indicate a satisfactory fit, and a lower AIC suggests a more parsimonious model in nested model comparisons. Nested models (differing in that the elements present in one, more general, model are omitted from another) are also compared by evaluating significance of a χ2 value equal to the difference of the respective χ2 values (of the more general model and a submodel), with the number of degrees of freedom equal to the difference between the respective numbers of degrees of freedom. Analyses were conducted using SPSS® for Windows® release 13, and Amos™ 5.
3. Results
3.1 Parental SUD and phenotypic development
3.1.1.Parental SUD
The number of affected parents (NAP) was computed for all individuals for whom SUD diagnoses of both parents were assessed (460 out of 478 subjects at the baseline assessment, all missing diagnostic information is maternal). Fifty percent of fathers and 18% of mothers were diagnosed with a SUD. Only 13% of affected mothers (5% of the total mothers' sample) had nonaffected husbands, whereas 69% of affected fathers had nonaffected wives. In 15% of families, both parents were affected (NAP=2), in 37%, either the father or the mother was affected (NAP=1), and in 48%, none of them (NAP=0). These figures indicate homogamy (mate similarity) for SUD liability, particularly among female addicts, consistent with our prior data (Vanyukov et al., 1994, 1996).
3.1.2. Sexual maturation and parental SUD
The results of Cox regression analysis (Table 1) indicate positive association of the rate of sexual maturation with NAP. When NAP was treated as a quantitative variable, congruent with its meaning as an index of familial SUD load, the hazard ratio was HR=1.42 (P=0.006). Stratification by ethnicity did not change the result (HR=1.45, P=0.005). The results of an analysis where NAP was treated as a categorical variable to evaluate the dose effect, with “no affected parents” as a reference category, suggest that the effect of this variable was mostly due to the difference between the individuals who had both parents affected and children with one or no parents with SUD (those with NAP=2 had twice the rate of maturation of those with NAP=0 in the age interval measured). A trend in the same direction was, however, observed for those with one vs. no affected parents.
Table 1.
Cox regression analysis of the influence of parental SUD load on sexual maturation (Tanner staging) rate
| B | SE | Wald | df | P | HR | 95.0% CI for HR | ||
|---|---|---|---|---|---|---|---|---|
| Lower | Upper | |||||||
| Non-stratified | ||||||||
| |
.353 |
.124 |
8.030 |
1 |
.005 |
1.423 |
1.115 |
1.816 |
| Stratified | ||||||||
| |
.370 |
.129 |
8.268 |
1 |
.004 |
1.448 |
1.125 |
1.864 |
| NAP categorical | ||||||||
| NAP | 8.140 | 2 | .017 | |||||
| NAP(1) | .326 | .222 | 2.142 | 1 | .143 | 1.385 | 0.895 | 2.142 |
| NAP(2) | .712 | .252 | 7.982 | 1 | .005 | 2.039 | 1.244 | 3.341 |
Note: NAP, number of affected parents; HR, hazard ratio (exp[B])
3.1.3. CD and SUD development and parental SUD
NAP was related to both rates (Table 2). In both cases the results of stratified analyses did not considerably differ from the non-stratified data. Parallel to the results obtained for sexual maturation, the largest effect was observed for two affected parents. It was also especially pronounced for conduct disorder, an earlier event than SUD (in the 41 individuals who had developed both CD and SUD, the former preceded the latter on average by 2.6 years, P<0.00001). (As follows from comparison, the diagnosis of CD was present in 56% of those who developed SUD, but only in 7% of those who did not.) The presence of a CD diagnosis increased the hazard for SUD development 9-fold (HR=9.08; 95% CI: 5.698−14.454; P<1×10−6). The presence of CD was also associated with the increased rate of sexual maturation, HR=1.70 (1.105−2.629; P=0.016).
Table 2.
Cox regression analysis of the influence of parental SUD load (NAP) on the rate of SUD and CD development
| Dependent | B | SE | Wald | df | P | HR | 95.0% CI for HR | ||
|---|---|---|---|---|---|---|---|---|---|
| |
|
|
|
|
|
|
|
Lower |
Upper |
| SUD rate | Non-stratified | ||||||||
| |
.487 |
.172 |
8.005 |
1 |
.005 |
1.628 |
1.161 |
2.281 |
|
| Stratified | |||||||||
| |
.438 |
.177 |
6.107 |
1 |
.013 |
1.549 |
1.095 |
2.192 |
|
| NAP categorical | |||||||||
| NAP | 9.454 | 2 | .009 | ||||||
| NAP(1) | .262 | .320 | .669 | 1 | .413 | 1.300 | .694 | 2.435 | |
| |
NAP(2) |
1.011 |
.333 |
9.229 |
1 |
.002 |
2.748 |
1.431 |
5.274 |
| CD rate | |||||||||
| Non-stratified | |||||||||
| |
.840 |
.160 |
27.598 |
1 |
.000000 |
2.315 |
1.693 |
3.167 |
|
| Stratified | |||||||||
| |
.802 |
.162 |
24.365 |
1 |
.000001 |
2.229 |
1.621 |
3.065 |
|
| NAP categorical | |||||||||
| NAP | 25.510 | 2 | .000003 | ||||||
| NAP(1) | 1.195 | .326 | 13.436 | 1 | .000247 | 3.303 | 1.744 | 6.258 | |
| NAP(2) | 1.732 | .345 | 25.136 | 1 | .000001 | 5.651 | 2.872 | 11.122 | |
3.2 Testosterone and phenotypic development
The level of testosterone (T) in the blood predictably grew over the assessments at visits 1 through 3, from mean±SD=1091.6±1524.6 to 2830.3±2178.5 to 6448.0±2335.1 pg/mL, respectively. It also correlated with respective Tanner stage measurements (Spearman's ρ, which did not differ for the genital and pubic hair development, were 0.5, 0.6, and 0.3, all P<0.001).
