Abstract
Previous studies have shown that exposure to intense noise causes outer hair cells (OHCs) to die, primarily through the process of apoptotic degeneration. The current study was designed to examine the regulatory role of mitochondrial bioenergetic function in controlling the initiation and execution of the apoptotic process of OHCs. Chinchilla cochleae were treated with 3-nitropropionic acid (3-NP, 20 mM, or 50 mM), an irreversible inhibitor of succinate dehydrogenase (SDH), to inhibit the mitochondrial energy production before and after exposure to 75 pairs of impulses at 155 dB pSPL. Comparison of the noise-exposed cochleae treated with and without 3-NP revealed that the inhibition of SDH activity delayed nuclear degradation in apoptotic OHCs. However, the initiation of apoptosis appeared to be undeterred. There was no major shift of cell death pathways from apoptosis to necrosis, although a small portion of OHCs showed signs of secondary necrosis. Collectively, the results of the study suggest that, while the mitochondrial energetic function plays an important role in regulating the apoptotic process, its dysfunction has a limited influence on the suppression of apoptotic induction in OHCs following exposure to intense noise.
Keywords: Apoptosis, Necrosis, Mitochondria, Impulse noise, Outer hair cell, Succinate dehydrogenase
1. Introduction
In the past few years, several studies have revealed that apoptosis is involved in outer hair cell (OHC) demise after exposure to an intense noise (Bohne et al., 2007; Hu et al., 2000; Nicotera et al., 2001; Niu et al., 2003; Shizuki et al., 2002; Wang et al., 2002; Yang et al., 2004; Ylikoski et al., 2002). Apoptosis has been found to be the primary cell death pathway leading to rapid expansion of the HC lesion after exposure to intense noise (Hu et al., 2002). Inspection of noise-damaged cochleae has also revealed the presence of necrotic death of OHCs (Hamernik et al., 1974; Kellerhals, 1972 Yang et al., 2004).
Several factors that influence the ratio of the apoptotic and necrotic generation have been identified. Hu et al. (2000) reported that the intensity of the noise is a factor affecting the trend in apoptotic and necrotic incidence. When the cochleae were exposed to a high level noise (120 dB SPL), OHCs appeared to die primarily through apoptosis. In contrast, when the cochleae received a relatively lower level of noise exposure (105 dB SPL), necrosis appeared to be the dominant type of cell death. Another factor that affects the ratio of apoptosis and necrosis is the post-exposure time at which the cochleae are examined. Hu et al. (2002) reported that apoptosis was the major driving force behind the rapid expansion of the cochlear lesion during the first two days of post-exposure pathogenesis. Yang et al. (2004) reported that apoptosis and necrosis contributed equally to the continuous generation of dying OHCs at the late phase of cochlear pathogenesis. Although the coexistence of apoptotic and necrotic OHCs has been observed for some time, the cellular mechanisms that regulate cell death propensity toward apoptosis or necrosis are still not known.
Apoptosis is an active death process, requiring an adequate energy supply to fuel the apoptotic machinery at the early phase of the death process (Majno et al., 1995). There is increasing evidence showing that the intracellular energy status plays a pivotal role in determining whether a cell dies by apoptosis or necrosis (Eguchi et al., 1997; Leist et al., 1997; Nicotera et al., 1998). When the cellular ATP level is maintained above a threshold level, a cell dies by apoptosis. However, if the cellular ATP level falls below the threshold level, the cell dies by necrosis (Sokolova et al., 2004).
Mitochondria are the primary sites of cellular energy production. A recent study has shown the maintenance of mitochondrial energy production during the period of apoptotic initiation in the OHC (Hu, 2007). However, whether the mitochondrial energy production is a crucial factor that controls the cell’s decision to die by apoptosis or necrosis is not known, nor is the role of mitochondrial energy production in regulating the apoptotic process, once the process is initiated. To address these issues, we used an established model of mitochondrial dysfunction induced by local application of 3-nitropropionic acid (3-NP), an irreversible inhibitor of succinate dehydrogenase (SDH), to produce mitochondrial dysfunction (Alston et al., 1977; Coles et al., 1979; Ludolph et al., 1992), and investigated the effects of the mitochondrial energetic deficiency on the generation of apoptosis and necrosis following exposure to intense noise. Specifically, the following questions were addressed: In the noise-damaged cochlea, did reduction of the mitochondrial energy production: (1) prevent the induction of apoptosis; (2) shift OHC death from apoptosis to necrosis; (3) disrupt the execution of the apoptotic process?
2. Materials and Methods
2.1. Subjects and noise exposure
Fifty-eight adult chinchillas (450-650 g) were used in the study. Among these animals, 37 animals were exposed to noise and the remaining 21 animals without exposure to noise served as normal controls. All procedures involving use and care of the animals were reviewed and approved by the State University of New York at Buffalo Institutional Animal Care and Use Committee.
An impulse noise was used to generate acoustic trauma. The impulse noise consisted of a series of 75 pairs of impulses (1 second between each pair) generated by a D/A converter on a signal processing board (Loughborough TMS 32020). The level of impulses was calibrated with a 0.25 inch condenser microphone (Larson Davis, 2520) and microphone amplifier (Larson Davis 824A) and the output displayed on an oscilloscope (Tectronix TDS1002). The peak level of the noise was 155 dB peak sound pressure level (pSPL).
We selected a high level of noise for the current study for several reasons. First, exposure to a high level of noise is a common health hazard seen in industrial, recreation and military activities. Therefore, the study on the cell death resulting from exposure to such high level of noise has both biological and clinical significances. Second, the same level of noise has been used in our previous studies (Harris et al., 2005; Hu et al., 2006). We have a wealth of data on the magnitudes of hearing loss and hair cell loss resulting from the noise exposure. By using a consistent noise level in our current study, we could compare the results of the current study with those from our previous studies. Finally, compared with the exposure to a low level of noise, exposure to a high level of noise produces a greater number of damaged (dying) hair cells in the cochlea, which facilitates the observation of the patterns of cell death.
2.2. Disruption of the mitochondrial energetic function
SDH was selected as a target enzyme for inhibition of the mitochondrial energy production because hair cells contain strong SDH activity (Chen et al., 2000) and SDH activity is preserved during the major course of OHC apoptosis following exposure to intense noise (Hu, 2007). SDH is a respiratory enzyme in the Krebs cycle, converting succinic acid to fumaric acid. SDH also forms a part of complex II in the electron transport chain that is the primary source of ATP production within mitochondria.
SDH activity was inhibited by 3-NP, an irreversible SDH inhibitor produced on moldy crops by the fungus Arthrinium sp. 3-NP (Sigma, USA) was dissolved in a normal artificial perilymph (142 mM NaCl, 5.37 mM KCl, 1.47 mM MgCl2, 2 mM CaCl2, and 10 mM HEPES) to generate a stock solution (400 mM). The stock solution was stored at a temperature of -20 °C for less than one month. Before each application, the stock solution was diluted with the artificial perilymph to produce a working concentration (20 or 50 mM).
