Abstract
The processed products of the proopiomelanocortin gene (ACTH, α-MSH, β-MSH, γ-MSH, etc.) interact with five melanocortin receptors, the MC1R, MC2R, MC3R, MC4R, and MC5R to modulate and control many important biological functions crucial for good health both peripherally (as hormones) and centrally (as neurotransmitters). Pivotal biological functions include pigmentation, adrenal function, response to stress, fear/flight, energy homeostasis, feeding behavior, sexual function and motivation, pain, immune response, and many others, and are believed to be involved in many disease states including pigmentary disorders, adrenal disorders, obesity, anorexia, prolonged and neuropathic pain, inflammatory response, etc. The roeianocortin-3 receptor (MC3R) is found primarily in the brain and spinal cord and also in the periphery, and its biological functions are still not well understood. Here we review some of the biological functions attributed to the MC3R, and then examine in more detail efforts to design and synthesize ligands that are potent and selective for the MC3R, which might help resolve the many questions still remaining about its function. Though some progress has been made, there is still much to be done in this critical area.
INTRODUCTION
The five melanocortin receptors and their corresponding agonist ligands which are derived from the precursor protein proopiomelanocortin (POMC) and the endogenous antagonist protein ligands agouti and agouti related protein (AGRP) have been the subject of scientific investigation for over 100 years, primarily for their function in melanopigmentation and adrenal function [1–4] and disorders related to pigmentation, stress and melanoma cancer. More recently, however, interest in both the melanotropin ligands and melanocortin receptors has greatly accelerated due to the cloning of the receptors in the 1990s, and the discovery of a number of important functions for the ligands and their receptors in a wide variety of biological activities including feeding behavior, obesity, anorexia, cachexia, sexual function and motivation, pain, immune response, cardiovascular function and many others [5,6]. And new discoveries of the involvement of this system in an ever wider variety of biological activities and behaviors including many diseases of the periphery and the central nervous system (CNS)are being made.
The major focus of this paper is on the melanocortin-3 system. The melanocortin-3 receptor (MC3R) is located on POMC neurons in the CMS, especially in the arcuate nucleus and within other areas of the brain [7] including the hypothalamus, and in several peripheral organs as well. It often is co-localized with the MC4R, but its precise function in obesity, cachexia, and related feeding behaviors is still not well understood. Initially (while still an orphan receptor) the MC3R was linked to non-insulin-dependent diabetes mellitus on human chromosome 20q which we now know is the MC3R [8,9], and there is some evidence that the MC3R is an inhibitory autoreceptor on POMC neurons including the stimulation of food intake by peripheral administration of an MC3R specific agonist [10], and inhibition of spontaneous action of POMC neurons after application of a MC3R agonist [11]. Interestingly and paradoxically in the MC3R knockout mice an obesity syndrome is observed with reduced lean body mass and increased fat mass for both sexes, and with no apparent hyperphagia and with apparent normal energy expenditure [12,13,14]. Interestingly the MC3R knockout mice appear to be resistant to high fat diet-induced insulin resistance [14]. These and other findings suggest that development of peptide and non-peptide ligands that are selective for the MC3R may herald the arrival of exciting new classes of drugs for the treatment of a variety of disease states that involve melanotropin peptides and melanocortin receptors. In addition they can serve as valuable ligands for sorting out the detailed mechanism(s) of action of these ligands and their receptors which are poorly understood.
THE MELANOCORTIN-3 RECEPTOR (MC3R) – OVERALL CONSIDERATIONS
The MC3R is expressed in the central nervous system, peripheral tissues and immune cells, with initial studies highlighting expression in brain, gut, and placenta but none were detected in the adrenal gland or melanocytes [15], This lack of expression in the adrenal glands is not surprising given the fact that MSH peptides do not increase circulating corticosterone levels, through activation of the hypothalamic pituitary adrenal axis (HPA) [16]. As already discussed, a potential role in energy metabolism has been postulated for the MC3R since in MC3R null mice there is an increased fat mass and higher ratio of weight gain to food intake [13]. Other studies have demonstrated a central role in modulating the host inflammatory response with receptor detection on peritoneal [16–18] and knee joint macrophages [19]. Activation of MC3R in the heart has been shown to exhibit a protective effect in ischaemic-reperfusion injury [20,21]. Thus the MC3R can be viewed as a fine tuner of specific mechanisms operating during inflammation, cardiovascular function and energy metabolism.
In the central nervous system, the melanocortin type 3 receptor (MC3R) is located on POMC neurons and other areas of the hypothalamus and often is co-localized with the melanocortin type 4 receptor (MC4R). The MC4R which regulates food intake is activated and modulated by the endogenous agonist, α-melanocyte stimulating hormone (α-MSH), a peptide processed from POMC, and also is modulated by an endogenous antagonist, agouti-related protein (AGRP) (orexigenic) [22]. Leptin, an adipocyte-derived hormone, acts on POMC and AGRP neurons in the arcuate nucleus of the hypothalamus, resulting in increased α-MSH and decreased AGRP [23]. Several lines of evidence have indicated that activation of the MC4R by α-MSH or synthetic peptide agonists reduces food intake, but that suppression of MC4R signaling by AGRP or synthetic melanocortin-4 receptor antagonists increases food intake and diminishes the hypophagic response to leptin [24,25]. Thus, the central melanocortin pathway is extremely important for normal energy homeostasis, and energy homeostasis via this pathway is highly susceptible to quantitative variations in MC3R/MC4R expression.
Melanocortin receptors belong to the superfamily of seven transmembrane G protein-coupled receptors (GPCRs). The hMCRs transduce signals by coupling to the heterotrimeric Gs protein and subsequent activation of adenylate cyclase. In many GPCRs, signaling is rapidly attenuated within minutes of agonist exposure, a process termed desensitization [26,27]. This process is associated with phosphorylation of serine/threonine residues in the third internal loop or carboxyl tail of the GPCR by G protein-coupled receptor kinases (GRKs) or second-messenger-dependent kinases [28]. There is no direct evidence for the hMC3R phosphorylation site yet. Phosphorylation by GRKs allows the GPCRs to interact with cytoplasmic proteins, the arrestins, which uncouple the GPCRs from G proteins [28,29]. For the hMCRs, arrestins target the receptor to clathrin-coated vesicles, and the receptor is sequestered into an intracellular vesicular compartment, probably endosomes, after pinching off the vesicles from the plasma membrane by dynamin [30]. After sequestration, the GPCRs may recycle to the surface membrane after resensitization [31,32] or undergo lysosomal degradation [33]. Certain GPCRs can be targeted selectively to lysosomes leading to down-regulation of the GPCR by the same membrane pathway that mediates internalization [33]. Down-regulation of GPCRs in various neural cell types is of particular interest because this may lead to certain diseases, such as addiction [34], and opiate tolerance and dependence [35]. More generally any down-regulation/desensitization, which occurs for GPCRs in response to agonists, leads to a decrease in drug efficacy.
Despite the central importance of the MC3R/MC4R in energy homeostasis, feeding behavior and the existence of an endogenous agonist and antagonist of the melanocortin system, highly selective MC3R agonists and antagonists, which could have important applications in treatment of cachexia and type II diabetes, and in evaluating the role of the MC3R in these conditions, are not yet available. Design of selective MC3R/MC4R agonists and antagonists thus becomes of critical importance. Our research group has been working on the design and synthesis of selective melanotropins for many years. Describing in a single review all existing approaches that have been taken to obtain ligands that target human melanocortin receptors (hMCRs) is not possible. Rather we will examine a few different approaches investigators have taken for design of selective ligands for the MC3R which reflect the current knowledge. We hope this will address the fundamental and the methodological issues which can promote rational design of more selective hMCR ligands. In this regard, understanding the molecular mechanisms underlying hMCRs function is also important in the design of ligands with the desired pharmacological profile. Knowledge of the current biostructural data is also important since it is becoming increasingly clear that stereostructural differences between the closely related receptors, such as the MC3R in relation to the MC1R, MC4R and MC5R, will be an important aspect of any successful development of a selective ligand for biological and biomedical applications [36].