Parallel to the results relating the risk for the disorder to the rate of sexual maturation as measured by Tanner staging, the T level at visits 1 and 2 was related to the rate of CD development, independently from parental SUD (Table 3 shows the results of the Cox regression analysis). A trend in the same direction (P=0.074) was observed for the T level at visit 3. No correlation was observed between the NAP and T levels. No interaction effect was found for parental SUD and the T level in their influence on the phenotypic development. Similarly, for the SUD development rate, the most significant relationship was observed for the T level at visit 2, with a trend for visit 1 and no effect detected for visit 3.
Table 3.
Influence of testosterone on the rate of CD and SUD development
| Dependent | Visit | B | SE | Wald | df | P | HR | 95.0% CI for HR | |
|---|---|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||||
| CD rate | |||||||||
| 1 | |||||||||
| |
.244 |
.118 |
4.283 |
1 |
.038 |
1.276 |
1.013 |
1.608 |
|
| 2 | |||||||||
| |
.385 |
.175 |
4.827 |
1 |
.028 |
1.470 |
1.042 |
2.072 |
|
| 3 | |||||||||
| |
|
.716 |
.401 |
3.184 |
1 |
.074 |
2.046 |
.932 |
4.489 |
| SUD rate | |||||||||
| 1 | |||||||||
| |
.203 |
.115 |
3.129 |
1 |
.077 |
1.225 |
.978 |
1.533 |
|
| 2 | |||||||||
| |
.603 |
.201 |
9.013 |
1 |
.003 |
1.827 |
1.233 |
2.708 |
|
| 3 | |||||||||
| .321 | .362 | .788 | 1 | .375 | 1.379 | .678 | 2.804 | ||
3.3 The role of peer deviance
To address the hypothesis that one mechanism for the observed relationships is earlier detachment from parents, affiliation with deviant peers, and thus earlier access to illicit drugs in earlier maturing individuals, we examined the relationships of peer deviance in this framework. Consistent with the hypothesis, the relationship between familial SUD load and peer delinquency (PD) strengthened linearly over time (ρ1=0.132, P=0.005, n=455; ρ2=0.171, P=0.001, n=394; ρ3=0.252, P=2×10−6, n=340; the subscript indicates visit), as did the relationship between PD and the CD outcome (ρ1=0.207, P=6×10−5, n=369; ρ2=0.332, P=1×10−6, n=320; ρ3=0.465, P = 1×10−6; n=278), as well as with the SUD outcome (ρ1=0.228, P=1×10−5, n=369; ρ2=0.304, P=1×10−6, n=320; ρ3=0.339, P=1×10−6; n=278).
Table 4 presents correlations between PD, testosterone level and Tanner stage. The T level at visit 1 (T1) was related to peer delinquency at visit 2 (PD2), but not at visit 1, and the visit 2 T level (T2) is related to PD at the same age and that at visit 3. It is noteworthy that PD at visit 1 (PD1) was related to T2. The relationship of PD with TS is more consistent than that with T.
Table 4.
Correlations between peer delinquency, testosterone level, and Tanner stage
| PD1 | PD2 | PD3 | T1 | T2 | T3 | |
|---|---|---|---|---|---|---|
| PD1 | 1 | .02 | .19** | .07 | ||
| PD2 | .36*** | 1 | .27*** | .28*** | .03 | |
| PD3 | .31*** | .47*** | 1 | .06 | .12 | .06 |
| TS1 | .16*** | .23*** | .08 | .48*** | .42*** | .24** |
| TS2 | .26*** | .35*** | .17* | .42*** | .59*** | .21** |
| TS3 | .03 | .14* | .05 | .24** | .21** | .30*** |
Notes: PD — peer delinquency; T — testosterone; TS — Tanner stage. Numbers following letters indicate subjects' visits.
P<0.05;
P<0.01;
P<0.001
A non-trivial relationship exists between age and T and PD in this sample. Age correlated cross-sectionally with both variables (correlation coefficients for T and PD at visits 1, 2 and 3, respectively: 0.516, P=1.8×10−24, and 0.157, P=0.021; 0.519, P=1.4×10−19, and 0.278, P=2.6×10−8; and 0.153, P=0.023, and 0.082, ns). Therefore, the visit 2 T-PD correlation might be due to their common relationship to age at that visit. Nevertheless, it would be inappropriate to model age as merely a nuisance variable and regress it out of both T and PD to evaluate their true association, because age variation at this developmental stage is in fact a proxy indicator of developmental changes, of which variation in the T level is another indicator. Indeed, T2 was a significant mediator of the age-PD2 relationship (Sobel test P=0.018 for the compound path). The path coefficients (standardized partial regression coefficients) were, respectively, 0.511 (P<0.001) and 0.165 (P=0.015) for the age→T2 and T2→PD2 components of the mediation chain. The direct age→PD2 path was also significant (0.192, P=0.001), leaving the possibility of other significant mediators of this developmental relationship, in addition to the testosterone level.