3-NP was locally applied to the cochleae at either one of two time points: 16 hrs before or immediately after exposure to an intense impulse noise. Application of 3-NP before the noise exposure allowed us to assess the effect of mitochondrial inhibition on the initiation of apoptosis, whereas the post-noise application enabled us to investigate the effect of mitochondrial dysfunction on the execution phase of apoptosis. When 3-NP was applied before the noise exposure, the compound was applied on the round window, so that the structural integrity of the inner ear was preserved. When applied after the noise exposure, the compound was perfused directly into the cochlea to enhance a fast diffusion of the compound into OHCs.
• Round window application of 3-NP
The 3-NP solution was applied 16 hrs before exposure to the noise to allow a thorough diffusion of the compound into the cochlea. The chinchilla was anesthetized with a mixture of ketamine (35 mg/kg) and acepromazine (0.5 mg/kg). Both left and right bullae were exposed through a posterior approach. A small hole was made in the posterior surface of the bulla to visualize the round window. Approximately 15 μl of the 3-NP solution (either 20 or 50 mM) was dropped onto the right round window using a micro-syringe and an equal volume of the artificial perilymph solution was dropped onto the left round window, serving as a control. The hole on the bulla was sealed with dental cement and the skin incision was sutured. Sixteen hours after the compound application, the animals were exposed to the impulse noise.
• Cochlear perfusion with 3-NP
The 3-NP solution was applied within 30 min after the noise exposure. The animal was anesthetized with a mixture of ketamine (35 mg/kg) and acepromazine (0.5 mg/kg) immediately after the noise exposure. Both the left and right cochleae were surgically exposed through a conventional ventral approach. Two small openings were drilled in the bony shell over the scala vestibuli and the scala tympani in the basal turn of the cochlea. Approximately 20 μl of either the 3-NP solution (20 mM) or the control artificial perilymph was gently perfused into the cochlea through the hole on the scala tympani within a period of 3 min. The infused solution was allowed efflux through the hole on the scala vestibuli. After the cochlear perfusion, the openings on the bony shell of the cochlea were sealed with a tissue adhesive (Vetbond Tissue Adhesive 3M, USA). The contralateral cochlea received the same treatment, but with an alternative solution (either 3-NP or artificial perilymph solution), which differed from that used for the first cochlea.
2.3. Auditory evoked potential measurement
Inferior colliculus potentials were examined in six animals to determine the functional impact of the 3-NP treatment. The animals were treated with the 3-NP solution (20 mM) on the round window of one ear and the control solution in the contralateral ear. The testing was performed before and 15 hrs after the 3-NP treatment. The inferior colliculus potentials were elicited by tone pips at octave intervals from 1-8 kHz with a method that has been previously reported in detail (Hu et al., 1997b). The threshold of the inferior colliculus potential was defined as the mid-point between the lowest level at which a clear response was seen and the next lower level where no response was seen.
2.4. Pathological examination
All the noise-exposed animals were sacrificed at 2 hrs after the noise exposure. The normal control animals (without exposure to the noise) were sacrificed with the same time schedule used for the noise-exposed animals. The cochleae were collected for pathological examinations. A series of dependent variables were examined. Tables 1 and 2 show the number of cochleae used for each experimental parameter.
Table 1.
The number of cochleae used for assessment of the 3-NP effects in the control animals without exposure to the noise
| SDH | PI | |
|---|---|---|
| Artificial perilymph | 4 | 4 |
| 3-NP RW (20 mM) | 6 | 4 |
| 3-NP RW (50 mM) | 5 | 6 |
| 3-NP CP (20 mM) | 5 | 6 |
Note: RW: round window application. CP: Cochlear perfusion
Table 2.
The number of cochlear used for each pathological parameter in noise exposed groups
| PI | Cas-3 + PI | |
|---|---|---|
| 3-NP RW (20 and 50 mM) | 19 | 5 |
| Perilymph RW | 19 | 5 |
| 3-NP CP (20 mM) | 9 | 4 |
| Perilymph CP | 9 | 4 |
Note: RW: Round window application.
CP: Cochlear perfusion
2.4.1. SDH staining and assessment
SDH activity was examined to assess the effectiveness of the 3-NP treatment. SDH activity was evaluated with a colorimetric assay using nitroblue tetrazolium (NBT) monosodium salt (Hu, 2007). Briefly, the animals were sacrificed at the defined time after the noise exposure. Cochleae were quickly removed from the skull and perfused through the round window with the SDH staining solution. After the SDH staining, the cochleae were fixed with 10% buffered formalin. After dissection, the organs of Corti were further stained with propidium iodide (PI) to visualize OHC nuclei.
For each cochlea, the optical density of SDH reaction was measured with image processing software (ImageJ from NIH). This density was expressed in the form of the mean gray level, where the mean gray level = sum of gray value / number of pixels measured. Specifically, the sections of the organ of Corti from the 3-NP treated cochleae and the corresponding sections from control cochleae treated with the artificial perilymph were photographed with identical parameters using a digital camera (Nikon, Coolpix 995). The images were transformed into gray images. The gray level of the image pixels enclosed within the contour of about 30 OHCs was measured and then averaged. To reduce the influence of systematic errors, the gray level in the area of selected OHCs was normalized by comparing the OHC value with that measured in the nearby pillar cell region where no detectable SDH activity could be found. Using this approach instead of measuring the absolute gray level of SDH staining reduced the influence of systemic variations, such as microscopic illumination, the thickness of specimens, and digital camera noises.
2.4.2. Nuclear staining of OHCs with PI
PI (Molecular Probes, Inc.) was used to assess the membrane permeability and visualize the nuclear morphology. Based on the status of the membrane integrity and the morphological change of nuclei, the viability of OHCs and modes of cell death were determined.
PI staining solution (5 μg/ml in 10 mM PBS) was gently perfused into the cochleae through the round window and the perfused solution was allowed efflux through the oval window. After the perfusion, the PI solution was allowed to remain in the cochleae for 10 min at room temperature. After the staining, the cochleae were fixed with 10% buffered formalin. In certain cochleae, PI staining was performed together with another staining method either for SDH or caspase-3.
The criteria for identification of apoptotic and necrotic OHCs have been reported in previous publications (Nicotera et al., 2003; Yang et al., 2004). The following is a brief description of these criteria.
Viable OHCs
Viable OHCs were defined as the OHCs lacking PI fluorescence in the nuclei. These cells also exhibited the normal shape, size and texture of the nuclei.
Apoptotic OHCs
In PI stained specimens, OHCs showing condensed or fragmented nuclei were considered apoptotic cells. The nature of apoptosis was further confirmed by caspase staining.
Necrotic OHCs
The principle criterion for detecting necrotic OHCs was nuclear swelling with malformed shapes and loss of normal nuclear textures. Necrotic cells also featured a strong PI uptake due to membrane disruption.
Secondary necrotic OHCs
The dying OHCs that exhibited both apoptotic (caspase-3 activity) and necrotic (nuclear swelling) phenotypes were identified as secondary necrotic OHCs.