GENERAL CONSIDERATIONS FOR THE DESIGN OF hMC3R SELECTIVE AGONISTS AND ANTAGONISTS
Although numerous native peptides have great potential for medical applications, and over 50% of all current drugs are peptide or peptidomimetic-based, often peptides have to be modified to enhance certain properties including metabolic stability, selectivity, and bioavailability, to be suitable therapeutic agents. Thus design of modified peptides for peptidomimetic and non-peptide applications has dramatically advanced during the last two decades [e.g. 37–42], One of the most challenging aspects of this research is rational design based on 3D-topographical features of the peptide pharmacophore. The first strategy in this approach is to identify the core structural features which are necessary for receptor/acceptor recognition. This is usually accomplished by truncation of the whole sequence; systematic single amino acid modifications of the peptide such as glycine, alanine, D-amino acid, etc. scans; ring size scans, bulky amino acid scans, etc. [e.g. 41,43]. Once the structure activity relationships (SAR) of the peptide ligand have been elucidated, the conformation-activity relationships have to be examined. For this purpose, a wide variety of physical/chemical methods are employed. For example, the three-dimensional arrangement of critical side chain groups and backbone conformations can be analyzed via NMR spectroscopy [44,45], X-ray crystallography [46], circular dichroism measurements [47], and computational methods [48]. The next step is to find a suitable non-peptide scaffold which can impart the same 3D topographic structural features as the peptide based on conformational analysis, computational chemistry and 3D modeling. The derived scaffold then can be used to replace the peptide drug pharmacophore and position the crucial structural elements correctly in 3D space. Finally, the structure can be refined by evaluating the biological responses of designed ligands. The general scheme for the de novo design of peptide drugs has been outlined in a recent review [41]. Understanding the conformation-activity relationships of biologically active peptides can provide important guidance in the design of peptide drugs and accelerate the process from native peptides to biologically active peptide drug or to peptidomimetics and small molecules.
STRUCTURE-ACTIVITY RELATIONSHIPS OF LINEAR MSH-DERIVED PEPTIDES
The endogenous MC3R agonist: γ-MSH H-Tyr-Val-Met-Gly-His-Phe-Arg-Trp-Asp-Arg-Phe-Gly-OH [49] was discovered by homologous screening of the various core sequences of POMC that contain the critical tetrapeptide sequence -His-Phe-Arg-Trp- [50–51]. Biological screening demonstrated that γ-MSH was selective for the hMC3R by up to 100 fold, compared to α-MSH [49]. Grieco et al. used a D-amino acid scan of the γ-MSH sequence to obtain the first highly selective agonist for the hMC3R (H-Tyr-Val-Met-Gly-His-Phe-Arg-D-Trp-Asp-Arg-Phe-Gly-OH; EC50 = 0.33 nM; hMC4R/hMC3R = 300, hMC5R/hMC3R = 250) [43]. Furthermore, replacement of the oxidizable Met3 residue with Nle increased the stability of the peptide [52], which is being widely used as a ligand for the hMC3R in a variety of ongoing pharmacological studies and in animal model studies related to the hMC3R. Interestingly, when the Phe6 residue of γ-MSH was substituted with D-Nal(2′), a substitution known to convert α-MSH analogues to antagonists at the hMC3R and hMC4R [53], the resulting analogue (H-Tyr-Val-Nle-Gty-His-D-Nal(2′)-Arg-Trp-Asp-Arg-Phe-Gly-NH2) exhibited potent hMC3R/hMC5R antagonist activity, while retaining potent full agonist activity at the hMC4R [54].
Hybridization of α-MSH with γ-MSH [52] proved to be an effective approach toward the design of selective hMC3R ligands, yielding a potent hMC3R selective antagonist (H-Tyr-Val-Nle-Gly-His-D-Phe-Arg-D-Nal(2′)-Asp-Arg-Phe-Gly-NH2; IC50 = 6 nM), which also showed potent agonist activities at the hMC1R, hMC4R and hMC5R (Table 1).
Table 1.
Melanotropin Peptide Analogues Selective for Human Melanocortin-3 Receptor
| a) Agonists/Partial Agonists | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ref. | Sequence | hMC1R | hMC3R | hMC4R | hMC5R | ||||||||
| IC50,nM | EC50,nM | % max effect |
IC50,nM | EC50,nM | % max effect |
IC50,nM | EC50,nM | % max effect |
IC50,nM | EC50,nM | % max effect |
||
| [43] | H-Tyr-Val-Met-Gly-His-Phe-Arg-D-Trp-Asp-Arg-Phe-Gly-OH | ND | ND | ND | 6.7 ± 1 | 0.33 ± 0.01 | 100 | 600 ± 70 | 100 ± 11 | 99 | 340 ± 40 | 82 ± 10 | 97 |
| [69] | c[Nle-Arg-D-Phe-Arg-Trp-Glu]-NH2 | 1,400±10 0 | >10,000 | 0 | 140±10 | 1.2±0.2 | 112 | 270±26 | 174±18 | 63 | 68.0±2 | >10,000 | 0 |
| [62] | H-D-Phe-c[Asp-Pro-D-Phe-Arg-Trp-Lys)-NH2 | ND | ND | ND | 3.7±0.05 | 4.87±0.5 | 61 | 700±100 | >10,000 | 0 | 1,100±180 | >10,000 | 0 |
| b) Antagonists | |||||||||||||
| Ref. | Sequence | hMC1R | hMC3R | hMC4R | hMC5R | ||||||||
| IC50,nM | EC50,nM | % max effect | IC50,nM | EC50,nM | % max effect | IC50,nM | EC50,nM | % max effect | IC50,nM | EC50,nM | % max effect | ||
| [60] | c[(O)C-(CH2)3,-C(O)-His-D-Nal(2′)Arg-Trp-Lys]-NH2 | ND | ND | ND | 5.9±0.19 | >10,000 | 0 | 220±13 | 1,200±200 | 22.8 | 26±1.4 | 1.01±0.09 | 81 |
