Abstract
Prostate cancer is the second most common cause of cancer death in men in the United States. Patients with prostate cancer are initially treated with surgical resection, radiation or antiandrogen therapy. After an initial remission however, the majority of prostate tumors evolve into a highly aggressive, metastatic androgen-independent state for which successful therapy has not yet been established. During recent years, new perspectives have emerged towards the development of preventive and therapeutic approaches for prostate cancer. The quinazoline-based α1-blockers have been shown to have antitumor efficacy against prostate cancer cells via their potency to induce apoptosis and anoikis via an α1-adrenoceptor-independent mechanism. Specifically, doxazosin and terazosin can induce apoptosis, inhibit invasion and migration of prostate cancer cells and endothelial cells and reduce their adhesion potential to extracellular matrix components (ECM), thus enhancing their susceptibility to anoikis. In this review we discuss recent evidence suggesting the apoptotic efficacy of quinazoline-based α1 adrenoceptor antagonists, doxazosin and terazosin and we speculate on the therapeutic promise of these drugs as novel antitumor agents against prostate cancer. From a drug discovery perspective, separation of the effect of doxazosin on apoptosis in prostate cancer cells from its original pharmacological activity in normal prostate cells, will provide a molecular basis to develop a novel class of apoptosis-inducing agents through lead optimization.
Keywords: Prostate cancer, adrenergic alpha-antagonists, doxazosin, terazosin, apoptosis, anoikis, vascularity
INTRODUCTION
In the United States, the incidence of prostate cancer has been increasing in recent years, with an estimated 230,110 new cases and 29,900 deaths from the disease in the year 2004.[1] With more than 75% of new cases diagnosed in patients older than 65 years, the incidence of prostate cancer is expected to rise with the changing population age structure. Enhanced public awareness and increasing detection in PSA screening has significantly contributed to the increased incidence of the disease. As a result of the described age migration, prostate cancer is becoming a disease of middle age, with an increasing number of new cases being diagnosed in men in their fifties.[2] Prostate cancer is notoriously considered slow-growing as a tumor, with doubling times for local disease calculated at 2–4 years. Prostate tumors, however, eventually emerge as androgen-independent and highly metastatic advanced disease, severely affecting patient morbidity and mortality.[3] Currently available treatments include surgery, androgen ablation therapy and radiotherapy-however, tumor relapse occurs frequently. Intense investigative efforts focus on exploiting the apoptotic signaling pathways towards a therapeutic benefit in patients with both androgen-dependent and androgen-independent prostate cancer.[4]
Alpha1 adrenoceptor antagonists are considered first-line medical treatment in the conservative management of patients with lower urinary tract symptoms (LUTS) due to benign prostatic hyperplasia (BPH).[5] In an entirely different clinical setting, α1-adrenoceptor antagonists can be co-administered as complementary antihypertensive drugs in combination with either a calcium channel blocker or angiotensin-converting enzyme (ACE) inhibitor for an antihypertensive effect.[6]
Several multicenter, double-blind, randomized, placebo-controlled studies have established that terazosin (Hytrin), doxazosin (Cardura), and tamsulosin (Flomax) (Figure 1) are relatively safe drugs, with dizziness as the major side effect.[7,8,9] Their therapeutic efficacy has been classically attributed to the blockade of the α1a adrenoceptor, the predominant receptor subtype in the human prostate stroma, that reduces prostate smooth muscle tone and thereby inhibits the dynamic component of the obstruction [10]. During ongoing prostatic hyperplasia however, the clinical response to doxazosin and terazosin is sustained for a longer duration than would be expected if acute α1-adrenoceptor blockade were its only mechanism of action.
Figure 1.
Chemical structures of alpha1 adrenoreceptor antagonists terazosin and doxazosin and the sulphonamide-derivative tamsulosin.
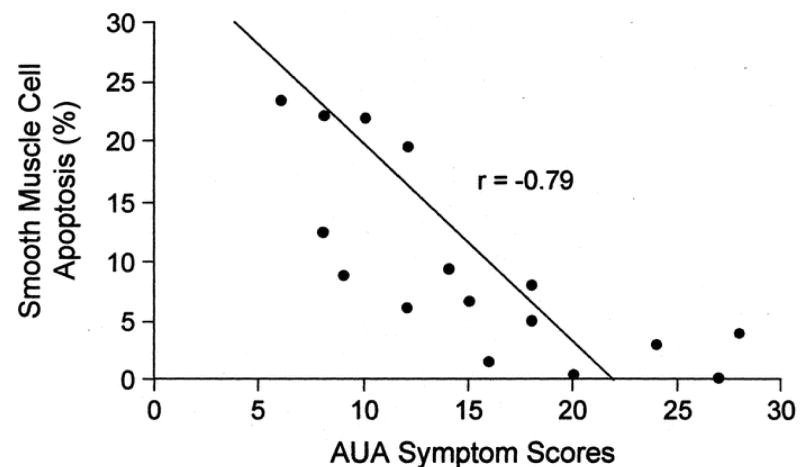
A differential mechanism of action of α1-adrenoceptor antagonists against prostate growth was recently identified, from retrospective clinical studies documenting that doxazosin treatment of BPH patients resulted in an increased prostatic cell apoptosis [11]. Two features were of noteworthy significance (biochemically and clinically) from this original study. At the biochemical level, doxazosin had no effect on the rate of proliferation, indicating that the anti-growth action was exclusively targeted in activating apoptosis. At the clinical front, the increased apoptotic index among the stromal cellular components of the prostate correlated with improved LUTS (Fig. 2). These observations provided an initial, yet strong support for the premise that apoptosis induction is a potential molecular mechanism underlying the long-term efficacy of doxazosin in the management of BPH. A subsequent retrospective study documented that terazosin can also induce apoptotic cell death of prostatic stromal and epithelial cells in treated patients compared to untreated controls within 1 month of treatment, without affecting cell proliferation.[12] These findings gained direct support from an earlier report in an experimental model of BPH, the murine prostate reconstitution model system, where doxazosin was shown to be an effective in vivo apoptosis inducer in epithelial cells, without affecting their proliferative capacity.[13]
Figure 2.