To illustrate the general extent of cochlear damage, the sizes of the central lesions in the organs of Corti were measured in the 3-NP-treated and the perilymph-treated control ears following exposure to the impulse noise. The central lesion was defined as the cochlear section in which the number of damaged OHCs was equal to or greater than 50% of the total number of OHCs in that region. Identification of the central lesions was achieved by inspecting PI stained nuclei. The sizes of the lesions were measured with a reticle placed in an eyepiece of the microscope. Because the central lesion was composed primarily of apoptotic OHCs, the size of the central lesion reflected the magnitude of the apoptotic pathology in the organ of Corti.
To assess the incidences of apoptosis and necrosis, the numbers of apoptotic and necrotic cells were quantified. The number of necrotic cells was directly counted with the criteria described above. The number of apoptotic cells was estimated by subtracting the sum of the numbers of necrotic and viable OHCs from the total number of OHCs normally occupying the cochlear region.
2.4.3. Detection of active Caspase-3
The activity of caspase-3 was detected using the fluorescent probe, FAM-DEVD-FMK (Intergen Company, USA). The basic labeling procedure has been previously published (Hu et al., 2002). Briefly, the animal was anesthetized with a mixture of ketamine (35 mg/kg) and acepromazine (0.5 mg/kg) at the defined time after the noise exposure. Both the left and right cochleae were surgically exposed through a conventional ventral approach. Two small openings were drilled in the bony shell over the scala vestibuli and the scala tympani in the basal turn of the cochlea. Through the opening on the scala tympani, the cochleae were perfused with approximately 20 μl of the freshly prepared solution, and the excessive staining solution was allowed efflux through the opening on the scala vestibuli. The organ of Corti was incubated in the same staining solution for 50 min. Then, the cochleae were perfused with the PI solution for 10 min. After the staining, the cochleae were collected for dissection.
2.4.4. Microscopic observation and image collection
All the specimens were observed with a microscope with bright field for SDH observation or epifluorescence illumination for inspection of PI and caspase-3 labeling. The cochlear regions containing active dying OHCs (apoptotic and necrotic) were further observed with a confocal microscope (Bio-Rad, MRC 1024), with a method that has been reported previously (Hu et al., 2002). The ability of confocal microscopy to conduct a series of optical sections enabled us to separate OHC nuclei and supporting cell nuclei (Hu et al., 2006). To prevent cross-talk and bleed-through of two fluorescences, the double labeled specimens were imaged using sequential acquisition of different fluorophores.
2.5. Statistics
The current experimental procedures had two features that improved the clarity of the results. First, all experiments had appropriate controls. Second, the noise exposure caused HC damage that was localized to the second cochlear turn. Therefore, there was a within-cochlea control, i.e., the cochlear tissue at the apex.
The statistical means of threshold shifts of IC potentials between the 3-NP-treated and the perilymph-treated control ears were compared using a two-way ANOVA, with one factor being the treatment and the other factor being frequency. All noise-induced threshold shifts were calculated relative to the thresholds measured before the 3-NP treatment.
The statistical method used for comparison of the numbers of apoptotic and necrotic OHCs between the 3-NP-treated and the perilymph-treated control ears was the chi-square distribution. The comparison of the gray levels of SDH activity before and after 3-NP treatment and among different concentrations was conducted using a one-way ANOVA or t-test. For all the statistical analyses, the significance value was set at p < .05.
3. Results
3.1. Effects of the 3-NP treatment in the normal control cochleae without exposure to noise
In the first part of the experiment, we examined the 3-NP effects on three parameters of the normal cochleae, including SDH activity, HC viability and the auditory function, with two concentrations of 3-NP (20 mM or 50 mM) and two routes of drug delivery (round window application or cochlear perfusion).
3.1.1. SDH activity following the round window application of 3-NP
Figs. 1A, 1B, and 1C show typical examples of SDH staining in the second cochlear turns, corresponding to the cochlear section where the maximal noise-induced HC damage takes place, from three cochleae collected at 16 hrs after the application of the artificial perilymph solution and the 3-NP solution on the round window. Fig. 1A shows a normal control cochlea that received only the vehicle solution. The dark staining in OHCs indicates strong SDH activity. Fig. 1B shows a marked reduction of SDH staining in a cochlea treated with 20 mM of 3-NP. Fig. 1C shows the further reduction of SDH activity as the concentration of the 3-NP solution increases from 20 mM to 50 mM.
Figure 1.
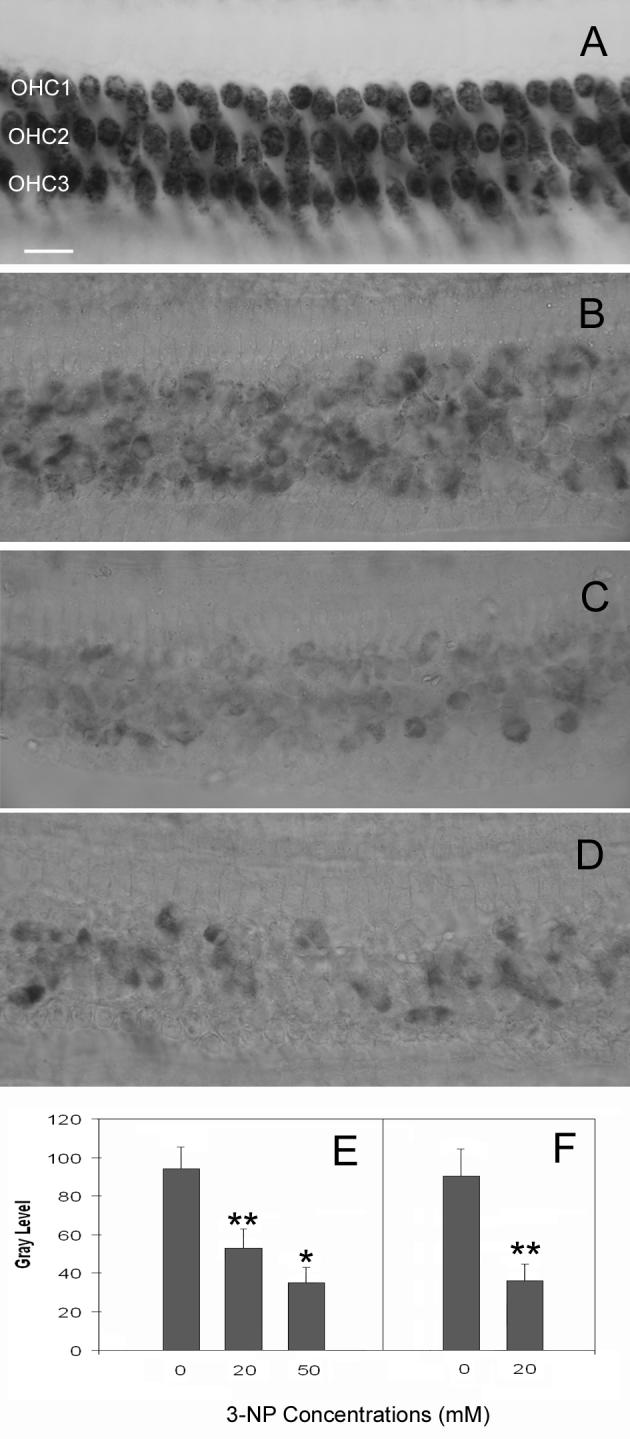
Comparison of the levels of SDH reduction. A: SDH staining in a normal control cochlea that received only the artificial perilymph solution. The dark staining in OHCs indicates strong SDH activity. B: SDH activity following the treatment of 20 mM of 3-NP on the round window. Notice a significant reduction of SDH staining. C: SDH staining after the treatment of 50 mM of 3-NP on the round window. SDH activity appears further reduced. D: SDH activity following cochlear perfusion of the 3-NP solution (20 mM). SDH staining is substantially reduced to a level similar to that induced by the round window application of 3-NP at 50 mM. E: Comparison of the average gray levels of SDH staining among the cochleae treated with the artificial perilymph solution (control), 20 mM or 50 mM of 3-NP solutions on the round window. F: The comparison of the gray levels of SDH staining between the 3-NP perfused ears and the artificial perilymph-perfused control ears. OHC1, OHC2, and OHC3 represent the first, second and third row of OHCs respectively. * indicates p < .05 and ** indicates p < .01. Bar: 20μm.