| [61] | Ac-Nle-c[Asp-Tic-D-Nal(2′)Arg-Trp-Lys]-NH2 | ND | ND | ND | 6.7±0.6 | >10,000 | 0 | 3.7±0.3 | >10,000 | 0 | 1.24±0.13 | 2.05±0.2 | 102 |
| [61] | Ac-Nle-c[Asp-Acpc-D-Na1(2′)-Arg-Trp-Lys]-NH2 | ND | ND | ND | 2.5±0.2 | >10,000 | 0 | 270±30 | >10,000 | 0 | 4.1±0.5 | 390±50 | 50 |
| [63] | Ac-c[Cys-Glu-Pro-D-Nal(2′)-Arg-Trp-Cy5]-Pro-Pro-Lys-Asp-NH2 | ND | ND | ND | 31±10 | >10,000 | 0 | 17,000 | >10,000 | 0 | 97,000 | >10,000 | 0 |
| [63] | Ac-c[Pen-Glu-His-D-Nal(2′)-Arg-Trp-Cys]-Pro-Pro-Lys-Asp-NH2 | ND | ND | ND | 3.0±0.5 | >10,000 | 0 | 750±100 | >10,000 | 0 | 94±11 | >10,000 | 0 |
| [52] | H-Tyr-Val-Nle-Gly-His-D-Phe-Arg-D-Nal(2′)-Asp-Arg-Phe-Gly-OH | 0.7±0.1 | 2.0±0.2 | 87 | 6.0±0.5 | >10,000 | 0 | 6,0±1 | 36±4 | 100 | 5.0±1 | 200±20 | 67 |
| [69] | c[Nle-Val-D-Nal(2′)-Arg-Trp-Glu]-NH2 | 4,000 | >10,000 | 0 | 1.7±0.1 | >10,000 | 0 | 180±20 | 16±0.3 | 13 | 2.2±0.5 | >10,000 | 0 |
| [71] | Ac-Nle-c[Asp-Aba-D-Phe-Arg-Trp-Lys]-NH2 | >10,000 | >10,000 | 0 | 50±6 | >10,000 | 0 | >10,000 | >10,000 | 0 | 2,900±300 | >10,000 | 0 |
GLOBAL AND LOCAL CONFORMATIONAL CONSTRAINTS IN THE DESIGN OF hMC3R SELECTIVE PEPTIDES
The conversion of the linear α-MSH into a cyclic peptide to enhance potency, metabolic stability, and to test hypotheses regarding a β-turn structure for the bioactive conformation of melanotropin ligands was an early innovation in the development of approaches to peptide ligand design. In the case α-MSH and its fragment analogues, the Cys4,Cys10(Cys11) analogues were examined over 25 years ago [55,56] and this evolved using computational methods, molecular dynamic simulations, conformational analysis via NMR and molecular modeling to the development of cyclic lactam analogues of α-MSH such as the truncated analogue Ac-Nle4-c[Asp5,D-Phe7,Lys10]α-MSH(4–10)-NH2 (MTII) [57,58] which is used worldwide for the study of melanocortin 1, 3, 4 and 5 receptors for both in vitro and in vivo studies because of its high potency, metabolic stability, and excellent biodistribution properties [59]. More recently we have examined a number of other cyclization strategies to obtain melanocortin receptor selective ligands. As an earlier effort to develop highly selective and potent agonists and/or antagonists for the hMC receptors, a new group of linker arms and a backbone to side chain cyclization strategy were employed (Fig. 1). A key analogue was found to have the desired selectivity and potency at the hMC3 receptors which were implicated to play pivotal roles in energy homeostasis and other biological effects in this study. Structure-activity studies [60] have shown that replacing the succinyl linker arm of 1 by an o-phthalic acid group and substituting a D-Nal(2′)7 residue in place of D-Phe7 results in a potent antagonist 7 (Fig. 1) at the hMC4 receptor. Furthermore, increasing the 23-membered lactam ring of 1 by one carbon atom (succinic →glutaric acid linker) gives a highly selective and potent antagonist 9 for the hMC3 receptor (Fig. 1). This cyclization strategy showed that a 23 membered ring is favored for maintaining good binding at all subtypes of melanocortin receptors [60]. Increasing the ring size or introducing an aromatic linker are likely to lead to more selective melanotropins. Thus, the selective MC3R antagonist: c[(O)C-(CH2)3-C(O)-His-D-Nal(2′)-Arg-Trp-Lys)]-NH2 showed good biological activity as an antagonist with a pA2= 10.6 and IC50 = 5.6 nM (24 membered ring, (hMC4R/hMC3R = 38)) [60].
Fig. (1).
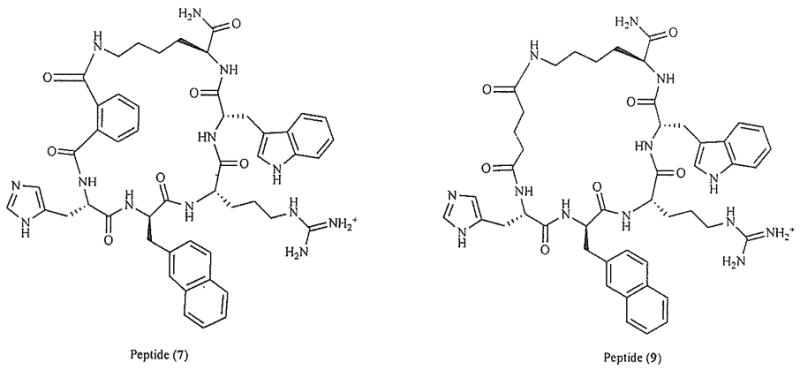
Key analogues of 1, Peptide 7 synthesized by introducing an o-phthalic acid in the cyclization linker of 1; Peptide 9 synthesized by replacing D-Phe7 with D-Nal(2′)7, and by replacing the cyclization linker of 1 by a glutaric acid linker [60].
In another approach, the template of SHU-9119, Ac-Nle4-c[Asp5-His6-D-Nal(2′)7-Arg8-Trp9-Lys10]-NH2), a potent but non-selective hMC3R/hMC4R antagonist [53] was modified in position 6 with nonconventional amino acids (Fig. 2) [61]. The biological results of this series of compounds clearly indicate that a conformational restriction with bulky amino acids in position 6 in a cyclic peptide can be used to obtain selective antagonists for the hMC3R and the hMC4R (pA2 = 9.1–9.8) [61]. Furthermore, modification of the MT-II template by aNle4 → Xaa4 substitutions (Xaa = aromatic amino acid) resulted in improved hMC4R/hMC3R selectivities, notably yielding a highly hMC3R-selective partial agonist PG-951 (H-D-Phe4-c[Asp5-His6-D-Phe7-Arg8-Trp9-Lys10]-NF2, IC50 = 3.7 nM, EC50 = 4.87 nM, 61% cAMP stimulation) (Table 1) [62].
Fig. (2).
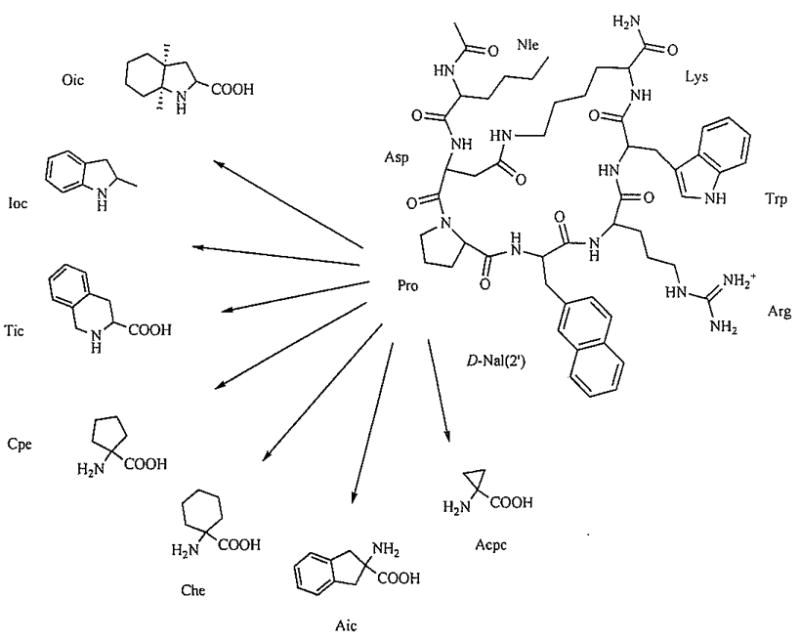
Structure of the potent monocyclic peptide Ac-Nle-c[Asp-Pro-D-Nal(2′)-Arg-Trp-Lys]NH2, referred to as PG-901, and the conformationally constrained amino acids [61].
Disulfide cyclization of the native γ-MSH around the pharmacophore sequence His-Phe-Arg-Trp provided no further leads toward the enhancement of hMC3R selectivity [54]. On the other hand, such cyclization in the α-MSH/β-MSH hybrid series yielded potent and highly selective hMC3R antagonists Ac-c[Cys-Glu-Pro-D-Nal(2′)-Arg-Trp-Cys]-Pro-Pro-Lys-Asp-NH2, and Ac-c[Pen-Glu-His-D-Nal(2′)-Arg-Trp-Cys]-Pro-Pro-Lys-Asp-NH2 (IC50 = 31 and 3 nM, respectively) (Table 1) [63].