Correlation of doxazosin induced prostatic stromal smooth muscle cell apoptosis with BPH symptom score improvement in 17 patients. BPH symptoms were graded using AUA symptom scoring system with lower scores indicating less severity/bother. Reprinted with permission from Kyprianou et al, 1998 [11].
A series of in vitro studies that followed provided further validation of the concept of apoptosis induction by α1-adrenoceptor antagonists and demonstrated that their apoptotic action was not limited to benign cells, but human prostate cancer cells, both androgen-independent and androgen-sensitive can also undergo apoptosis in response to doxazosin [14,15]. Furthermore, two lines of evidence from these studies established the apoptotic action being independent of α1 adrenoreceptor action. First, transfection-mediated overexpression of α1-adrenoceptor in human prostate cancer cells (that lack endogenous α1-adrenoceptor) did not yield any significant changes in the sensitivity of prostate cancer cells to doxazosin-mediated apoptosis. Second, the apoptotic potency was specific to the quinazoline-based antagonists doxazosin and terazosin (pointing to a class effect), while tamsulosin, an agent with a distinct chemical structure (sulfonamide, Fig. 1), did not elicit any apoptotic effect against prostate cells.
This review is an attempt, based on the information gathered thus far, to present the pharmacomolecular profile of the recently recognized antitumor action of the quinazoline-based α1 adrenoceptor antagonists against prostate tumor growth via induction of apoptosis and inhibition of angiogenesis. Pharmacological exploitation of these apoptotic properties of doxazosin and terazosin is expected to lead to the development of novel, safe, and effective treatment options for patients with advanced prostate cancer.
Apoptosis Induction by Quinazolines: Targeting Survival Independent of α1-Adrenoceptors
Apoptosis represents a powerful weapon against advanced prostate cancer, as both hormone-dependent and hormone-independent cells retain the ability to undergo cell death in response to androgen deprivation or chemotherapeutic drugs/ionizing irradiation, respectively.[4] The extrinsic pathway of apoptotic signaling consists of cell surface death receptors, such as Fas,[16] which are associated with a number of intracellular regulatory proteins, the end result being apoptosis activation through caspase 8 proteolytic processing. The intrinsic pathway involves the mitochondria as the protagonist, which upon accumulation of effector molecules causing mitochondrial membrane permeabilization (MMP), determine the apoptotic fate of the cell by releasing cytochrome c leading to the activation of the caspase cascade.[17] The endoplasmic reticulum (ER) has also been recently implicated as a contributor to apoptosis through mobilization of ER calcium stores, calcium release and sensitization of mitochondria to apoptotic stimuli [18]. Activation of the caspase cascade (family of cysteine proteases) is intimately involved in the execution of apoptosis in response to various stimuli, with cleavage of structural and functional proteins involved in cell cycle regulation and DNA repair [19,20]. The bcl-2 oncoprotein is an apoptosis suppressor in human tumors[21,22] via its ability to block loss of mitochondrial membrane potential and cytochrome c release, and consequently inhibit caspase-9 activation.[23] In prostate cancer, bcl-2 overexpression is associated with poor prognosis[24] and emergence of hormone refractory disease.[25]
It is of major mechanistic interest that the in vitro antitumor activity of doxazosin and terazosin against prostate cancer cells was apparently mediated via an α1-adrenoceptor-independent mechanism.[14,15] Tamsulosin, an α1-adrenoreceptor antagonist that belongs to a distinct class of sulphonamides (Fig. 1), does not exert an apoptotic action against prostate cancer cells. The therapeutic potential of the apoptotic activity of doxazosin stems from further studies documenting that the combination of doxazosin with chemotherapeutic agents (such as adriamycin and etoposide) or ionizing irradiation, results in an augmented cytotoxic effect in prostate cancer cells [26,27]. These findings provide a compelling molecular basis for the development of feasible combination approaches of doxazosin with existing modalities for the effective treatment of androgen independent prostate cancer.