The average optical densities of SDH staining were measured in the cochleae treated with the artificial perilymph, 20 mM, or 50 mM of 3-NP solutions (Fig. 1E). Compared to the artificial perilymph-treated cochleae, there is a significant reduction in SDH staining (p < .01) in the cochleae that received the 20 mM or the 50 mM of 3-NP treatment. There is also a significant difference in the gray levels of SDH staining between the cochleae treated with 20 mM and with 50 mM of 3-NP (p < .05). This observation indicates that the round window application of the 3-NP solution effectively suppressed SDH activity in the OHCs.
3.1.2. SDH activity following the cochlear perfusion of 3-NP
Fig. 1D shows a typical pattern of SDH activity in a cochlea collected 2 hrs after the cochlear perfusion of the 3-NP solution at 20 mM. SDH staining is substantially reduced to a level similar to that observed in the cochleae that received the round window application of the 3-NP solution at 50 mM. Fig. 1F shows the comparison of the average optical densities of SDH staining between the 3-NP perfused ears and the vehicle-perfused control ears. The gray level of the SDH staining in the 3-NP-treated ears is significantly reduced (p < .01).
3.1.3. Auditory function following the 3-NP treatment
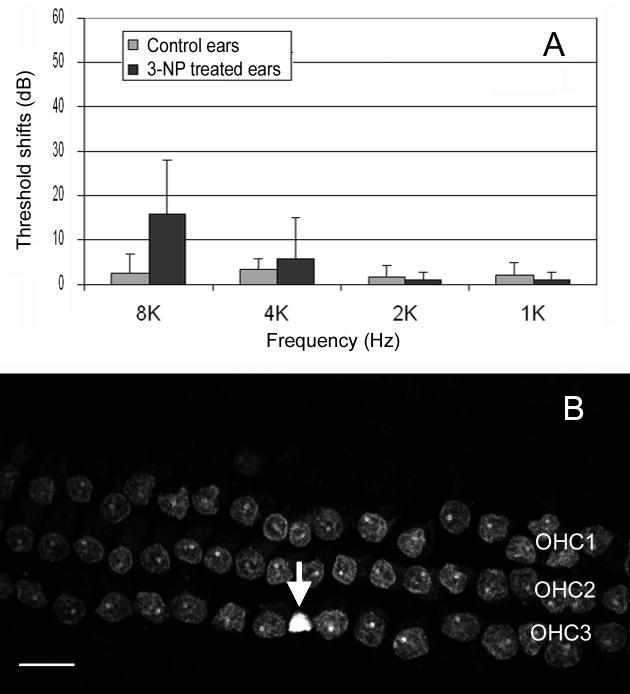
To evaluate the effect of the 3-NP treatment on the auditory function and to better explain the consequences of the observed SDH reduction, the auditory thresholds were estimated using the auditory evoked inferior colliculus potentials measured before and 15 hrs after application of the 3-NP solution (20 mM) on the round window. Fig. 2A shows the comparisons of threshold shifts between the 3-NP-treated ears and the control ears. There are no significant threshold shifts in the control ears following the sham surgery, indicating that the surgery itself did not affect hearing sensitivity. In the 3-NP-treated ears, there is an average of a 15.5 dB threshold shift at 8 kHz, whereas there is no threshold shift in other tested frequencies. However, the threshold shift at 8 kHz was not statistically significant due to large individual variation. The appearance of the threshold shift at 8 kHz in certain animals was likely caused by the effect of SDH inhibition, rather than by the middle ear disturbance due to the drug application. If the hearing loss was caused by middle ear disturbance, we would expect to see a low frequency hearing loss in those ears. Additionally, the control ears would have shown threshold shifts as well.
Figure 2.
A: The comparison of the threshold shifts of the inferior colliculus potentials between the ears treated with and without 3-NP. The 3-NP treated ears have an average of 15.5 dB greater threshold shift at 8 kHz. However, the shift is not statistically significant. B: The morphology of OHC nuclei stained by PI in the basal part of a cochlea following application of 50 mM of 3-NP on the round window. The arrow points to a condensed nucleus. OHC1, OHC2, and OHC3 represent the first, second and third row of OHCs respectively. Bar: 15 μm.
3.1.4. Impact of the 3-NP treatment on OHC viability
OHC viability was examined with PI staining to further assess the effect of the 3-NP treatment on the normal OHCs. In the normal cochleae without the 3-NP treatment, microscopic observation of the organ of Corti revealed no PI positive cells (data not shown), indicating no OHC death in normal cochleae.
When the cochleae were treated with 20 mM of 3-NP on the round window, the three rows of OHCs appeared slightly disarranged in the basal part of the cochlea, suggesting the occurrence of body swelling of the cells. Additionally, some nuclei became malformed. However, most of the OHCs excluded PI, except for a few cells (less than 10) in the basal extreme of the cochlea, which showed a strong uptake of PI and swollen nuclei, indicating that these cells were dying. In the cochleae treated with a cochlear perfusion of 20 mM of 3-NP, there was no sign of cell death in any cochlear turns, possibly due to the fact that these cochleae were examined at 2 hrs after the drug exposure, whereas the cochleae receiving the round window 3-NP application were examined at 16 hrs after the drug application.
When 50 mM of 3-NP was applied on the round window, malformed nuclei were seen in both the first and second cochlear turns. In the first turn, about 3-10% of OHCs had a strong PI fluorescence. Above the first cochlear turn, positively PI stained cells were barely seen. Fig. 2B shows an example of the basal section of the organ of Corti from a cochlea treated with 50 mM of 3-NP. To illustrate the nuclear morphology in both dying and viable cells, the cochlea was stained after the cochlear fixation. Therefore, the nuclei of both viable and dying cells are visible. An arrow shows a condensed nucleus. The neighboring cell nuclei appear slightly malformed.