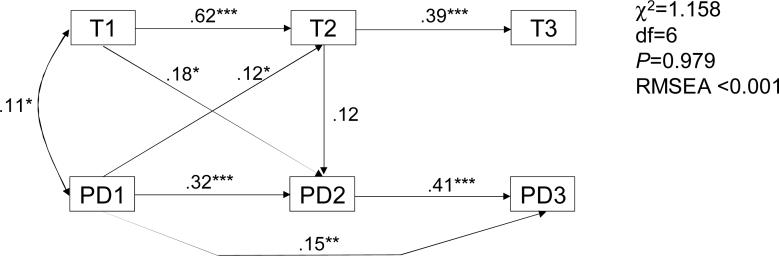
The longitudinal T-PD relationships were further explored using path analysis. The best-fitting model relating T and PD values across time is presented in Figure 1. Only significant paths are shown. As can be seen, the physiological process indicated by the correlation between T1 and T3 was, as expected, completely mediated by T2; the indirect path is highly significant (Sobel test P=9×10−8). In turn, the T2-PD2 association does not seem to result from the direct relationship (only a trend observed at P=0.100), but may be accounted by for the sum of compound paths PD2→PD1→T2 and PD2→T1→T2. Whereas the direct path PD1→PD3 was significant (P=0.002), this relationship was also mediated in part by PD2 (Sobel test P=1.3×10−7). It should be noted that, whereas the T2→PD2 direct path approached significance in this model (path coefficient 0.121, P=0.103), the reversal of its direction also did not change the model fit and only slightly reduced the effect estimate (0.088, P=0.104).
Figure 1.
Path analysis of the developmental relationships between testosterone and peer deviance (*p<.05; **p<.01; ***p<.001; error terms omitted). T1, T2, and T3 are testosterone level at visits 1, 2, and 3, respectively; PD1, PD2, and PD3 are peer delinquency levels at the same visits.
Adding NAP to the model as the upstream variable, resulting in good fit (χ2=5.235, df=9, P=0.813, RMSEA<.001), suggested no direct effect of the SUD familial load on the testosterone levels, but showed its direct contribution to PD at all time points. While this direct effect remained approximately the same across the time points, the total NAP effects increased (0.171, 0.252, 0.292), accounted for by the compound (mediated) paths including the previous PD values, and consistent with the growing NAP-PD correlations. The reversal of the direction of the T1→PD1 path in the model did not result in an appreciable deterioration of the model fit (from P=0.861 to P=0.813). The effect of T2 on PD2 appeared to be more pronounced in this model (0.141, P=0.053). It is noteworthy that the mediated path NAP→PD1→T1 approached significance (Sobel test P=0.088), as did the NAP→PD1→T2 path (P=0.062).
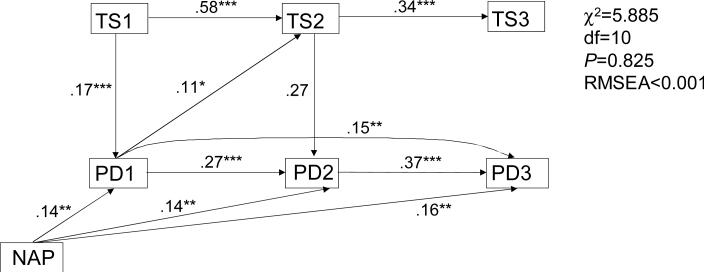
Replacing the testosterone level in the path analysis with another indicator of physiological maturation, the Tanner stage, did not substantially change the pattern of relationships. As follows from the Figure 2, presenting the best-fitting model, the paths connecting Tanner stages were comparable to those relating the testosterone levels. The middle time point TS value, TS2, as expected, completely mediated the relationship between TS1 and TS3. The model involving Tanner staging, however, was much less tolerant to reversals of the paths and thus more suggestive of the direction of relationships between the two processes (physiological maturation and peer affiliation). In particular, the reversal of the TS1→PD1 path resulted in poorly failing models (P <.001) regardless of the directions of other paths. This analysis, testing the relationship between the temporal continuities in the bivariate distributions, does not, however, take into account the changes (growth) in the variables' values over time. To examine these changes, a parallel growth curve analysis was conducted.
Figure 2.
Path analysis of the developmental relationships between familial SUD load, Tanner staging and peer deviance. TS1, TS2 and TS3 are Tanner stage values at visits 1, 2 and 3, respectively.