Putative mechanisms underlying doxazosin-mediated apoptosis include induction of transforming growth factor-β1 (TGF-β1) signaling and upregulation of the cell cycle regulators p21 and p27 and, NF-κB inhibitor I Ba gene expression. TGF-β1 serves as a potent regulator of prostate proliferation and apoptosis given its ability to inhibit cell proliferation and induce apoptosis.[28] TGF-β signaling is elicited via interaction of TGF-β (ligand) with its transmembrane receptors, TβRI and TβRII, which have serine-threonine kinase activity.[29] Intracellular downstream signaling is mainly mediated by the Smad family of proteins, with Smad4 translocation to the nucleus.[30] Studies from our laboratory have revealed upregulation of a few TGF-β1 downstream signaling effectors, such as Smad4, TGF-β-inducible early-response gene 1 (TIEG1) and IκBα in PC-3 cells in response to doxazosin treatment.[31] Interestingly, TGF-β1 protein expression was significantly elevated in prostate specimens from BPH patients treated with terazosin in correlation with increased apoptosis among the same cell populations.[32] An independent report implicated expression of TGF-β1 as a possible means of apoptosis induction in primary cultures of prostate cancer cells, showing that doxazosin-treated cells undergoing apoptosis produced more TGF-β1 than untreated cells, an effect that was reversed by a neutralizing TGF-β1 antibody.[33] Further support for the functional involvement of TGF-β signaling in quinazoline-mediated apoptotic action against prostate cancer cells, stems from a recent study by Xu et al [34], demonstrating that terazosin-induced apoptosis in human prostate cancer cells, PC-3 and DU-145, is associated with p27Kip1 upregulation (a cycle dependent kinase inhibitor and an intracellular effector of TGF-β apoptotic signaling) and proceeds via an Rb and p53 independent pathway. Moreover, a new mechanistic insight has recently been gained from a structural dissection studies of the effect, demonstrating that the apoptosis-inducing property of doxazosin in androgen-independent prostate cancer cells proceeds by targeting the intracellular Akt activation survival pathway.[35]. In vivo administration of doxazosin at pharmacologically relevant doses resulted in a significant reduction in tumor volume of the prostate cancer xenografts (Fig. 3) via caspase-3 induction.[14] These observations provide an appealing rationale towards the undertaking of a placebo-controlled randomized clinical trial to establish the antitumor efficacy of α1-adrenoceptor antagonists in prostate cancer patients.
Figure 3.
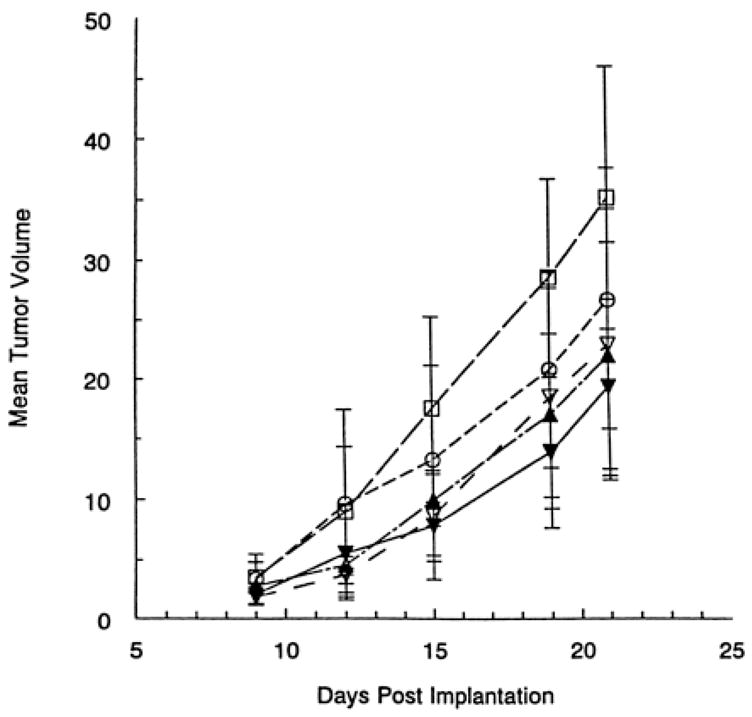
In vivo effect of doxazosin on prostate cancer. Doxazosin treatment resulted in a significant suppression of the prostate tumor xenograft growth compared with controls (P <0.05). ▼, 100 mg/kg doxazosin; ○, 10 mg/kg doxazosin; ▲, 30 mg/kg doxazosin; ▽, 3 mg/kg doxazosin; □, vehicle control (sterile water). Reprinted with permission from [14].
It is important to consider the growing evidence that certain quinazoline-based tyrosine kinase inhibitors act as antitumor agents possessing antiangiogenic, antiproliferative or apoptotic effects against a variety of cancer cell types. Rapidly gaining popularity due to its antitumor properties is the EGFR inhibitor ZD1839 (gefitinib, “Iressa”), a drug that has been shown to induce apoptosis and inhibit proliferation of prostate cancer cells in vitro,[36] as well as many other cancer cell types,[37] and is currently undergoing clinical evaluation for the treatment of prostate cancer.[38,39] In view of the established ability of the quinazoline-based α1 adrenoceptor antagonists to induce apoptosis in prostate cancer cells, it is tempting to consider that the intrinsic quinazoline component may confer tyrosine kinase activity, accounting for the apoptotic activity of these agents in prostate cancer.[40]
The Anoikis Effect: Targeting Angiogenesis with α1-Blockade?