Collectively, the observation of the 3-NP treatment in normal cochleae without exposure to the noise indicates that the application of 3-NP through either the round window approach or the direct cochlear perfusion could effectively inhibit SDH activity in the cochlear section where the initial noise-induced damage takes place.
3.2. Effects of the 3-NP treatment on induction of OHC apoptosis following exposure to intense noise
To examine the effect of the mitochondrial dysfunction on initiation of the noise-induced apoptosis, the size of the central cochlear lesions and the numbers of apoptotic and necrotic OHCs was documented in the cochleae that received the 3-NP treatment (either 20 or 50 mM) on the round window before the noise exposure.
3.2.1. Sizes of the central lesions
The sizes of the central lesions differed considerably among individual cochleae. However, there was no systematic difference between the cochleae treated with 20 mM and 50 mM of 3-NP. Therefore, the data obtained from the two conditions were collectively analyzed. Fig. 3A shows the comparison of the average sizes of the central lesions between the ears treated with 3-NP and the ears treated with the artificial perilymph on the round window. There is a trend toward a reduction in the size of the cochlear lesions in the 3-NP-treated ears following exposure to the noise. However, the reduction is not statistically significant (p > .05). This result indicates that inhibition of the mitochondrial energetic function did not attenuate noise-induced OHC damage in the early phases of the cochlear pathogenesis.
Figure 3.
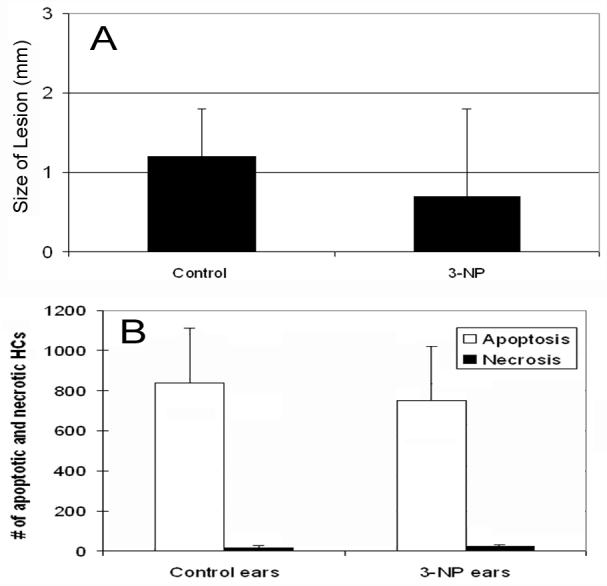
A: The comparison of the average sizes of the central lesions between the 3-NP-treated ears and the ears without the 3-NP treatment following exposure to the impulse noise. B: The comparison of the numbers of apoptotic and necrotic nuclei in the center of the cochlear lesion between the 3-NP-treated and the perilymph-treated control cochleae. There is no significant increase in the number of necrotic cells after the 3-NP treatment (p>0.05).
3.2.2. The numbers of apoptotic and necrotic cells
To determine whether the inhibition of the mitochondrial function affects the generation of apoptosis, we quantified the numbers of apoptotic and necrotic cells in the cochlear lesions. Fig. 3B shows the comparison of the numbers of apoptotic and necrotic nuclear lesions between the ears treated with 3-NP and the ears treated with the artificial perilymph on the round window. Overall, the cochlear lesions of both the 3-NP-treated and the perilymph-treated cochleae were dominated by the apoptotic pathology. Necrotic OHCs accounted for only a small fraction of the overall cell death. There was no significant increase in the number of necrotic cells after the 3-NP treatment (p > .05). This result indicates that compromising the mitochondrial energy production does not suppress the initiation of apoptosis. There was no major shift of apoptosis to necrosis in the OHCs with the compromised mitochondrial energetic function.
To confirm the nature of apoptosis in the OHCs with condensed or fragmented nuclei and to determine whether some of OHCs with necrotic nuclei possessed certain apoptotic characteristics, we examined caspase-3 activity in OHCs. In the normal controlled cochleae without exposure to the noise, there was no caspase-3 activity in OHCs (data not shown).
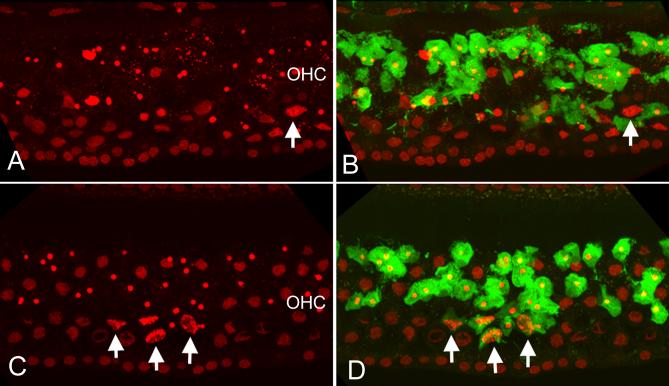
In the perilymph-treated control cochleae, exposure to the noise caused rapid activation of caspase-3. Strong caspase-3 activities were observed in the OHCs exhibiting nuclear condensation or fragmentation (Fig 4B). However, there was no caspase-3 activity in the dying cells with swollen nuclei (an example indicated by the arrow in 4B).
Figure 4.
Double staining of PI and caspase-3 activity in a 3-NP treated ear and a control ear without the 3-NP treatment following exposure to the noise. A: Nuclear morphology of OHCs in the perilymph-treated control ear. B: The same cochlear section showing both the nuclear morphology and the caspase-3 fluorescence (green). Notice that strong caspase-3 activity is observed in the OHCs exhibiting nuclear condensation or fragmentation. However, there is no caspase-3 activity in the dying cells with swollen nuclei (indicated by an arrow in A and B). C and D: the PI fluorescence (C) and PI plus caspase-3 (D) in a 3-NP-treated cochlea. Similar to the perilymph-treated control cochlea (see A and B), the 3-NP treated cochlea shows caspase-3 activity in all the OHCs having condensed nuclei. However, certain OHCs with necrotic nuclei also exhibit caspase-3 activity (arrows). The labels of OHC in A and B indicate the OHC region.
Examination of the cochleae treated with 3-NP on the round window revealed that the OHCs with mitochondrial energetic dysfunction continued to exhibit caspase-3 activity in a pattern similar to that of the noise-exposed cochleae treated with artificial perilymph. All the OHCs having condensed or fragmented nuclei showed strong caspase-3 activity. However, the OHCs with necrotic nuclei had diverse patterns of caspase-activity. Over two-thirds of the OHCs showing necrotic nuclei lacked caspase-3 activity. A small portion of OHCs with necrotic nuclei showed caspase-3 activity (arrows in Figs. 4C and 4D). This finding of caspase-3 activity in OHCs having swollen nuclei following the 3-NP treatment suggests that inhibition of mitochondrial energy production drove a small portion of cells to die through a secondary necrotic pathway.