Introduction of the χ-constrained phenylproline analogues into the His6 position of the MT-II template resulted in topographical changes that lead to increased hMC5R selectivity, which indicated that hMC3 and hMC4 receptors are more sensitive to steric effects and χ-conformational constraints than the hMC5 receptor [64]. Another example of using χ-constrained amino acids that lead to selective partial agonists and antagonists at the hMC3R was obtained by introducing (o-Phe)-Phe at the D-Phe position of MT-II. yielding a selective partial antagonist of the MC3R (Ac-Nle-c[Asp-His-(o-Phe)Phe-Arg-Trp-Lys]-NH2) [65]. This is a typical example showing that introducing an alkyl or aryl group on the aromatic ring of an aromatic amino acid residue, particularly in the ortho position can significantly restrict its conformation in χ2 space. The χ2 torsional angle can be efficiently restricted by the interaction between the aryl moiety and the β-hydrogens of the amino side-chain in the o-substituted phenylalanine and finally increase the selectivity of melanocortin receptors.
USING NMR BASED MODELING AND COMPUTATIONAL METHODS FOR MELANOCORTIN RECEPTOR SELECTIVE LIGANDS
Computational methods in conjunction with NMR structural determination and molecular modeling are a very useful tool in peptide and peptidomimetic design considerations, with the caveat that for novel structures, current force fields might not give a completely accurate picture of the structure or energetics of the ligands. Along with the NMR structure of AGRP (the endogenous antagonist of melanotropin) (PDB: 1HYK) [66] and MTII (potent agonist) [67], the 3D pharmacophore of human melanocortin receptor ligands has been partially deciphered. Results from these biophysical and chemical studies can be crucial for designing novel selective agonist and antagonist melanotropins [52].
Recent conformation-activity relationship studies also have yielded some important structural clues for development of hMC3R-selective ligands. Thus, Ying et al. [67] determined that solution structures of the non-selective agonist MT-II, hMC3R/hMC4R antagonist SHU9119, hMC4R-selective agonist VJH085 and hMC3R-selective antagonist MK-9 all feature (β-turn-like motifs spanning the His6 and D-Phe/D-Nal(2′)7 residues. This structural feature was hypothesized to be very important for receptor-ligand recognition and interaction. Grieco et al. had found that replacement of the His6 residue in SHU9119 template with a variety of conformationally constrained amino acids leads to substantially improved receptor selectivity (Table 1) [61, 68]. On the basis of these results, Grieco et al. suggested that the improvement in receptor selectivity was due to differences between the hMC3R and hMC4R binding pockets, which are able to accommodate amino acid residues with different conformational profiles.
Recently, our laboratories have produced several potent and selective hMC3R agonists and hMC3R/hMC5R antagonists by placing a bulky hydrophobic Nle residue next to the melanocortin pharmacophore Xaa-Phe-Arg-Trp in a cyclic γ-MSH-derived template (Fig. 3] [69]. The observed hMC3R selectivity was attributed to steric interference between the Nle4 side chain and the Arg7 binding space, based on MCMM-LMCS/OPLS-AA simulations, as well as relatively high rigidity of the 20-membered cyclic lactam template. Similar results were obtained when Nle was placed within the melanocortin pharmacophore, in position 6 of a cyclic α-MSH template, yielding an hMC3R antagonist cyclo (5β→10ε)-(succinyl5-Nle6-D-Phe7-Arg8-Trp9-Lys10)-NH2 (IC50 = 84 nM) and an hMC3R/hMC5R antagonist cyclo(5β→10ε)-(succinyl5-Nle6-D-Nal(2′)7-Arg8-Trp9-Lys10)-NH2 (IC50 = 12 and 17 nM, respectively: pA2 = 8.3 (hMC3R) and 8.7 (hMC5R)) [70] These findings underscore the significance of steric factors in melanocortin receptor selectivity, and suggest that introduction of bulky residues in the direct proximity to the melanocortin pharmacophore is an effective approach to design of novel hMC3R-selective agonists and antagonists. Congruent findings were reported by Ballet et al for the MT-II/SHU9119 cyclic Lactam template, where the His6-Xaa7 residues were replaced with the 4-amino-1,2,4,5-tetrahydro-2-benzazepin-3-one-Xaa (Aba-Xaa) building blocks (Fig. 4) [71] resulting in a highly hMC3R selective antagonist Aba-2 (Ac-Nle4-c[Asp5-Aba6-D-Phe7-Arg8-Trp9-Lys10)-NH2, IC50 = 50 nM) and a hMC3R/hMC5R antagonist Aba-4 (Ac-Nle4-c[Asp5-Aba6-D-Nal(2′)7-Arg8-Trp9-Lys10)-NH2, IC50 = 43 and 87 nM, respectively) (Table 1). The authors hypothesized that the observed hMC3R receptor selectivity may be caused by either steric influence of the Aba block or its unique conformational features, or, possibly, both.
Fig. (3).
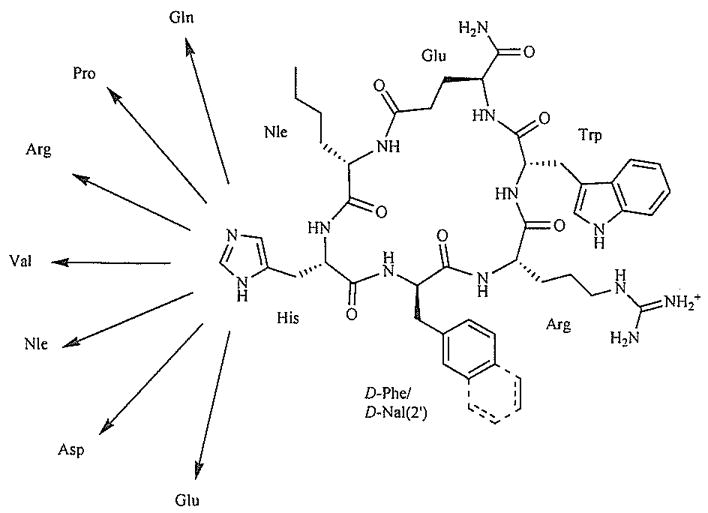
Design of the γ-MSH-derived cyclic lactam scaffold [69].
Fig. (4).
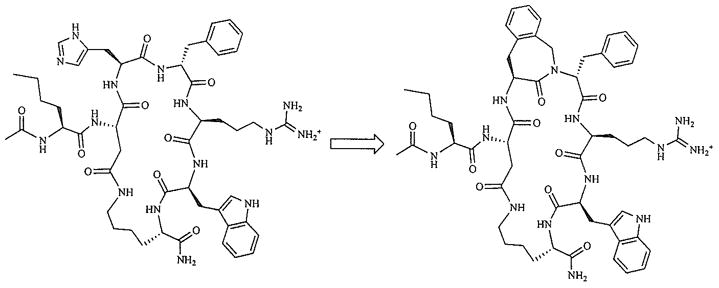
Design of the MT-II-derived Aba analogues [71].
Deletion of His6 from the sequence of cyclic α-MSH analogues, reported by Bednarek et al. [72], yielded a hMC4R-selective antagonist MBP10 (cyclo(6β->10ε)-(succinyl6-D-Nal(2′)7-Arg8-Trp9-Lys10)-NH2). It was suggested that the tripeptide core D-Nal(2′)-Arg-Trp was sufficient for high binding affinity toward the hMC4R, whereas the tetrapeptide core His-D-Nal(2′)-Arg-Trp is required for high binding affinity toward the hMC3R and hMC5R. Contrary to this hypothesis, our recent results [70] suggest that the tripeptide sequence D-Phe/D-Nal(2′)-Arg-Trp is sufficient for high binding affinity and agonist activity not only at the hMC4R but also at the hMC3R, as exemplified by cyclic peptides AVM75, cyclo(6β→10ε)-(2,3-pyrazinedicarbonyl6-D-Phe-Arg8-Trp9-Lys10)-NH2, and AVM76, cyclo(6β→10ε)-(2,3-pyrazinedicarbonyl6-D-Nal(2′)7-Arg8-Trp9-Lys10)NH2, which exhibit a potent and selective partial agonist (EC50 = 27 nM, 70% cAMP stimulation) and antagonist (IC50 = 23 nM) activities at the hMC3R, respectively (Fig. 5). These findings strongly suggest that the observed high receptor selectivities in ‘tripeptide-pharmacophore’ analogues are due to different conformational and steric properties of the linker arm, rather than different pharmacophore sequence requirements of the melanocortin receptor subtypes. Thus, manipulation of the linker structure is proving to be a powerful tool in the development of highly selective melanotropin peptides.