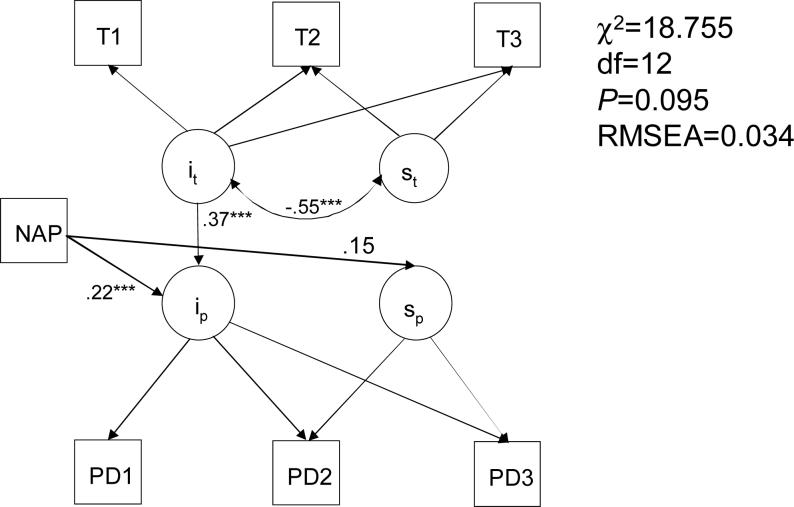
The results of fitting a parallel growth model (χ2=16.815, df=9, P=0.052, RMSEA=0.043), relating testosterone and peer deviance growth trajectories, supported the conclusion that the connection between the two processes was largely due to the direct relationship between the outset values (intercept parameters) of the two growth trajectories (subjects with higher outset T levels had more deviant peers at that age), with a possible connection between the intercept parameter of the PD growth (ip) and the slope of the T model (st) via its intercept (it), due to the correlation between the latter two (−0.552). The reversal of the significant it→ip path (0.403, p <.001) in this model, which could be suggested by the results of the above path analysis, did not lead to acceptable fit. Adding NAP to the model (Figure 3) as the upstream covariate resulted in a model with satisfactory fit, supporting significant relationship between NAP and ip (path coefficient 0.222, P <.001), the relationship between NAP and sp approaching significance (0.151, P=0.117), and the significant it→ip path. As in the above model, a significant negative correlation was estimated between the T intercept and slope (−0.551, P<.001), consistent with the expectation that growth in the testosterone level is contingent on the closeness of the outset value to a mature level. Growth models employing Tanner staging did not have satisfactory fit, likely because of the temporally nonlinear character of the relationship between the two processes.
Figure 3.
Growth curve modeling of the relationship between familial SUD load, testosterone level and peer delinquency
The results of Cox regression analysis indicated that, in parallel with the increasing contribution of the familial SUD load to PD shown above, the latter is, increasingly with age, related to the rate of SUD development (HR [95% CI] for the three PD measures were, respectively, 1.663 [1.277−2.165], P=0.0016, 2.144 [1.603−2.867], P=2.7×10−7, and 3.242 [2.244−4.683], P=3.7×10−10).
4. Discussion
This study is a continuation of research testing a model of SUD etiology that relates the transmissibility of SUD liability to the rate of physiological maturation and psychological regulation (Tarter et al., 1999; Kirillova et al., 2001). It is well established that the heritability of liability to SUD is largely non-drug-specific (Tsuang et al., 1998; Kendler et al., 2003a). There is also a substantial genetic overlap between liabilities to SUD and externalizing behavior (Kendler et al., 2003b), including conduct disorder (CD) symptoms (Button et al., 2006). The genetic commonality between heritable disinhibitory/externalizing behavioral traits and SUD liability (Young et al., 2000; Krueger et al., 2002) is consistent with behavior dysregulation as a major component of SUD liability (Tarter et al., 1999, 2004).
A prior review article, pointing out the relationship between early menarche and higher SUD severity in females, hypothesized that maturational status may also be related to the risk for SUD in males (Tarter et al., 1999). The relationship, in contrast to that in females, was, however, hypothesized to connect higher SUD risk with slower maturation in boys. While noting the scarcity of empirical evidence, that supposition was based on contradictory data for delayed pubertal maturation and antisocial behavior, as well as on an earlier preliminary finding on a subsample of participants in the current study, suggesting lower testosterone levels in sons of SUD fathers compared to sons of controls (Dawes et al., 1999). Only baseline data, however, were used in that study, with subjects in prepuberty (age ∼10−12), in contrast to the longitudinal data of the current study. The marginally significant (P=0.04) result was not replicated on a larger sample in the current study, as the group differences did not reach significance at any of the three age points available (data not shown).
The results reported here are consistent, however, with our more recent previous study (Kirillova et al., 2001), which showed that parents' SUD liability (number of affected parents) is positively related to their sons' rates of sexual maturation. Whereas parental SUD is unsurprisingly related to the rates of offspring's CD and SUD development, the rate of sexual maturation is also itself related to the risk for CD, thus suggesting that parental contribution to the latter is in part mediated by the maturation rate.
This parental contribution can be genetic and/or environmental. On the genetic side, both CD and SUD liabilities are significantly heritable and genetically correlated (Button et al., 2006; Krueger et al., 2002). Genetic variation elevating the rate of physiological maturation may lead to earlier and incomplete (or suboptimal) brain maturation, particularly in the prefrontal cortex (PFC) that matures last and plays an important role in behavior regulation. For instance, low executive cognitive functioning, subserved by the PFC, is associated with antisociality (Giancola et al., 1998). Similar effects may result from environmental stress (Teicher et al., 2002). Germane to these hypotheses, cortical thickness in individuals with “superior”, in contrast to “high” and “average”, intelligence peaked later (11 vs. 7−8 years), perhaps reflecting a later end of synaptic proliferation, and then decreased faster (Shaw et al., 2006a). Children with ADHD, on the other hand, start with lower thickness, further dropping over adolescence overall in parallel with the changes in controls (Shaw et al., 2006b). The CD-related brain volumetric data (Kruesi et al., 2004), while similar, did not reach significance (perhaps due to a small sample).