3.3. Effects of the 3-NP treatment on the execution of apoptosis
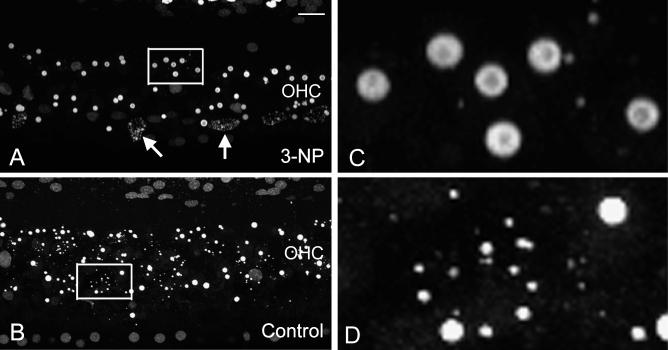
To investigate the influence of the 3-NP treatment on the process of apoptotic execution, the progression of nuclear degradation was observed. Overall, the perilymph-treated control ears exhibited diversity in the extent of nuclear degradation with a large quantity of small nuclear fragments. In contrast, the formation of small nuclear fragments was less common in the 3-NP-treated ears. Fig. 5 shows typical examples of nuclear morphologies in a 3-NP-treated cochlea and a contralateral control ear of the same animal. Notice that in the 3-NP-treated cochlea (Fig. 5A; see also 5C for a high magnification view), nuclear condensation appears to be the dominant nuclear pathology. Only a few cells show nuclear fragmentation (arrows). In contrast, the perilymph-treated control ear exhibits a large quantity of nuclear fragments (Fig. 5B; see also 5D for a high magnification view). To quantify the difference, the average sizes of nuclei were compared between the 3-NP-treated ears and the perilymph-treated control ears. The comparison showed that the average size of apoptotic nuclei in the perilymph-treated control cochleae was significantly smaller than that in the 3-NP-treated cochleae (5.4 ± 7.6 μm2 vs. 11.2 ± 12.1 μm2, p < .01). The finding of significantly fewer nuclear fragments in the 3-NP-treated ears suggests the delay of the progression of nuclear degradation following mitochondrial inhibition because the onset of nuclear condensation precedes the onset of nuclear fragmentation (Hu et al., 2006).
Figure 5.
Examples of nuclear morphologies in a 3-NP treated cochlea (A) and an artificial perilymph-treated control ear (B) after the noise exposure. Both cochlear sections show a large number of apoptotic nuclei. However, the extent of nuclei shrinkage is different. The 3-NP-treated ear is dominated by nuclear condensation. Only a few cells show nuclear fragmentation (arrows). In contrast, the perilymph-treated control ear exhibits a large quantity of small nuclear fragments. C and D show the high-magnification views of the regions indicated by the squares in A and B, respectively. Bar: 20 μm. The labels of OHC in A and B indicate the OHC region.
4. Discussion
In the current study, we used an established method, inhibition of SDH activity with 3-NP, to reduce mitochondrial ATP production. This approach has been widely used in previous studies of both in vitro and in vivo subjects to generate an experimental model of mitochondrial dysfunction (Alston et al., 1977; Coles et al., 1979; Ludolph et al., 1992). Studies have shown that the resulting level of ATP suppression following a 3-NP treatment is dose- and time-dependent. For example, Pang, et al. (1997) reported that application of a concentration of 3-NP (5 mM) reduces the cellular ATP level in cultured rat hippocampal neurons by about 25% at 2 hrs after the 3-NP treatment. In the current study, we intended to use the highest dose allowed without causing rapid cell death to generate maximal suppression of SDH activity. Our pilot study showed that treatment of 3-NP at 100 mM resulted in a widespread OHC death in the cochlea. Based on this observation, we used the concentration of 50 mM as the maximal dose. This near-lethal dose of the 3-NP treatment resulted in a small amount of OHC death in the basal portion of the cochlea. It should be noted that the 3-NP induced cell death should not be confused with the cell death caused by exposure to the intense noise because the former was confined primarily in the basal extreme of the cochlea, whereas the noise-induced cell death started from the second cochlear turn.
In the current study, instead of directly measuring the ATP level in OHCs, we monitored the 3-NP effect by observing SDH activity. With this approach, we were unable to evaluate the precise level of the ATP alteration in OHCs following the 3-NP treatment. However, based on the physiological and pathological impacts of the 3-NP treatment, we postulate that the 3-NP treatment generated a significant reduction in the mitochondrial ATP production. The experimental evidences supporting this postulation include: (1) SDH staining revealed a marked reduction of SDH activity in the cochleae treated with the 3-NP; (2) the loss of the auditory function was observed in certain cochleae treated with 20 mM of 3-NP. It is expected that the treatment of 50 mM of 3-NP would generate a greater functional impact; (3) the loss of OHC viability in the basal part of the cochlea was observed when 50 mM of 3-NP was applied. These findings suggest a significant reduction of the mitochondrial function in the 3-NP treated cochleae, particularly in those cochleae receiving 50 mM of the 3-NP treatment. Collectively, the 3-NP treatment used in the current study appeared to generate a near-lethal mitochondrial dysfunction in OHCs. This level of mitochondrial dysfunction provides us with an ideal model to assess the role of mitochondrial dysfunction in the generation of apoptosis following exposure to intense noise.
4.1. Persistence of apoptotic initiation in the OHCs with suppressed SDH activity following exposure to intense noise
The first question to be addressed in the current study was the role of the mitochondrial energetic function in initiation of OHC apoptosis following exposure to intense noise. The study revealed that the mitochondrial dysfunction induced by the inhibition of SDH activity did not induce a major shift of cell death from apoptosis to necrosis.
The failure to halt the induction of OHC apoptosis following the 3-NP treatment suggests the presence of a residual ATP supply to the apoptotic machinery even when the mitochondrial energetic function is substantially interrupted. The residual energy production may derive from two sources. The first is the surviving mitochondria. Although the 3-NP treatment could cause substantial inhibition of SDH activity, a small amount of SDH might still function. The second source of ATP production is cytosolic glycolysis. In non-cochlear tissues, the role of glycolysis in the generation of apoptosis has been studied. Atlante et al (2005) reported that complete suppression of the oxidative phosphorylation by oligomycin caused a decrease of the ATP content in cells undergoing apoptosis, but cells could still die by apoptosis. When the ATP level was further decreased by suppressing both oxidative phosphorylation and glycolysis, cell death shifted to necrosis. Similarly, a study by Volbracht et al. (1999) showed that the application of ATP-depleting agents prevented apoptotic death. However, repletion of ATP by enhanced glycolysis restored all apoptotic features. These studies suggest that the continuous functioning of glycolysis in a cell can generate enough ATP to fuel the apoptotic process.