Fig. (5).
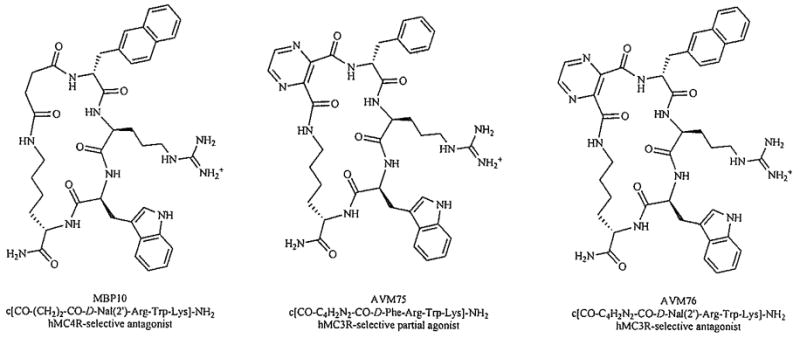
Development of highly selective ‘tripeptide-pharmacophore’ analogues via modulation of the linker arm [70, 72].
DEVELOPMENT OF hMC3R-SELECTIVE SMALL MOLECULE LIGANDS
Though many commercial pharmaceutical companies have been examining small molecules that can bind to the melanocortin receptors, surprisingly few of these ligands appear to have high potency and selectivity for the MC3R. It is worth noting that a number of publications of small-molecule research report little or no data for the MC3R.
One of the most popular strategies to emerge in the pursuit of small-molecule ligands for the melanocortin receptors has been the use of so-called “privileged structure” motifs, presumably biased towards GPCR activity, in combination with a D-tetrahydro-isoquinoline carboxyl (Tic)-D-para-chloro(p-Cl)-phenylalanine (Phe) dipeptide consensus sequence. A series of heterocycle-substituted cyclohexylpiperidine structures emerged from this work [73]. Interestingly, analogue 1 (Fig. 6) with L(S) stereochemistry at the pCl-Phe asymmetric center, has the highest affinity for the MC3R. Significant activation of the MC3R occurs only with the more common D-Tic-D-pCl-Phe dipeptide, found to be optimal for MC4R activity. The most potent ligand (2) is a partial agonist with an N-methyl substitution to the heterocycle within the privileged structure portion of the molecule.
Fig. (6).
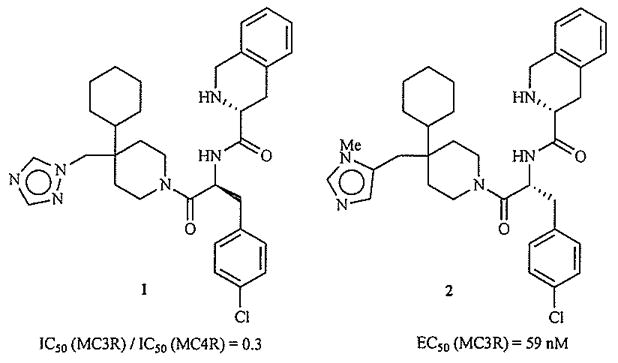
Most MC3R-selective (1) and potent (2) ligands from ref. [73].
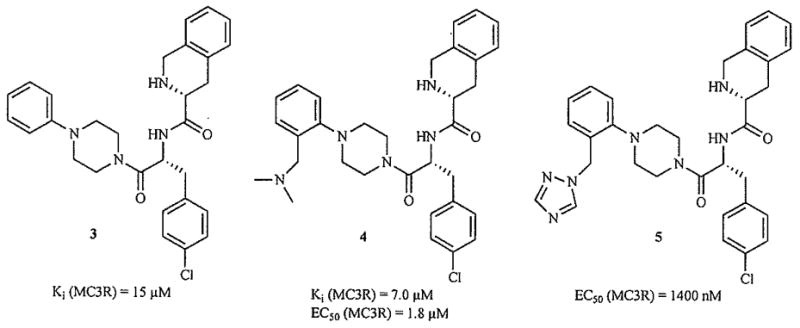
A number of other putatively GPCR-biased privileged structures have been appended to the D-Tic-D-pCl-Phe sequence. Three examples of substituted arylpiperazines are shown in Fig. 7, but their affinities for the hMC3R are in the micromolar range [74–76].
Fig. (7).
Compounds with modifications to both the privileged structure and the dipeptide consensus sequence have been reported. Two examples are shown in Fig. 8 [77]. Interestingly, in this series only the analogue N-methylated at the II nitrogen of the imidazole shows no activity at the MC3R. This substitution is likely to change the orientation and hydrogen-bonding properties of this heterocycle, and illustrates the importance of the histidine or histidine-mimicking side chain in MC3R binding and activation.
Fig. (8).
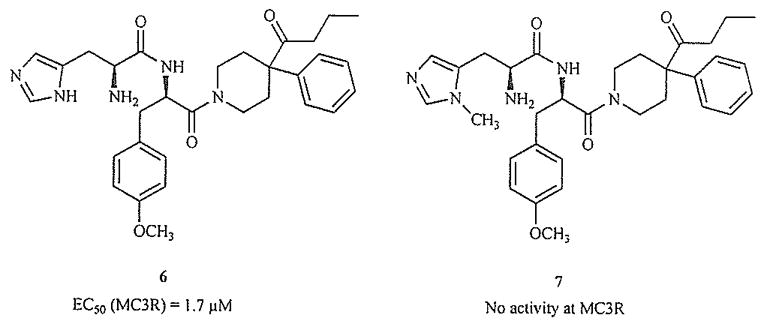
N-Methylation changes functional activity [77].
The compounds in Fig. 9 provide further support for this assertion [78]. Varying the position of N-methylation in the His-mimicking piperazine produces the only analogue which significantly activates the MC3R.
Fig. (9).
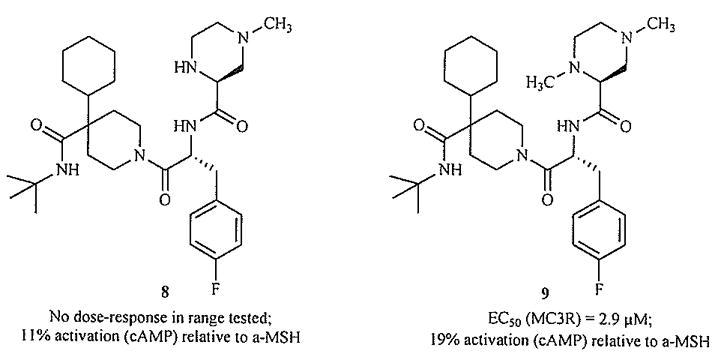
N-Methylation changes functional activity [78].
Clearly the privileged structure approach has had a major impact on research efforts aimed at developing melanocortin-targeted small molecules. Molecular modeling provides a rationale for the activity of this molecular template, as the pharmacophore elements found in the small molecules appear to overlap with the side-chain functionality found in the key residues of bioactive peptide ligands [79].
A logical alternative approach to creating small molecule ligands for these receptors, then, is the use of the experimentally-derived structures of known active ligands to rationally design new molecules. In the first report of work employing this strategy [80], the solution structure of an active cyclic peptide ligand related to MT-II was first determined using NMR-derived constraints. Models of a variety of ring systems appended with the appropriate side chain functionality were overlapped with the peptide NMR structure, and compounds demonstrating the best overlap were synthesized. This study seems to indicate that in some compounds, changing or removing any of the pharmacophore elements will cause the complete loss of MC3R activity (Fig. 10). Generally, it illustrates the potential to generate active compounds by a ligand-based design approach.
Fig. (10).