Both genetic and environmental factors may contribute to earlier maturation in the offspring of SUD parents, promoting affiliation with deviant peers. In females, familial stress, as predicted by the Belsky et al., (1991) theory, was associated with earlier menarche (Moffitt et al., 1992; Wierson et al., 1993; Ellis and Garber, 2000). The data on males in this respect are scarce. This study suggests that similar mechanisms may operate in males and contribute to the transmission of the risk for SUD. The Belsky et al. theory also does not exclude the possibility of an alternative, genetic, explanation of variation in development (Comings et al., 2002), although the latter work was related to the androgen receptor gene that is X-linked and thus cannot mediate the father-son transmission.
The complexity of the system is further exacerbated by the possibility that certain genotypes, regardless of whether they are themselves associated with increased behavioral dysregulation, can facilitate the effect of adverse environment (Caspi et al., 2002). The influence of genes and vertically transmitted environment, which underlie the transmissibility of SUD liability, on the trajectory of individual behavioral development may be realized not only directly through phenotypic variation, but also via their contribution to the formative aspects of peer environment. The effect of parental SUD liability phenotype on peer deviance, growing over time (due to both immediate and lagged effects, as follows from our data), is consistent with both growing detachment from parents and the increasingly active homophilic choice of peers. Inasmuch as homophily results from the individual's selection into, and/or his choice of, a behaviorally similar peer group, this phenotypic correlation will induce higher genetic (and environmental) similarity between the members of the group, such as a gang,—akin to the effects of assortative mating (Vanyukov, 2003; Guo, 2006). This will further augment the active genotype-environment correlation, insofar as the environmental choice (peer behavior) will be in part genetically mediated. Sharing the same friends is more likely between monozygotic than dizygotic twins (Walden et al., 2004), indicating genetic contribution to peer selection. The same work showed that peer deviance, including substance use, along with parent-child relationship problems, contribute to substance use variation in adolescents as environmental factors. The models employed therein were not designed to test mediation of parental influence by peer deviance (and did not include parental SUD), leaving that mediation possibility open. Such possibility is consistent with our results of path and growth curve analysis indicating influence of parental SUD on the offspring's deviant peer affiliation trajectory. The relationships between parental SUD, peer deviance and the indicators of physiological maturation suggest a mechanism for active genotype-environment correlation.
This mechanism, along with the straightforward developmental connections between the values of a variable at different time points, includes an apparent effect of the testosterone level (and Tanner stage) and peer deviance on each other's development. Our finding suggesting the visit 1 Tanner stage (TS) influences peer deviance, and not the opposite, may suggest the choice for the direction of the path for the other indicator of sexual maturation used, the testosterone level (T), and PD at that time point. The more pronounced relationship of PD with the TS, compared to the T level, as evidenced also by higher correlations for TS, is consistent with our prior findings. A possible explanation is that the visible signs of maturation, which depend not only on the hormone level, are of greater importance for behavior regulation and peer selection than the testosterone level per se (Kirillova et al., 2001).
The reciprocal relationship between testosterone and social interactions, e.g., social dominance/potency, has been noted previously (Mazur and Booth, 1998; Rowe et al., 2004; Archer, 2006; Reynolds et al., in press). This relationship was found to be moderated by peer deviance: it was stronger among boys who did not have deviant peers, decreasing with growth in peer deviance, although leaving a possibility that different indicators of dominance may need to be used in different contexts (Rowe et al., 2004). The latter work did not, however, seek to determine the relationship between testosterone (or pubertal maturation) and peer deviance.
Testosterone was positively associated with externalizing behavior in boys of different ages (Archer, 2006), consistent with our data that support not only its association with the rate of CD and SUD development, but also the association between the rate of sexual maturation and the risk for CD. In turn, CD is strongly related with the risk for SUD, thus possibly connecting the latter with the testosterone level and maturation rate in adolescents. The finding that both testosterone and peer deviance in younger (mean age ∼11) children are cross-predictive of the next visit's (age ∼13) values extends the data on the reciprocity of the influence of testosterone and peer environment. The direct contribution of the parental SUD load to peer deviance and its growth is consistent with detachment from parents (possibly more pronounced if the latter have SUD), as well as with direct and indirect vertical environmental transmission (the contagion effect of parental deviance and the adverse environmental consequences of parental SUD promoting substance use in the offspring), and with genetic mechanisms of SUD inasmuch as the environmental choice is heritable.