Cells, including tumor epithelial cells and endothelial cells, rely on cell-extracellular matrix signals, as well as cell-cell interactions that offer structural support and survival signals.[41] The term anoikis (homelessness in Greek) is used to describe apoptosis induction through loss of cell attachment to its natural surrounding environment.[42] Resistance to anoikis is required for tumor cells to detach from the primary tumor and metastasize, and believed to be conferred overexpression of oncogenes such as ras, raf and src.[43] Furthermore, bcl-2 overexpression has been implicated in anoikis resistance, being abundant in keratinocytes rapidly adhering (via integrins β1 and α6β4) to collagen IV.[44] The finding that the quinazolines doxazosin and terazosin can induce anoikis in prostate cancer epithelial cells and endothelial cells by inhibiting their attachment to gelatin/collagen is of special mechanistic interest that begs for further exploration.[45] Transfection-mediated bcl-2 overexpression was able to provide only partial protection from quinazoline-based α1-antagonist-mediated anoikis. Since enforced bcl-2 expression in highly metastatic PC-3 variants failed to protect them from anoikis, one has also to consider that bcl-2 independent mechanisms could be operational.[46] Intracellular effectors of the integrin pathway such as FAK, Akt and catenin, are critical components of the anchorage-mediate survival signaling that is potentially targeted by the quinazolines. A reduction in focal adhesion kinase (FAK) expression has been recently shown in prostate cancer cells undergoing apoptosis in response to doxazosin.[47] FAK, which significantly enough is upregulated in metastatic prostate cancer,[48] is activated by binding to the cytoplasmic domain of the β1 integrin, a key step in adhesion-mediated survival signaling.[49] Therefore overexpression of FAK and Akt may block anoikis in prostate cancer cells and adjacent-endothelial cells despite loss of ECM anchorage.[50] Further support for such a concept stems from studies in other cellular systems that FAK activation[51] and attachment-mediated activation of Akt survival pathway protects cells from anoikis.[52]
The vascular endothelial growth factor (VEGF), as a key mediator of the angiogenic response [53] provides an additional molecular target for the action of quinazolines in the prostate. An immunohistochemical analysis of human prostate specimens (from BPH patients) demonstrated that in addition to increasing the apoptotic index of prostate tumor cell populations, terazosin treatment also resulted in a significant reduction in microvessel density (MVD) in prostate tissue.[54] Furthermore, a recent study documented the ability of terazosin to inhibit VEGF induced angiogenesis in vivo by reducing tube formation of human umbilical vein endothelial cells.[55] In resonance with this evidence are the findings by Keledjian et al demonstrating that doxazosin induces a downregulation of VEGF in human prostate cancer and endothelial cells.[45] Both the quinazolines, doxazosin and terazosin (but not tamsulosin) decrease prostate cancer cell adhesion and inhibit their invasion potential in vitro. VEGF transmits signals required for stimulation of vessel growth: vasorelaxation, increase in vascular permeability, endothelial cell migration, survival and proliferation, upon binding to VEGF receptors [56, 57]. In prostate cancer there is an increase in VEGF expression (compared to the normal gland) that correlates with tumor stage, grade, microvessel density and clinical outcome.[58] Thus it is worth considering that the quinazoline-based α1-adrenoceptor antagonists might soon join the group of potent angiogenesis inhibitors currently in clinical trials for prostate cancer patients, that includes thalidomide, genistein, and celecoxib.
Chemoprevention of prostate cancer is rapidly becoming the center of intense research efforts with special focus on the use of selenium, vitamins (in particular vitamins E, D, A and C), and soy intake, via their potential apoptotic action.[59] The initial report on the Prostate Cancer Prevention Trial (PCPT)[60] revealed an approximate 25% reduction in prostate cancer incidence over the seven-year follow-up period in patients receiving finasteride, a type 1 5α-reductase inhibitor that reduces dihydrotestosterone (DHT) levels, compared to placebo controls. A similar randomized study, Reduction by Dutasteride in Prostate Cancer (REDUCE), is currently under way for dutasteride.[59] Interestingly enough, the ability of the quinazoline-based α1 adrenoceptor antagonists doxazosin and terazosin in their own right to affect prostate growth by targeting both stromal and epithelial cell apoptosis might assist the interpretation of the prospective European doxazosin combination therapy (PREDICT) study.[61] This clinical study showed that the combination of finasteride and doxazosin did not provide any therapeutic benefit within the first two years over doxazosin alone with respect to symptom improvement. Thus, the newly-recognized apoptotic/antiangiogenic properties of the quinazolines provide a new molecular basis that calls for initiation of a chemoprevention trial to explore the potential beneficial impact of doxazosin and terazosin on prostate cancer development and progression.