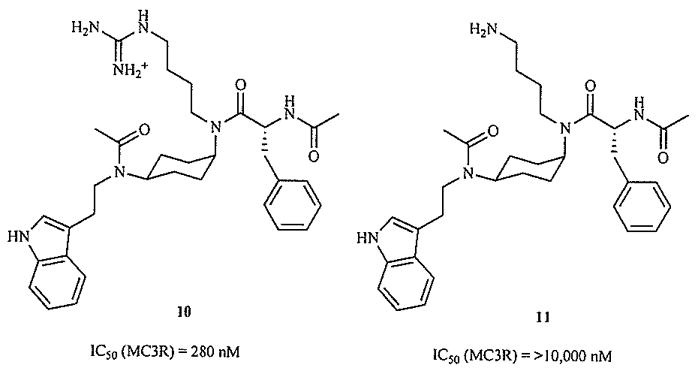
Removing functional groups eliminates MC3R binding of rationally designed molecules [80].
Our own rational design of small molecules for the melanocortin receptors relied on a similar approach [81]. Instrumental to achieving this goal was the determination of an NMR solution structure for MT-II [67], against which potential new structures could be superimposed. Modeling indicated that our amino acid-derived scaffold could mimic the β-turn backbone of the peptide, and facilitated the prediction of which positional and stereochemical isomers were most likely to be active. All members of a test set of eight compounds were found to be high-affinity antagonists, and most subtype-selective. In this series, there appears to be a significant similarity in the binding requirements for the MC1R and MC3R, as typified by the nanomolar binding of 13 to both subtypes (Fig. 11). Demonstrating the crucial nature of the stereochemistry of these ligands, inversion of the C6 center to produce the diastereomeric 12 yields complete MC5R selectivity. This work further demonstrates that a ligand-based rational design approach may be a highly efficient means of producing hMC3R-active molecules.
Fig. (11).
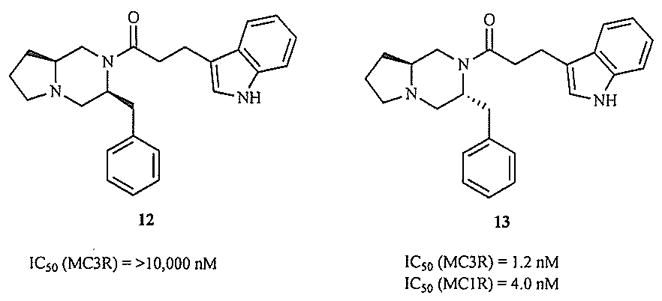
Stereochemistry crucial for MC3R activity in rationally designed molecules [81].
More traditional approaches, such as the optimization of activity for a compound series originally derived from a screening hit, have also led to the discovery of novel ligands for the MCRs. An example of a pyridazinone-based molecule [82] derived in this way is shown in Fig. (12).
Fig. (12).
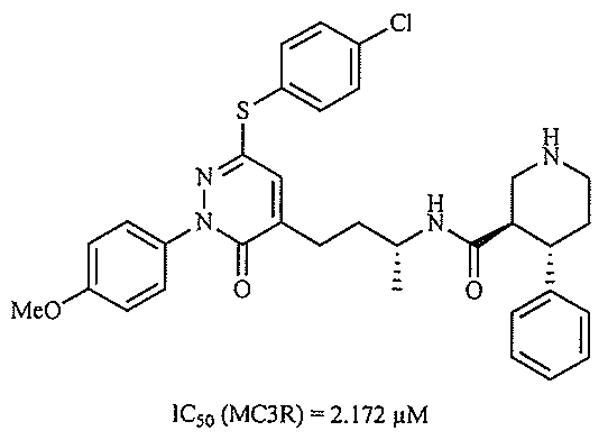
Ligand optimized from screening hit [82].
DATA BASE SEARCH FOR DRUG DESIGN
Computational tools to search chemical structure databases are useful for finding leads early in a drug discovery program. Similarity searches are among the most diverse and most useful, and have become a standard tool for target specific library design [83, 84]. The underlying theory is that, given a compound with desired biological activities, compounds that are similar to it in structure are likely to have similar activity. In a common practice of focused library design, the investigator provides a set of chemical structures as a “probe” for searches of a database of compounds, and finds those that are most similar, and then submits them for testing. Similarity searches can be done on the basis of either 2D or 3D structure. 2D similarity searches are computationally very inexpensive and rapid; their popularity is related to the trend of applying high-throughput medicinal chemistry methodologies.
Successful approaches to the design of focused compound libraries have recently been reported for the melanocortin 4 receptor and other GPCRs [73, 85–88]. They range from 2D simulation algorithms, to the analysis of ligand-receptor spatial arrangements and to neural network learning QSAR systems. With this variety of tools, it now appears possible to design libraries that are enriched in compounds possessing the desired target-specific properties.
THE hMC3R STRUCTURE AND RELATED ASPECTS OF LIGAND-RECEPTOR INTERACTION
Comparison of the human melanocortin receptor sequences revealed that most of the conserved amino acids are located within the putative transmembrane helices, with the most sequence deviations occurring within the C-terminus, first extracellular and third intracellular loops, whereas the other extra- and intracellular loops as well as the amino-terminus show regions of substantial similarity [89].
Chen et al. examined the significance of 19 transmembrane domain amino acids for molecular recognition and receptor signaling (Fig. 13) [90]. The hMC3R residues E131, D154, D158, and H298 were found to be homologous to the hMC1R residues E94, D117, D121, and H260, and to the hMC4R residues E100, D122, D126, and H264, respectively [91–93]. The existence of an ionic pocket formed by amino acid residues E131 in transmembrane domain 2 [TM2) and D154, D158 in TM3 of hMC3R and a second hydrophobic binding pocket consisting of F295, F296, and H298 in TM6 of hMC3R was hypothesized, as replacement of these amino acids with alanine resulted in dramatically reduced NDP-α-MSH binding affinity and agonist potency (Fig. 14). Furthermore, substitutions of D121 in TM2 and D332 in TM7 with alanine resulted in the complete loss of ligand binding, ligand induced receptor activation, and cell surface protein expression. These findings suggest that TM3 and TM6 are important for NDP-α-MSH binding, while D121 in TM2 and D332 in TM7 are crucial for receptor signaling. Interestingly, unlike MC1R and MC4R, only TM6 of hMC3R seems to be involved in a hydrophobic binding pocket, as mutations of F216A, F233A, F234A, F288A, F290A, and F318A did not significantly alter NDP-α-MSH binding affinity and receptor activity. Tao and Segaloff also described the hMC3R mutation 1183N, found in patients with high fat contents, which completely abolishes agonist-mediated receptor activation [93].
Fig. (13).
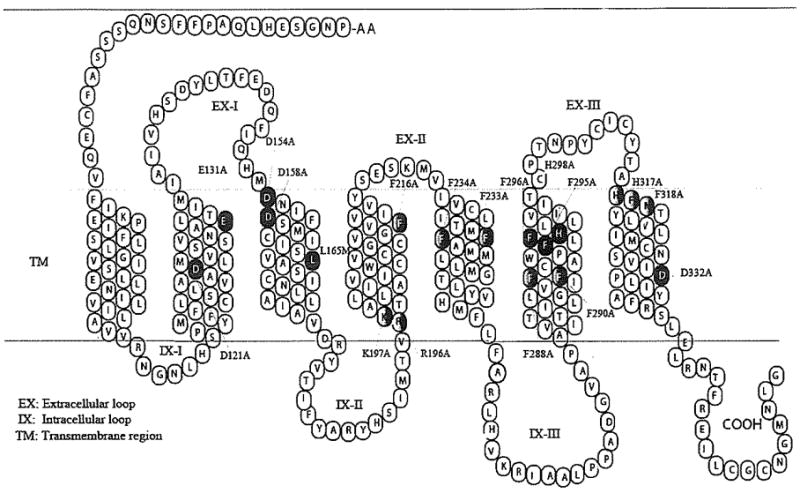
Two-dimensional representation of the hMC3R sequence and secondary strucmre [90].