Some caveats should be noted. The offspring sample has not yet passed through the age of risk for SUD, and the sampling was protracted, resulting in censored cases. Additional censoring occurs due to the incomplete observation of the survival time, because sexual maturation (Tanner stage 5) was timed according to the age at the visit rather than the actual (unobserved) age of reaching stage 5 (interval-censored data). Such data might substantially bias results of binary outcome analysis, such as logistic regression. They are, however, well handled by the Cox model we employed (Afifi and Clark, 1990; Hosmer and Lemeshow, 1999). The other types of longitudinal analysis used provide convergent support to the results. In this research, we did not concurrently study the role of other hormones and numerous factors that contribute to pubertal development and brain remodeling in adolescence, forming basis for behavior regulation involved in CD and SUD risk (Sisk and Foster, 2004). Despite noted parallels, these findings may not extend to females, which need to be studied separately. Whereas the sample decreased over the consecutive visits, attrition is unlikely to bias the results, since there are no differences between the subjects who have and have not been available for assessment at visit 4 for any of the analyzed variables (data not shown).
In sum, this study supports the hypothesis that the familial SUD risk transmission in males may be in part mediated by a developmental process involving earlier sexual maturation, growing homophilic affiliation with delinquent peers, and behavioral dysregulation manifesting in CD.
Acknowledgments
This research was supported by grants P50DA005605, R01DA019157, K02DA018701 and K02DA017822 from the National Institute on Drug Abuse. We are thankful to Dr. Judith S. Gavaler, Barbara Dilettuso, Kathleen Hartle and the CEDAR staff for their help and support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Afifi AA, Clark V. Computer-Aided Multivariate Analysis. Van Nostrand Reinhold Company; New York, NY: 1990. [Google Scholar]
- Archer J. Testosterone and human aggression: an evaluation of the challenge hypothesis. Neuroscience & Biobehavioral Reviews. 2006;30:319–345. doi: 10.1016/j.neubiorev.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Aroian LA. The probability function of the product of two normally distributed variables. Annals of Mathematical Statistics. 1944;18:265–271. [Google Scholar]
- Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. Journal of Personality and Social Psychology. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Belsky J, Steinberg L, Draper P. Childhood experience, interpersonal development, and reproductive strategy: an evolutionary theory of socialization. Child Development. 1991;62:647–670. doi: 10.1111/j.1467-8624.1991.tb01558.x. [DOI] [PubMed] [Google Scholar]
- Button TM, Hewitt JK, Rhee SH, Young SE, Corley RP, Stallings MC. Examination of the causes of covariation between conduct disorder symptoms and vulnerability to drug dependence. Twin Research and Human Genetics. 2006;9:38–45. doi: 10.1375/183242706776402993. [DOI] [PubMed] [Google Scholar]
- Caspi A, McClay J, Moffitt TE, Mill J, Martin J, Craig IW, Taylor A, Poulton R. Role of genotype in the cycle of violence in maltreated children. Science. 2002;297:851–854. doi: 10.1126/science.1072290. [DOI] [PubMed] [Google Scholar]
- Comings DE, Muhleman D, Johnson JP, MacMurray JP. Parent-daughter transmission of the androgen receptor gene as an explanation of the effect of father absence on age of menarche. Child Development. 2002;73:1046–1051. doi: 10.1111/1467-8624.00456. [DOI] [PubMed] [Google Scholar]
- Dawes MA, Dorn LD, Moss HB, Yao JK, Kirisci L, Ammerman RT, Tarter RE. Hormonal and behavioral homeostasis in boys at risk for substance abuse. Drug and Alcohol Dependence. 1999;55:165–176. doi: 10.1016/s0376-8716(99)00003-4. [DOI] [PubMed] [Google Scholar]
- Ellis BJ, Graber J. Psychosocial antecedents of variation in girls' pubertal timing: maternal depression, stepfather presence, and marital and family stress. Child Development. 2000;71:485–501. doi: 10.1111/1467-8624.00159. [DOI] [PubMed] [Google Scholar]
- Giancola PR, Mezzich AC, Tarter RE. Executive cognitive functioning, temperament, and antisocial behavior in conduct-disordered adolescent females. Journal of Abnormal Psychology. 1998;107:629–641. doi: 10.1037//0021-843x.107.4.629. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL. Brain development during childhood and adolescence: a longitudinal MRI study. Nature Neuroscience. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Graber JA, Seeley JR, Brooks-Gunn J, Lewinsohn PM. Is pubertal timing associated with psychopathology in young adulthood? Journal of the American Academy of Child and Adolescent Psychiatry. 2003;43:718–726. doi: 10.1097/01.chi.0000120022.14101.11. [DOI] [PubMed] [Google Scholar]