Conclusions and Future Directions
Prostate tumor cells acquire multiple molecular strategies to evade apoptosis and promote angiogenesis during progression to advanced disease. Therefore manipulation and reconstitution of apoptotic pathways will potentially offer therapeutically attractive and molecularly feasible treatment options not only for prostate cancer but possibly for other human malignancies. Among the various survival tactics utilized by prostate cancer cells is a dysfunctional TGF-β signaling through loss of receptors and intranuclear effectors and upregulation of VEGF. Consequently targeting survival pathways mediated by TGF-β and anoikis signaling mediated by VEGF/integrins by the α1 adrenoceptor antagonists (widely used in the clinical management of BPH patients) comprise a viable approach for prostate cancer therapy. The quinazoline-based α1 adrenoceptor antagonists, doxazosin and terazosin, can induce apoptosis in benign and malignant prostate cells via activation of TGF-β signaling, reduce tumor vascularity in clinical prostate tumors possibly by sensitizing prostate cancer cells to anoikis and suppress prostate tumorigenic growth in vivo.[62] The rapidly growing evidence on doxazosin’s prostate anti-growth activity, directly supports the long-term action of α1 adrenoceptor antagonists in the management of BPH, and holds bright therapeutic promise for their use as potential antitumor agents in prostate cancer prevention and treatment. Separation of the effect of doxazosin/terazosin on apoptosis from their original (α1-blockade-based) pharmacological activity, provides molecular underpinnings for the pharmacological exploitation of these α1-adrenoceptor antagonists to develop a novel class of antitumor agents. Finally, it is worthwhile to reflect on the emerging evidence from the current chemoprevention trials (PCPT) [60] and the MTOPS (Medical Therapy of Prostatic Symptoms) study [63], to justify similar chemoprevention trials for α1 adrenoceptor antagonists in prostate cancer.
References
- 1.JEMAL A, TIWARI RC, MURRAY T, et al. Cancer statistics, 2004. CA Cancer J Clin. 2004;54(1):8–29. doi: 10.3322/canjclin.54.1.8. [DOI] [PubMed] [Google Scholar]
- 2.HANKEY BF, FEUER EJ, CLEGG LX, et al. Cancer surveillance series: interpreting trends in prostate cancer—part I: Evidence of the effects of screening in recent prostate cancer incidence, mortality, and survival rates. J Natl Cancer Inst. 1999;91(12):1017–24. doi: 10.1093/jnci/91.12.1017. [DOI] [PubMed] [Google Scholar]
- 3.ALBERTSEN PC, FRYBACK DG, STORER BE, KOLON TF, FINE J. Long-term survival among men with conservatively treated localized prostate cancer. JAMA. 1995;274(8):626–31. [PubMed] [Google Scholar]
- 4.KYPRIANOU N. Apoptosis: therapeutic significance in the treatment of androgen-dependent and androgen-independent prostate cancer. World J Urol. 1994;12(6):299–303. doi: 10.1007/BF00184107. [DOI] [PubMed] [Google Scholar]
- 5.DJAVAN B, MARBERGER M. A meta-analysis on the efficacy and tolerability of α1-adrenoceptor antagonists in patients with lower urinary tract symptoms suggestive of benign prostatic obstruction. Eur Urol. 1999;36(1):1–13. doi: 10.1159/000019919. [DOI] [PubMed] [Google Scholar]
- 6.SEVER PS. Alpha 1-blockers in hypertension. Curr Med Res Opin. 1999;15(2):95–103. doi: 10.1185/03007999909113369. [DOI] [PubMed] [Google Scholar]
- 7.LEPOR H, AUERBACH S, PURAS-BAEZ A, et al. A randomized multicenter placebo controlled study of the efficacy and safety of terazosin in the treatment of benign prostatic hyperplasia. J Urol. 1992;148(5):1467–74. doi: 10.1016/s0022-5347(17)36941-0. [DOI] [PubMed] [Google Scholar]
- 8.CHAPPLE CR, CARTER P, CHRISTMAS TJ, et al. A three-month double-blind study of doxazosin as treatment for benign prostatic obstruction. Br J Urol. 1994;74(1):50–6. doi: 10.1111/j.1464-410x.1994.tb16546.x. [DOI] [PubMed] [Google Scholar]
- 9.LEPOR H. Phase III multicenter placebo-controlled study of tamsulosin in benign prostatic hyperplasia. Tamsulosin Investigator Group. Urology. 1998;51(6):892–900. doi: 10.1016/s0090-4295(98)00126-5. [DOI] [PubMed] [Google Scholar]
- 10.FORRAY C, BARD JA, WETZEL JM, et al. The alpha 1-adrenergic receptor that mediates smooth muscle contraction in human prostate has the pharmacological properties of the cloned human alpha 1c subtype. Mol Pharmacol. 1994;45(4):703–8. [PubMed] [Google Scholar]