Fig. (14).
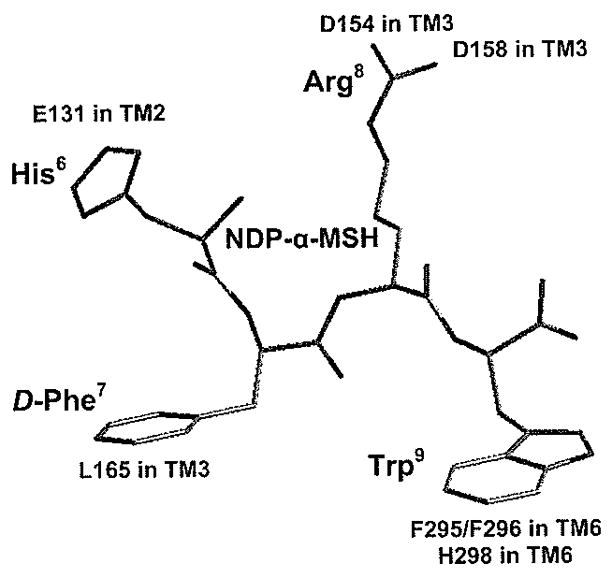
Two-dimensional representation of a proposed three-dimensional model illustrating the synthetic melanocortin NDP-α-MSH docked inside the hMC3R. Two receptor binding pockets are hypothesized, the first being a predominantly ionic pocket formed by D154 and D158, and the second is a hydrophobic pocket formed by aromatic residues in TM6 [90].
POTENTIAL THERAPEUTIC USES FOR THE MC3R
Until the discovery and cloning of the MC3R, MC4R and MC5R receptors in the 1990s most of the work with POMC related peptides (β-endorphin not included) centered around the possible roles of α-MSH in pigmentation, pigmentary disorder, and melanoma cancer, and the role(s) of ACTH in adrenal function. Though much critical research still is ongoing in these areas, since then a large number of academic, pharmaceutical and biotechnology groups have been examining the role(s) primarily of α-MSH and related peptides, peptidomimetics and non-peptide ligands on the MC3R, MC4R and MC5R. It is not our intention here to review this rapidly expanding literature (see e.g. refs. [5, 6] for examples of many areas under investigation). In this review we will concentrate on those aspects related to the MC3R.
In the last 15 years, major advances in our understanding of the regulation of body weight and energy homeostasis have been reported [59, 94]. It is now widely understood that the disorders of leptin feedback or hypothalamic melanocortin signaling can lead to pathological weight gain and diabetes in humans [e.g. 95, 96]. Downstream of leptin signaling, the interplay between melanocortin peptides and neuropeptide Y (NPY) has been examined [e.g. 97] and it has been demonstrated that melanocortin peptide administration within the PVH leads to the regulation of feeding behavior as well as energy expenditure. The central melanocortin system modulates energy homeostasis through the actions of the agonist α-MSH, a POMC cleavage product, and the endogenous antagonist AGRP on the MC3R and MC4R [98,99]. The MC3R is primarily expressed in the brain in the regions of the hypothalamus and limbic system, as well as in the placenta and gut [100,101]. On the other hand, MC4R is widely expressed throughout the brain [15,102]. Additionally, the control of food intake has been suggested to be modulated through the MC4R [103], whereas the MC3R and MC5R have not been linked to any specific actions on feeding behavior. Several investigators [12,13,104] have attempted to solve this puzzle by studying MC3R-deficient mice and to comparing these mice with mice deficient for both the MC3R and MC4R genes. It was suggested that inactivation of the mouse MC3R leads to increased fat mass, reduced lean mass and higher feed efficiency than their wild type littermates. Furthermore, MC3R−/− mice also exhibited a unique metabolic syndrome leading to ≈50% increase in adipose mass, an unusual increase in respiratory quotient and reduced energy expenditure [13].
The role of MC3R also has been proposed for anti-inflammation as well as cardiovascular regulation. Getting et al. [105] have suggested that activation of MC3R by ACTH(4–10) suppresses cytokine release from microphages which then leads to the inhibition of inflammation. Furthermore, the MC3R is the only melanocortin receptor subtype for which γ2-MSH is selective [106] which makes this receptor an obvious leading candidate for mediation of cardiovascular regulation [107,108] and inhibition of TRH release [109]. Recently, Marks et al. [110] have shown that peripheral injections of D-Trp8-γ-MSH, a potent and selective MC3R agonist can stimulate food intake in mice which suggests that individuals affected with involuntary weight loss due to chronic diseases can be helped [111]. This is the first time such a physiological role for MC3R has been suggested where this receptor acts as an inhibitory autoreceptor of POMC neurons. Table 1 describes a number of potent and selective MC3R agonists and antagonists that might prove useful for the treatment of obesity, cachexia and cardiovascular, inflammatory, and other diseases.
CONCLUDING REMARKS
POMC is clearly a primordial gene whose presence is found in virtually all multicellular animals that have a central nervous system. From this perspective it perhaps is not surprising that the processed ligands from this gene and their target G-protein coupled receptor are involved in most aspects of human behavior, endocrine and central nervous system function. Though α-MSH, ACTH and some of the other processed peptides of POMC, especially from the pituitary gland, were among the first peptide hormones/neurotransmitters whose biological functions were evaluated in considerable detail at least with respect to melanopigmentary and adrenal function, it was the cloning of the receptors for these functions [i.e. MC1R and MC2R) and the additional three receptors (i.e. MC3R, MC4R, MC5R) that led to recognition of the central role of this system of ligands and receptors in numerous endocrine and CNS functions in normal health and disease. Though much of this research has focused on the MC4R and feeding behavior it has become increasingly clear that the MC3R is often as crucial as the MC4R in a variety of biological functions, and it seems clear that future research will have to evaluate the MC3R receptor system in much more detail, especially its relationships in various physiological and pharmacological situations where they and other neurotransmitter and hormonal systems are involved in health and disease. Clearly highly potent and selective ligands, both agonists and antagonists, will be needed to sort out the complex biology involved. Though we have developed some reasonably selective hMC3R ligands, there is clearly a great need for further development, and we plan to pursue this vigorously into the future.
Acknowledgments
The research done in our laboratory was supported primarily by a grant from the U.S. Public Health Service, National Institutes of Health DK17420. We thank Margie Colie for much help in preparing this review.