- Guo G. Genetic similarity shared by best friends among adolescents. Twin Research and Human Genetics. 2006;9:113–121. doi: 10.1375/183242706776402920. [DOI] [PubMed] [Google Scholar]
- Hosmer DW, Lemeshow S. Applied Survival Analysis: Regression Modeling of Time to Event Data. John Wiley and Sons; New York, NY: 1999. [Google Scholar]
- Kendler KS, Jacobson KC, Prescott CA, Neale MC. Specificity of genetic and environmental risk factors for use and abuse/dependence of cannabis, cocaine, hallucinogens, sedatives, stimulants, and opiates in male twins. American Journal of Psychiatry. 2003a;160:687–695. doi: 10.1176/appi.ajp.160.4.687. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Prescott CA, Myers J, Neale MC. The structure of genetic and environmental risk factors for common psychiatric and substance use disorders in men and women. Archives of General Psychiatry. 2003b;60:929–37. doi: 10.1001/archpsyc.60.9.929. [DOI] [PubMed] [Google Scholar]
- Kirillova GP, Vanyukov MM, Gavaler JS, Pajer K, Dunn M, Tarter RE. Substance abuse in parents and their adolescent offspring: The role of sexual maturation and sensation seeking. Journal of Child and Adolescent Substance Abuse. 2001;10:77–89. [Google Scholar]
- Kirisci L, Tarter RE, Reynolds M, Vanyukov M. Individual differences in childhood neurobehavior disinhibition predict decision to desist substance use during adolescence and substance use disorder in young adulthood: A prospective study. Addictive Behaviors. 2006;31:686–696. doi: 10.1016/j.addbeh.2005.05.049. [DOI] [PubMed] [Google Scholar]
- Kosten TA, Rounsaville BJ. Sensitivity of psychiatric diagnosis based on the best estimate procedure. American Journal of Psychiatry. 1992;149:1225–1227. doi: 10.1176/ajp.149.9.1225. [DOI] [PubMed] [Google Scholar]
- Krueger RF, Hicks BM, Patrick CJ, Carlson SR, Iacono WG, McGue M. Etiologic connections among substance dependence, antisocial behavior, and personality: modeling the externalizing spectrum. Journal of Abnormal Psychology. 2002;111:411–424. [PubMed] [Google Scholar]
- Kruesi MJ, Casanova MF, Mannheim G, Johnson-Bilder A. Reduced temporal lobe volume in early onset conduct disorder. Psychiatry Research. 2004;132:1–11. doi: 10.1016/j.pscychresns.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Loeber R. Peer Delinquency Scale. University of Pittsburgh, Department of Psychiatry; Pittsburgh, PA: 1989. [Google Scholar]
- MacKinnon DP, Lockwood CM, Hoffman JM, West SG, Sheets V. A comparison of methods to test mediation and other intervening variable effects. Psychological Methods. 2002;7:83–104. doi: 10.1037/1082-989x.7.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall W, Tanner I. Variation in pattern of pubertal changes in boys. Archives of Disease in Childhood. 1970;45:13–23. doi: 10.1136/adc.45.239.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazur A, Booth A. Testosterone and dominance in men. Behavioral and Brain Sciences. 1998;21:353–363. [PubMed] [Google Scholar]
- Moffitt TE, Caspi A, Belsky J, Silva PA. Childhood experience and the onset of menarche: a test of a sociobiological model. Child Development. 1992;63:47–58. doi: 10.1111/j.1467-8624.1992.tb03594.x. [DOI] [PubMed] [Google Scholar]
- Orvaschel H, Puig-Antich J. Schedule for Affective Disorder and Schizophrenia for School Age Children, Epidemiological version: Kiddie-SADS-E (K-SADS-E) (4th edition) Western Psychiatric Institute and Clinic; Pittsburgh, PA: 1987. [Google Scholar]
- Preacher KJ, Hayes AF. SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behavioral Research Methods, Instruments, & Computers. 2004;36:717–731. doi: 10.3758/bf03206553. [DOI] [PubMed] [Google Scholar]
- Reynolds M, Tarter R, Kirisci L, Kirillova G, Brown S, Clark D, Gavaler J. Testosterone levels and sexual maturation predict substance use disorders in adolescent boys: A prospective study. Biological Psychiatry. doi: 10.1016/j.biopsych.2006.07.008. in press. [DOI] [PubMed] [Google Scholar]
- Rowe R, Maughan B, Worthman CM, Costello EJ, Angold A. Testosterone, antisocial behavior, and social dominance in boys: pubertal development and biosocial interaction. Biological Psychiatry. 2004;55:546–52. doi: 10.1016/j.biopsych.2003.10.010. [DOI] [PubMed] [Google Scholar]
- Rubinow DR, Schmidt PJ. Androgens, brain, and behavior. American Journal of Psychiatry. 1996;153:974–984. doi: 10.1176/ajp.153.8.974. [DOI] [PubMed] [Google Scholar]
- Scarr S, McCartney K. How people make their own environments: a theory of genotype greater than environment effects. Child Development. 1983;54:424–435. doi: 10.1111/j.1467-8624.1983.tb03884.x. [DOI] [PubMed] [Google Scholar]
- Shaw P, Greenstein D, Lerch J, Clasen L, Lenroot R, Gogtay N, Evans A, Rapoport J, Giedd J. Intellectual ability and cortical development in children and adolescents. Nature. 2006a;440:676–679. doi: 10.1038/nature04513. [DOI] [PubMed] [Google Scholar]