- 11.KYPRIANOU N, LITVAK JP, BORKOWSKI A, ALEXANDER R, JACOBS SC. Induction of prostate apoptosis by doxazosin in benign prostatic hyperplasia. J Urol. 1998;159(6):1810–5. doi: 10.1016/S0022-5347(01)63162-8. [DOI] [PubMed] [Google Scholar]
- 12.CHON JK, BORKOWSKI A, PARTIN AW, ISAACS JT, JACOBS SC, KYPRIANOU N. Alpha 1-adrenoceptor antagonists terazosin and doxazosin induce prostate apoptosis without affecting cell proliferation in patients with benign prostatic hyperplasia. J Urol. 1999;161(6):2002–8. [PubMed] [Google Scholar]
- 13.YANG G, TIMME TL, PARK SH, WU X, WYLLIE MG, THOMPSON TC. Transforming growth factor beta 1 transduced mouse prostate reconstitutions: II. Induction of apoptosis by doxazosin. Prostate. 1997;33(3):157–63. doi: 10.1002/(sici)1097-0045(19971101)33:3<157::aid-pros2>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 14.KYPRIANOU N, BENNING CM. Suppression of human prostate cancer cell growth by alpha1-adrenoceptor antagonists doxazosin and terazosin via induction of apoptosis. Cancer Res. 2000;60(16):4550–5. [PubMed] [Google Scholar]
- 15.BENNING CM, KYPRIANOU N. Quinazoline-derived alpha1-adrenoceptor antagonists induce prostate cancer cell apoptosis via an alpha1-adrenoceptor-independent action. Cancer Res. 2002;62(2):597–602. [PubMed] [Google Scholar]
- 16.WAJANT H. The Fas signaling pathway: more than a paradigm. Science. 2002;296(5573):1635–6. doi: 10.1126/science.1071553. [DOI] [PubMed] [Google Scholar]
- 17.KROEMER G, REED JC. Mitochondrial control of cell death. Nat Med. 2000;6(5):513–9. doi: 10.1038/74994. [DOI] [PubMed] [Google Scholar]
- 18.BRECKENRIDGE DG, GERMAIN M, MATHAI JP, NGUYEN M, SHORE GC. Regulation of apoptosis by endoplasmic reticulum pathways. Oncogene. 2003;22(53):8608–18. doi: 10.1038/sj.onc.1207108. [DOI] [PubMed] [Google Scholar]
- 19.WOLF BB, GREEN DR. Suicidal tendencies: apoptotic cell death by caspase family proteinases. J Biol Chem. 1999;274(29):20049–52. doi: 10.1074/jbc.274.29.20049. [DOI] [PubMed] [Google Scholar]
- 20.THORNBERRY NA, LAZEBNIK Y. Caspases: enemies within. Science. 1998;281(5381):1312–6. doi: 10.1126/science.281.5381.1312. [DOI] [PubMed] [Google Scholar]
- 21.HOCKENBERY D, NUNEZ G, MILLIMAN C, SCHREIBER RD, KORSMEYER SJ. Bcl-2 is an inner mitochondrial membrane protein that blocks programmed cell death. Nature. 1990;348(6299):334–6. doi: 10.1038/348334a0. [DOI] [PubMed] [Google Scholar]
- 22.KYPRIANOU N, KING ED, BRADBURY D, RHEE JG. bcl-2 over-expression delays radiation-induced apoptosis without affecting the clonogenic survival of human prostate cancer cells. Int J Cancer. 1997;70(3):341–8. doi: 10.1002/(sici)1097-0215(19970127)70:3<341::aid-ijc16>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 23.CORY S, HUANG DC, ADAMS JM. The Bcl-2 family: roles in cell survival and oncogenesis. Oncogene. 2003;22(53):8590–607. doi: 10.1038/sj.onc.1207102. [DOI] [PubMed] [Google Scholar]
- 24.BUBENDORF L, SAUTER G, MOCH H, et al. Prognostic significance of Bcl-2 in clinically localized prostate cancer. Am J Pathol. 1996;148(5):1557–65. [PMC free article] [PubMed] [Google Scholar]
- 25.APAKAMA I, ROBINSON MC, WALTER NM, et al. bcl-2 overexpression combined with p53 protein accumulation correlates with hormone-refractory prostate cancer. Br J Cancer. 1996;74(8):1258–62. doi: 10.1038/bjc.1996.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.CAL C, USLU R, GUNAYDIN G, OZYURT C, OMAY SB. Doxazosin: a new cytotoxic agent for prostate cancer? BJU Int. 2000;85(6):672–5. doi: 10.1046/j.1464-410x.2000.00607.x. [DOI] [PubMed] [Google Scholar]
- 27.CUELLAR DC, RHEE J, KYPRIANOU N. Alpha1-adrenoceptor antagonists radiosensitize prostate cancer cells via apoptosis induction. Anticancer Res. 2002;22(3):1673–9. [PubMed] [Google Scholar]
- 28.MARTIKAINEN P, KYPRIANOU N, ISAACS JT. Effect of transforming growth factor-beta 1 on proliferation and death of rat prostatic cells. Endocrinology. 1990;127(6):2963–8. doi: 10.1210/endo-127-6-2963. [DOI] [PubMed] [Google Scholar]
- 29.DE CAESTECKER MP, PIEK E, ROBERTS AB. Role of transforming growth factor-beta signaling in cancer. J Natl Cancer Inst. 2000;92(17):1388–402. doi: 10.1093/jnci/92.17.1388. [DOI] [PubMed] [Google Scholar]