The current study provides no further evidence showing the separate contributions of residual mitochondrial oxidative phosphorylation and cytosolic glycolysis to the ATP synthesis in the apoptotic cells. However, considering the level of SDH inhibition, we think that the mitochondrial ATP production is not the primary contributor of the residual ATP production in the current experimental condition. We predict that if ATP production in OHCs were further reduced by suppression of cytosolic glycolysis on the top of mitochondrial inhibition, the apoptotic cell death would be completely suppressed. Confirmation of this hypothesis warrants further studies.
4.2. Disruption of the mitochondrial energy production drives a small portion of OHCs to die through secondary necrosis
An interesting finding of the current study was that certain dying cells with the necrotic nuclear morphology simultaneously exhibited caspase-3 activity, a key apoptotic phenotype, indicating that these cells entered a secondary necrotic pathway. This finding raises an important question as to why only these cells entered the secondary necrotic pathway, whereas most other OHCs continued to undergo the classic apoptotic pathway. Here we offer an explanation based on the consideration of the regulatory role of the cellular energy level in determining the ultimate fate of cells.
In the current study, the experimental treatments (3-NP and noise exposure) can, presumably, cause ATP reduction of various extents in individual OHCs. Considering the sources of ATP production in the OHC (Puschner et al., 1997), we speculate that if mitochondrial oxidative phosphorylation is substantially compromised, while the cytosolic glycolysis is preserved, the cell would enter the apoptotic pathway. However, if both oxidative phosphorylation and glycolysis are compromised, the cell would die by necrosis. If oxidative phosphorylation is compromised, whereas glycolysis is only partially disrupted, the cell would die by the pathway of secondary necrosis.
If the above speculation on the cause of the secondary necrosis is applicable to “naturally” occurring cochlear damage induced by exposure to intense noise, one would expect to see this type of the pathology in the perilymph-treated control cochleae because metabolic disruption following exposure to intense noise could generate variable levels of ATP reduction. However, so far, we have not seen the presence of individual OHCs possessing both apoptotic and necrotic phenotypes in the noise-damaged cochlea without the 3-NP treatment. The secondary necrosis appears to be a unique phenomenon that occurs only in the current experimental condition in which the cochleae were exposed to both noise and 3-NP. Therefore, it is possible that the secondary necrosis is regulated by more than one cellular mechanism, which deserves a close look in future studies.
4.3. Disruption of the mitochondrial energy supply slows the pace of progression of apoptosis
The current study demonstrated that dysfunction of mitochondrial energetic function delayed the progression of the nuclear disassembly in apoptotic OHCs. In the perilymph-treated control ears, small nuclear fragments were commonly seen in the center of the cochlear lesion. The presence of small nuclear fragments shortly after the noise exposure suggests rapid degradation of OHC nuclei. In contrast, small nuclear fragments were much less common in the 3-NP-treated ears. This observation suggests that the pace of progression of nuclear degradation was reduced following the disruption of the mitochondrial energetic function.
The cellular mechanism responsible for the delay of the apoptotic process following inhibition of mitochondrial ATP generation is not known. Because the generation of apoptotic phenotypes, including nuclear degradation, has been found to be related to caspase-3 activity (Janicke et al., 1998; Woo et al., 1998), the role of caspase-3 activity should be considered. There is evidence from studies of non-auditory tissues showing the dependence of caspase activation on the level of cellular ATP during apoptosis (Eguchi et al., 1999; Leist et al., 1999). These observations suggest that the slowdown of nuclear degradation observed in the current study is possibly due to an incomplete activation of caspase-3 following the reduction of the mitochondrial energy production. In the current study, we did not quantify the caspase-3 activity and therefore were unable to assess the precise role of caspase-3 in controlling the apoptotic execution. Nevertheless, our study did demonstrate caspase-3 activity in the 3-NP-treated cochleae, which may be responsible for the generation of apoptotic phenotypes of OHCs. Understanding the involvement of caspase-3 in the delayed apoptotic events requires further study using quantitative techniques to monitor the status of caspase-3 activity.
Acknowledgments
The authors thank Richard Salvi and Robert F. Burkard for their critical comments. The authors also thank Chiemi Tanaka, Andrew B. Knapp, Brian J. Sawka and Rebecca L. Utech for their help in preparing the manuscript. Research was supported by the Grant NIDCD 1R03 DC006181-01A1.
Abbreviations
- OHC
outer hair cell
- SDH
succinate dehydrogenase
- 3-NP
3-nitropropionic acid
- PI
propidium iodide
- ATP
adenosine triphosphate
- PBS
Phosphate Buffered Saline
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- Alston TA, Mela L, Bright HJ. 3-Nitropropionate, the toxic substance of Indigofera, is a suicide inactivator of succinate dehydrogenase. Proc Natl Acad Sci U S A. 1977;74:3767–71. doi: 10.1073/pnas.74.9.3767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atlante A, Giannattasio S, Bobba A, Gagliardi S, Petragallo V, Calissano P, Marra E, Passarella S. An increase in the ATP levels occurs in cerebellar granule cells en route to apoptosis in which ATP derives from both oxidative phosphorylation and anaerobic glycolysis. Biochim Biophys Acta. 2005;1708:50–62. doi: 10.1016/j.bbabio.2005.01.009. [DOI] [PubMed] [Google Scholar]
- Bohne BA, Harding GW, Lee SC. Death pathways in noise-damaged outer hair cells. Hear Res. 2007;223:61–70. doi: 10.1016/j.heares.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Chen GD, McWilliams ML, Fechter LD. Succinate dehydrogenase (SDH) activity in hair cells: a correlate for permanent threshold elevations. Hear Res. 2000;145:91–100. doi: 10.1016/s0378-5955(00)00076-9. [DOI] [PubMed] [Google Scholar]
- Coles CJ, Edmondson DE, Singer TP. Inactivation of succinate dehydrogenase by 3-nitropropionate. J Biol Chem. 1979;254:5161–7. [PubMed] [Google Scholar]