- Shaw P, Lerch J, Greenstein D, Sharp W, Clasen L, Evans A, Giedd J, Castellanos FX, Rapoport J. Longitudinal mapping of cortical thickness and clinical outcome in children and adolescents with attention-deficit/hyperactivity disorder. Archives of General Psychiatry. 2006b;63:540–549. doi: 10.1001/archpsyc.63.5.540. [DOI] [PubMed] [Google Scholar]
- Sisk CL, Foster DL. The neural basis of puberty and adolescence. Nature Neuroscience. 2004;7:1040–1047. doi: 10.1038/nn1326. [DOI] [PubMed] [Google Scholar]
- Sobel ME. Asymptotic intervals for indirect effects in structural equations models. In: Leinhart S, editor. Sociological Methodology. Jossey-Bass; San Francisco: 1982. pp. 290–312. [Google Scholar]
- Spitzer RL, Williams JB, Gibbon M. Instruction Manual for the Structured Clinical Interview for DSM–III–R. Biometrics Research Department, New York State Psychiatric Institute; New York: 1987. [Google Scholar]
- Tarter R, Kirisci L, Habeych M, Reynolds M, Vanyukov M. Neurobehavior disinhibition in childhood predisposes boys to substance use disorder by young adulthood: direct and mediated etiologic pathways. Drug and Alcohol Dependence. 2004;73:121–132. doi: 10.1016/j.drugalcdep.2003.07.004. [DOI] [PubMed] [Google Scholar]
- Tarter RE, Vanyukov M. Alcoholism: a developmental disorder. Journal of Consulting and Clinical Psychology. 1994;62:1096–1107. doi: 10.1037//0022-006x.62.6.1096. [DOI] [PubMed] [Google Scholar]
- Tarter RE, Vanyukov MM. Theoretical and operational framework for research into the etiology of substance use disorders. Journal of Child and Adolescent Substance Abuse. 2001;10:1–12. [Google Scholar]
- Tarter R, Vanyukov M, Giancola P, Dawes M, Blackson T, Mezzich A, Clark DB. Etiology of early age onset substance use disorder: a maturational perspective. Development and Psychopathology. 1999;11:657–683. doi: 10.1017/s0954579499002266. [DOI] [PubMed] [Google Scholar]
- Tarter RE, Vanyukov M, Kirisci L, Reynolds M, Clark D. Predictors of marijuana use in adolescents before and after licit drug use: Examination of the gateway hypothesis. American Journal of Psychiatry. 2006 doi: 10.1176/ajp.2006.163.12.2134. in press. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Andersen SL, Polcari A, Anderson CM, Navalta CP. Developmental neurobiology of childhood stress and trauma. Psychiatric Clinics of North America. 2002;25:397–426. doi: 10.1016/s0193-953x(01)00003-x. [DOI] [PubMed] [Google Scholar]
- Tsuang MT, Lyons MJ, Meyer JM, Doyle T, Eisen SA, Goldberg J, True W, Lin N, Toomey R, Eaves L. Co-occurrence of abuse of different drugs in men: the role of drug- specific and shared vulnerabilities. Archives of General Psychiatry. 1998;55:967–972. doi: 10.1001/archpsyc.55.11.967. [DOI] [PubMed] [Google Scholar]
- Vanyukov MM. Evolution, genes, and the environment: Neurobiological outcomes. In: Fishbein DH, editor. The Science, Treatment, and Prevention of Antisocial Behaviors: Application to the Criminal Justice System, Volume 2, Chapter 2. Civic Research Institute; Kingston, NJ: 2004. pp. 4–1.pp. 4–29. [Google Scholar]
- Vanyukov MM, Kirisci L, Tarter RE, Simkevitz HF, Kirillova GP, Maher BS, Clark DB. Liability to substance use disorders: 2. A measurement approach. Neuroscience & Biobehavioral Reviews. 2003a;27:517–526. doi: 10.1016/j.neubiorev.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Vanyukov MM, Moss HB, Tarter RE. Assortment for the liability to substance abuse and personality traits. Annals of the New York Academy of Sciences. 1994;708:102–107. doi: 10.1111/j.1749-6632.1994.tb24702.x. [DOI] [PubMed] [Google Scholar]
- Vanyukov MM, Neale MC, Moss HB, Tarter RE. Mating assortment and the liability to substance abuse. Drug and Alcohol Dependence. 1996;42:1–10. doi: 10.1016/0376-8716(96)01255-0. [DOI] [PubMed] [Google Scholar]
- Vanyukov MM, Tarter RE. Genetic studies of substance abuse. Drug and Alcohol Dependence. 2000;59:101–123. doi: 10.1016/s0376-8716(99)00109-x. [DOI] [PubMed] [Google Scholar]
- Vanyukov MM, Tarter RE, Kirisci L, Kirillova GP, Maher BS, Clark DB. Liability to substance use disorders: 1. Common mechanisms and manifestations. Neuroscience & Biobehavioral Reviews. 2003b;27:507–515. doi: 10.1016/j.neubiorev.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Walden B, McGue M, Iacono WG, Burt SA, Elkins I. Identifying shared environmental contributions to early substance use: the respective roles of peers and parents. Journal of Abnormal Psychology. 2004;113:440–50. doi: 10.1037/0021-843X.113.3.440. [DOI] [PubMed] [Google Scholar]
- Wierson M, Long PJ, Forehand RL. Toward a new understanding of early menarche: the role of environmental stress in pubertal timing. Adolescence. 1993;28:913–24. [PubMed] [Google Scholar]
- Young SE, Stallings MC, Corley RP, Krauter KS, Hewitt JK. Genetic and environmental influences on behavioral disinhibition. American Journal of Medical Genetics. 2000;96:684–695. [PubMed] [Google Scholar]