- 30.MASSAGUE J, WOTTON D. Transcriptional control by the TGF-beta/Smad signaling system. EMBO J. 2000;19(8):1745–54. doi: 10.1093/emboj/19.8.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.PARTIN JV, ANGLIN IE, KYPRIANOU N. Quinazoline-based alpha 1-adrenoceptor antagonists induce prostate cancer cell apoptosis via TGF-beta signalling and I kappa B alpha induction. Br J Cancer. 2003;88(10):1615–21. doi: 10.1038/sj.bjc.6600961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.GLASSMAN DT, CHON JK, BORKOWSKI A, JACOBS SC, KYPRIANOU N. Combined effect of terazosin and finasteride on apoptosis, cell proliferation, and transforming growth factor-beta expression in benign prostatic hyperplasia. Prostate. 2001;46(1):45–51. doi: 10.1002/1097-0045(200101)46:1<45::aid-pros1007>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 33.ILIO KY, PARK II, PINS MR, KOZLOWSKI JM, LEE C. Apoptotic activity of doxazosin on prostate stroma in vitro is mediated through an autocrine expression of TGF-beta1. Prostate. 2001;48(3):131–5. doi: 10.1002/pros.1091. [DOI] [PubMed] [Google Scholar]
- 34.XU K, WANG X, LING PM, TSAO SW, WONG YC. The alpha1-adrenoceptor antagonist terazosin induces prostate cancer cell death though a p53 and RB independent pathway. Oncol Rep. 2003;10 ( 5):1555–60. doi: 10.3892/or.10.5.1555. [DOI] [PubMed] [Google Scholar]
- 35.SHAW YJ, YANG YT, GARRISON JB, KYPRIANOU N, CHENG CS. Pharmacological exploitation of the α1-adrenoceptor antagonist doxazosin to develop a novel class of antitumor agents that block intracellular Akt activation. Manuscript submitted for publication. J Med Chem. 2004 doi: 10.1021/jm049752k. [DOI] [PubMed] [Google Scholar]
- 36.VICENTINI C, FESTUCCIA C, GRAVINA GL, ANGELUCCI A, MARRONARO A, BOLOGNA M. Prostate cancer cell proliferation is strongly reduced by the epidermal growth factor receptor tyrosine kinase inhibitor ZD1839 in vitro on human cell lines and primary cultures. J Cancer Res Clin Oncol. 2003;129(3):165–74. doi: 10.1007/s00432-003-0420-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.CIARDIELLO F, CAPUTO R, BIANCO R, et al. Inhibition of growth factor production and angiogenesis in human cancer cells by ZD1839 (Iressa), a selective epidermal growth factor receptor tyrosine kinase inhibitor. Clin Cancer Res. 2001;7(5):1459–65. [PubMed] [Google Scholar]
- 38.BLACKLEDGE G. Growth factor receptor tyrosine kinase inhibitors; clinical development and potential for prostate cancer therapy. J Urol. 2003;170(6 Pt 2):S77–83. doi: 10.1097/01.ju.0000095022.80033.d3. [DOI] [PubMed] [Google Scholar]
- 39.Phase II Trial of Bicalutamide (Casodex) Plus Gefitinib (Iressa, ZD1839) Versus Bicalutamide Plus Placebo in Patients with Localized Prostate Cancer Prior to Radical Prostatectomy. http://www.mskcc.org/mskcc/html/2270.cfm?IRBNO=02-110.
- 40.ANGLIN IE, GLASSMAN DT, KYPRIANOU N. Induction of prostate apoptosis by alpha1-adrenoceptor antagonists: mechanistic significance of the quinazoline component. Prostate Cancer Prostatic Dis. 2002;5(2):88–95. doi: 10.1038/sj.pcan.4500561. [DOI] [PubMed] [Google Scholar]
- 41.RUOSLAHTI E, REED JC. Anchorage dependence, integrins, and apoptosis. Cell. 1994;77(4):477–8. doi: 10.1016/0092-8674(94)90209-7. [DOI] [PubMed] [Google Scholar]
- 42.FRISCH SM, FRANCIS H. Disruption of epithelial cell-matrix interactions induces apoptosis. J Cell Biol. 1994;124(4):619–26. doi: 10.1083/jcb.124.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.GROSSMANN J. Molecular mechanisms of “detachment-induced apoptosis—Anoikis”. Apoptosis. 2002;7(3):247–60. doi: 10.1023/a:1015312119693. [DOI] [PubMed] [Google Scholar]
- 44.TIBERIO R, MARCONI A, FILA C, et al. Keratinocytes enriched for stem cells are protected from anoikis via an integrin signaling pathway in a Bcl-2 dependent manner. FEBS Lett. 2002;524(1–3):139–44. doi: 10.1016/s0014-5793(02)03040-5. [DOI] [PubMed] [Google Scholar]
- 45.KELEDJIAN K, KYPRIANOU N. Anoikis induction by quinazoline based alpha 1-adrenoceptor antagonists in prostate cancer cells: antagonistic effect of bcl-2. J Urol. 2003;169(3):1150–6. doi: 10.1097/01.ju.0000042453.12079.77. [DOI] [PubMed] [Google Scholar]