- Eguchi Y, Shimizu S, Tsujimoto Y. Intracellular ATP levels determine cell death fate by apoptosis or necrosis. Cancer Res. 1997;57:1835–40. [PubMed] [Google Scholar]
- Eguchi Y, Srinivasan A, Tomaselli KJ, Shimizu S, Tsujimoto Y. ATP-dependent steps in apoptotic signal transduction. Cancer Res. 1999;59:2174–81. [PubMed] [Google Scholar]
- Hamernik RP, Henderson D, Crossley JJ, Salvi RJ. Interaction of continuous and impulse noise: audiometric and histological effects. J Acoust Soc Am. 1974;55:117–21. doi: 10.1121/1.1928141. [DOI] [PubMed] [Google Scholar]
- Harris KC, Hu B, Hangauer D, Henderson D. Prevention of noise-induced hearing loss with Src-PTK inhibitors. Hear Res. 2005;208:14–25. doi: 10.1016/j.heares.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Hu BH. Delayed mitochondrial dysfunction in apoptotic hair cells in chinchilla cochleae following exposure to impulse noise. Apoptosis. 2007 doi: 10.1007/s10495-006-0027-7. [DOI] [PubMed] [Google Scholar]
- Hu BH, Henderson D. Changes in F-actin labeling in the outer hair cell and the Deiters cell in the chinchilla cochlea following noise exposure. Hear Res. 1997a;110:209–18. doi: 10.1016/s0378-5955(97)00075-0. [DOI] [PubMed] [Google Scholar]
- Hu BH, Henderson D, Nicotera TM. Involvement of apoptosis in progression of cochlear lesion following exposure to intense noise. Hear Res. 2002;166:62–71. doi: 10.1016/s0378-5955(02)00286-1. [DOI] [PubMed] [Google Scholar]
- Hu BH, Henderson D, Nicotera TM. Extremely rapid induction of outer hair cell apoptosis in the chinchilla cochlea following exposure to impulse noise. Hear Res. 2006;211:16–25. doi: 10.1016/j.heares.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Hu BH, Zheng XY, McFadden SL, Kopke RD, Henderson D. R-phenylisopropyladenosine attenuates noise-induced hearing loss in the chinchilla. Hear Res. 1997b;113:198–206. doi: 10.1016/s0378-5955(97)00143-3. [DOI] [PubMed] [Google Scholar]
- Hu BH, Guo W, Wang PY, Henderson D, Jiang SC. Intense noise-induced apoptosis in hair cells of guinea pig cochleae. Acta Otolaryngol. 2000;120:19–24. [PubMed] [Google Scholar]
- Janicke RU, Sprengart ML, Wati MR, Porter AG. Caspase-3 is required for DNA fragmentation and morphological changes associated with apoptosis. J Biol Chem. 1998;273:9357–60. doi: 10.1074/jbc.273.16.9357. [DOI] [PubMed] [Google Scholar]
- Kellerhals B. Acoustic trauma and cochlear microcirculation. An experimental and clinical study on pathogenesis and treatment of inner ear lesions after acute noise exposure. Adv Otorhinolaryngol. 1972;18:91–168. [PubMed] [Google Scholar]
- Leist M, Single B, Castoldi AF, Kuhnle S, Nicotera P. Intracellular adenosine triphosphate (ATP) concentration: a switch in the decision between apoptosis and necrosis. J Exp Med. 1997;185:1481–6. doi: 10.1084/jem.185.8.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leist M, Single B, Naumann H, Fava E, Simon B, Kuhnle S, Nicotera P. Nitric oxide inhibits execution of apoptosis at two distinct ATP-dependent steps upstream and downstream of mitochondrial cytochrome c release. Biochem Biophys Res Commun. 1999;258:215–21. doi: 10.1006/bbrc.1999.0491. [DOI] [PubMed] [Google Scholar]
- Ludolph AC, Seelig M, Ludolph AG, Sabri MI, Spencer PS. ATP deficits and neuronal degeneration induced by 3-nitropropionic acid. Ann N Y Acad Sci. 1992;648:300–2. doi: 10.1111/j.1749-6632.1992.tb24562.x. [DOI] [PubMed] [Google Scholar]
- Majno G, Joris I. Apoptosis, oncosis, and necrosis. An overview of cell death. Am J Pathol. 1995;146:3–15. [PMC free article] [PubMed] [Google Scholar]
- Nicotera P, Leist M, Ferrando-May E. Intracellular ATP, a switch in the decision between apoptosis and necrosis. Toxicol Lett. 1998;102-103:139–42. doi: 10.1016/s0378-4274(98)00298-7. [DOI] [PubMed] [Google Scholar]
- Nicotera T, Henderson D, Hu BH, Zheng XY. Noise exposure and mechanisms of hair cell death. In: Henderson D, Prasher D, Kopke R, Salvi R, Hamernik R, editors. Noise Induced Hearing Loss: Basic Mechanisms, Prevention and Control. Noise Research Network Publications; London: 2001. pp. 99–117. [Google Scholar]
- Nicotera TM, Hu BH, Henderson D. The caspase pathway in noise-induced apoptosis of the chinchilla cochlea. J Assoc Res Otolaryngol. 2003;4:466–77. doi: 10.1007/s10162-002-3038-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu X, Shao R, Canlon B. Suppression of apoptosis occurs in the cochlea by sound conditioning. Neuroreport. 2003;14:1025–9. doi: 10.1097/01.wnr.0000070830.57864.32. [DOI] [PubMed] [Google Scholar]
- Pang Z, Geddes JW. Mechanisms of cell death induced by the mitochondrial toxin 3-nitropropionic acid: acute excitotoxic necrosis and delayed apoptosis. J Neurosci. 1997;17:3064–73. doi: 10.1523/JNEUROSCI.17-09-03064.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puschner B, Schacht J. Energy metabolism in cochlear outer hair cells in vitro. Hear Res. 1997;114:102–6. doi: 10.1016/s0378-5955(97)00163-9. [DOI] [PubMed] [Google Scholar]
- Shizuki K, Ogawa K, Matsunobu T, Kanzaki J, Ogita K. Expression of c-Fos after noise-induced temporary threshold shift in the guinea pig cochlea. Neurosci Lett. 2002;320:73–6. doi: 10.1016/s0304-3940(02)00059-9. [DOI] [PubMed] [Google Scholar]
- Sokolova IM, Evans S, Hughes FM. Cadmium-induced apoptosis in oyster hemocytes involves disturbance of cellular energy balance but no mitochondrial permeability transition. J Exp Biol. 2004;207:3369–80. doi: 10.1242/jeb.01152. [DOI] [PubMed] [Google Scholar]
- Volbracht C, Leist M, Nicotera P. ATP controls neuronal apoptosis triggered by microtubule breakdown or potassium deprivation. Mol Med. 1999;5:477–89. [PMC free article] [PubMed] [Google Scholar]
- Wang J, Dib M, Lenoir M, Vago P, Eybalin M, Hameg A, Pujol R, Puel J. Riluzole rescues cochlear sensory cells from acoustic trauma in the guinea-pig. Neuroscience. 2002;111:635–648. doi: 10.1016/s0306-4522(02)00004-0. [DOI] [PubMed] [Google Scholar]
- Woo M, Hakem R, Soengas MS, Duncan GS, Shahinian A, Kagi D, Hakem A, McCurrach M, Khoo W, Kaufman SA, Senaldi G, Howard T, Lowe SW, Mak TW. Essential contribution of caspase 3/CPP32 to apoptosis and its associated nuclear changes. Genes Dev. 1998;12:806–19. doi: 10.1101/gad.12.6.806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang WP, Henderson D, Hu BH, Nicotera TM. Quantitative analysis of apoptotic and necrotic outer hair cells after exposure to different levels of continuous noise. Hear Res. 2004;196:69–76. doi: 10.1016/j.heares.2004.04.015. [DOI] [PubMed] [Google Scholar]
- Ylikoski J, Xing-Qun L, Virkkala J, Pirvola U. Blockade of c-Jun N-terminal kinase pathway attenuates gentamicin-induced cochlear and vestibular hair cell death. Hear Res. 2002;166:33–43. doi: 10.1016/s0378-5955(01)00388-4. [DOI] [PubMed] [Google Scholar]