- 46.BONDAR VM, MCCONKEY DJ. Anoikis is regulated by BCL-2-independent pathways in human prostate carcinoma cells. Prostate. 2002;51(1):42–9. doi: 10.1002/pros.10070. [DOI] [PubMed] [Google Scholar]
- 47.WALDEN P, NEIDER A, GLOBINA Y. Investigation of the non-adrenergic, apoptotic mechanism of action of doxazosin in the prostate. J Urol. 2002;167(suppl):215. # 869. [Google Scholar]
- 48.TREMBLAY L, HAUCK W, APRIKIAN AG, BEGIN LR, CHAPDELAINE A, CHEVALIER S. Focal adhesion kinase (pp125FAK) expression, activation and association with paxillin and p50CSK in human metastatic prostate carcinoma. Int J Cancer. 1996;68(2):164–71. doi: 10.1002/(sici)1097-0215(19961009)68:2<169::aid-ijc4>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 49.FRISCH SM, VUORI K, RUOSLAHTI E, CHAN-HUI PY. Control of adhesion-dependent cell survival by focal adhesion kinase. J Cell Biol. 1996;134(3):793–9. doi: 10.1083/jcb.134.3.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.FRISCH SM, SCREATON RA. Anoikis mechanisms. Curr Opin Cell Biol. 2001;13(5):555–62. doi: 10.1016/s0955-0674(00)00251-9. [DOI] [PubMed] [Google Scholar]
- 51.FRISCH SM, VUORI K, RUOSLAHTI E, CHAN-HUI PY. Control of adhesion-dependent cell survival by focal adhesion kinase. J Cell Biol. 1996;134(3):793–9. doi: 10.1083/jcb.134.3.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.KHWAJA A, RODRIGUEZ-VICIANA P, WENNSTROM S, WARNE PH, DOWNWARD J. Matrix adhesion and Ras transformation both activate a phosphoinositide 3-OH kinase and protein kinase B/Akt cellular survival pathway. EMBO J. 1997;16(10):2783–93. doi: 10.1093/emboj/16.10.2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.FERRARA N, GERBER HP, LECOUTER J. The biology of VEGF and its receptors. Nat Med. 2003;9(6):669–76. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 54.KELEDJIAN K, BORKOWSKI A, KIM G, ISAACS JT, JACOBS SC, KYPRIANOU N. Reduction of human prostate tumor vascularity by the alpha1-adrenoceptor antagonist terazosin. Prostate. 2001;48(2):71–8. doi: 10.1002/pros.1083. [DOI] [PubMed] [Google Scholar]
- 55.PAN SL, GUH JH, HUANG YW, CHERN JW, CHOU JY, TENG CM. Identification of apoptotic and antiangiogenic activities of terazosin in human prostate cancer and endothelial cells. J Urol. 2003;169(2):724–9. doi: 10.1097/01.ju.0000037731.83941.db. [DOI] [PubMed] [Google Scholar]
- 56.KARKKAINEN MJ, PETROVA TV. Vascular endothelial growth factor receptors in the regulation of angiogenesis and lymphangiogenesis. Oncogene. 2000;19(49):5598–605. doi: 10.1038/sj.onc.1203855. [DOI] [PubMed] [Google Scholar]
- 57.ERIKSSON U, ALITALO K. Structure, expression and receptor-binding properties of novel vascular endothelial growth factors. Curr Top Microbiol Immunol. 1999;237:41–57. doi: 10.1007/978-3-642-59953-8_3. [DOI] [PubMed] [Google Scholar]
- 58.STROHMEYER D, ROSSING C, BAUERFEIND A, et al. Vascular endothelial growth factor and its correlation with angiogenesis and p53 expression in prostate cancer. Prostate. 2000;45(3):216–24. doi: 10.1002/1097-0045(20001101)45:3<216::aid-pros3>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 59.DJAVAN B, ZLOTTA A, SCHULMAN C, et al. Chemotherapeutic prevention studies of prostate cancer. J Urol. 2004;171(2 Pt 2):S10-3. doi: 10.1097/01.ju.0000108221.63466.7d. discussion S13-4. [DOI] [PubMed] [Google Scholar]
- 60.THOMPSON IM, GOODMAN PJ, TANGEN CM, et al. The influence of finasteride on the development of prostate cancer. N Engl J Med. 2003;349(3):215–24. doi: 10.1056/NEJMoa030660. [DOI] [PubMed] [Google Scholar]
- 61.KIRBY RS, ROEHRBORN C, BOYLE P, et al. Efficacy and tolerability of doxazosin and finasteride, alone or in combination, in treatment of symptomatic benign prostatic hyperplasia: the Prospective European Doxazosin and Combination Therapy (PREDICT) trial. Urology. 2003;61(1):119–126. doi: 10.1016/s0090-4295(02)02114-3. [DOI] [PubMed] [Google Scholar]
- 62.TAHMATZOPOULOS A, KYPRIANOU N. Apoptotic impact of alpha1-blockers on prostate cancer growth: a myth or an inviting reality? Prostate. 2004;59(1):91–100. doi: 10.1002/pros.10357. [DOI] [PubMed] [Google Scholar]
- 63.MCCONNELL JD, ROEHRBORN CG, BAUTISTA OM, et al. The long-term effect of doxazosin, finasteride, and combination therapy on the clinical progression of benign prostatic hyperplasia. N Engl J Med. 2003;349(25):2387–98. doi: 10.1056/NEJMoa030656. [DOI] [PubMed] [Google Scholar]