References
- 1.Krieger DT, Ganong WF. ACTH and related peptides, structure, regulation and action. Ann NY Acad Sci. 1977;297:1–664. [PubMed] [Google Scholar]
- 2.Vaudry H, Eberle AN, editors. Ann NY Acad Sci. Vol. 680. 1993. The Melanotropic Peptides; pp. 1–684. [DOI] [PubMed] [Google Scholar]
- 3.Hadley ME, editor. The Melanotropic Peptides. I, II & III. CRC Press, Inc.; Boca Raton, FL: 1988. [Google Scholar]
- 4.Eberle AN, editor. The Melanotropins; Chemistry, Physiology and Mechanisms of Action. Karger, Basel; Switzerland: 1988. [Google Scholar]
- 5.Cone RD. The Melanocortin System. Ann NY Acad Sci. 2003;994:1–387. [Google Scholar]
- 6.Cone RD, editor. The Melanocortin Receptors. Humana Press; Totowa, NJ: 2000. [Google Scholar]
- 7.Kesterson RA. The melanocortin 3 receptor In The Melanocortin Receptors. In: Cone RD, editor. Chapter 13. Humana Press; Totowa, NJ: 2000. pp. 385–403. [Google Scholar]
- 8.Bell GI, Xiang KS, Newman MV, Wu SH, Wright LG, Fajans SS, Spielman RS, Cox NJ. Gene for non-insulin-dependent diabetes-mellitus (maturity onset diabetes of the young subtype) is linked to DNA polymorphism on human chromosome-20q. Proc Nat Acad Sci USA. 1991;88:1484–88. doi: 10.1073/pnas.88.4.1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamada Y, Xiang KS, Bell GI, Seino S, Nishi M. Dinucleotide repeat polymorphism in a gene on chromosome-20 encoding a 6-protein coupled receptor (D20S32E) Nucleic Acids Res. 1991;19:2519. doi: 10.1093/nar/19.9.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marks DL, Hruby V, Brookhart G, Cone RD. The regulation of food intake by selective stimulation of the type 3 melanocortin receptor (MC3R) Peptides. 2006;27:259–64. doi: 10.1016/j.peptides.2005.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cowley MA, Smart JL, Rubinstein M, Cerdan MG, Diano S, Horvath TL, Cone RD, Low MJ. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature. 2001;411:480–84. doi: 10.1038/35078085. [DOI] [PubMed] [Google Scholar]
- 12.Chen AS, Marsh DJ, Trumbauer ME, Frazier EG, Guan XM, Yu H, Rosenblum CI, Vongs A, Feng Y, Cao LH, Metzger JM, Strack AM, Camacho RE, Mellin TN, Nunes CN, Min W, Fisher J, Gopal-Truter S, Maclntyre DE, Chen HY, Van derPloeg LHT. Inactivation of the mouse melanocortin-3 receptor results in increased fat mass and reduced lean body mass. Nat Genet. 2000;26:97–102. doi: 10.1038/79254. [DOI] [PubMed] [Google Scholar]
- 13.Butler AA, Kesterson RA, Khong K, Cullen MJ, Pelleymounter MA, Dekoning J, Boetscher M, Cone RD. A unique metabolic syndrome causes obesity in the melanocortin-3 receptor-deficient mouse. Endocrinology. 2000;141:3518–21. doi: 10.1210/endo.141.9.7791. [DOI] [PubMed] [Google Scholar]
- 14.Sutton GM, Trevaskis JL, Hulver MW, McMillan RP, Markward NJ, Babin MJ, Meyer EA, Butler AA. Diet-genotype interactions in the development of the obese, insulin-resistant phenotype of C57BL/6J mice lacking melanocortin-3 or -4 receptors. Endocrinology. 2006;147:2183–96. doi: 10.1210/en.2005-1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gantz I, Miwa H, Konda Y, Shimoto Y, Tashiro T, Watson SJ, DelValle J, Yamada T. Molecular cloning expression and gene localization of a fourth melanocortin receptor. J Biol Chem. 1993;268:15174–79. [PubMed] [Google Scholar]
- 16.Getting SJ, Gibbs L, Clark AJL, Flower RJ, Perretti M. POMC gene derived peptides activate melanocortin type 3 receptor on murine macrophages suppress cytokine release and inhibit neutrophil migration in acute experimental inflammation. J Imnunol. 1999;162:7446–53. [PubMed] [Google Scholar]
- 17.Etting SJ, Allcock GH, Flower R, Perretti M. Natural and synthetic agonists of the melanocortin receptor type 3 possess anti-inflammatory properties. J Leukoc Biol. 2001;69:98–104. [PubMed] [Google Scholar]
- 18.Getting SJ, Christian HC, Lam CW, Gavins FN, Flower RJ, Schiöth HB, Perretti M. Redundancy of a functional melanocortin I receptor in the anti-inflammatory actions of melanocortin peptides, studies in the recessive yellow (c/c) mouse suggest an important role for melanocortin 3 receptor. J Immunol. 2003;170(6):3323–30. doi: 10.4049/jimmunol.170.6.3323. [DOI] [PubMed] [Google Scholar]
- 19.Getting SJ, Christian HC, Flower RJ, Perretti M. Activation of melanocortin type 3 receptor as a molecular mechanism for adrenocorticotropic hormone efficacy in gouty arthritis. Arthritis Rheum. 2002;46:2765–75. doi: 10.1002/art.10526. [DOI] [PubMed] [Google Scholar]
- 20.Getting SJ, Di Filippo C, Christian HC, Lam CW, Rossi F, D’Amico M, Perretti M. MC-3 receptor and the inflammatory mechanisms activated in acute myocardial infaret. J Leukocyte Biol. 2004;76:845–53. doi: 10.1189/jlb.0306175. [DOI] [PubMed] [Google Scholar]
- 21.Guarini S, Schiöth HB, Mioni C, Cainazzo M, Ferrazza G, Giuliani D, Wikberg JES, Bertolini A, Bazzani C. MC(3) receptors are involved in the protective effect of melanocortins in myocardial ischemia/reperfusion-induced arrhythmias. Naunyn Schmiedebergs . Arch Pharmacol. 2002;366:177–82. doi: 10.1007/s00210-002-0572-8. [DOI] [PubMed] [Google Scholar]
- 22.Schwartz M, Woods S, Porte DJ, Seeley R, Baskin D. Central nervous system control of food intake. Nature. 2000;404:661–71. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- 23.Ahima R, Flier J. Leptin Annu Rev Physiol. 2000;62:413–37. doi: 10.1146/annurev.physiol.62.1.413. [DOI] [PubMed] [Google Scholar]
- 24.Fan W, Boson B, Kesterson R, Hruby V, Cone R. Role of melanocortinergic neurons in feeding and the agouti obesity syndrome. Nature. 1997;385:165–68. doi: 10.1038/385165a0. [DOI] [PubMed] [Google Scholar]
- 25.Seeley R, Yagaloff K, Fisher S, Burn P, Thiele T, van Dijk G, Baskin D, Schwartz M. Melanocortin receptors in leptin effects. Nature. 1997;390:349. doi: 10.1038/37016. [DOI] [PubMed] [Google Scholar]
- 26.Chuang TT, Lacovelli L, Sallese M, De Blasi AG. Protein-coupled receptors heterologous regulation of homologous desensitization and its implications. Trends Pharmacol Sci. 1996;17:416–21. doi: 10.1016/s0165-6147(96)10048-1. [DOI] [PubMed] [Google Scholar]
- 27.Bunemann M, Hosey M. G-protein coupled receptor kinases as modulators of G-protein signaling. J Physiol. 1999;517:5–23. doi: 10.1111/j.1469-7793.1999.0005z.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lohse M, Benovic J, Codina J, Caron M, Lefkowilz R. β-Arrestin, a protein that regulates β-adrenergic receptor function. Science. 1990;248:1547–50. doi: 10.1126/science.2163110. [DOI] [PubMed] [Google Scholar]
- 29.Cai M, Stankova M, Pond SJK, Mayorov AV, Perry JW, Yamamura HI, Trivedi D, Hruby VJ. Real time differentiation of G-protein coupled receptor (GPCR) agonist and antagonist by two photon fluorescence laser microscopy. J Am Chem Soc. 2004;26:7160–61. doi: 10.1021/ja049473m. [DOI] [PubMed] [Google Scholar]
- 30.Zhang J, Barak LS, Winkler KE, Caron MG, Ferguson SSG. A central role for β-arrestins and clathrin-coated vesicle-mediated endocytosis in β2-adrenergic receptor resensitization. Differential regulation of receptor resensitization in two distinct cell types. J Biol Chem. 1997;272:27005–14. doi: 10.1074/jbc.272.43.27005. [DOI] [PubMed] [Google Scholar]
- 31.Oakley RH, Laporte SA, Holt JA, Barak LS, Caron MG. Association of β-arrestin with G protein-coupled receptors during clathrin-mediated endocytosis dictates the profile of receptor resensitization. J Biol Chem. 1999;274:32248–57. doi: 10.1074/jbc.274.45.32248. [DOI] [PubMed] [Google Scholar]
- 32.Tsao P, Cao T, von Zastrow M. Role of endocytosis in mediating downregulaution of G-protein-coupled receptors. Trends Pharmacol Sci. 2001;22:91–6. doi: 10.1016/s0165-6147(00)01620-5. [DOI] [PubMed] [Google Scholar]
- 33.Tsao PI, von Zastrow M. Diversity and specificity in the regulated endocytic membrane trafficking of G-protein-coupled receptors. Pharmacol Ther. 2001;89:139–47. doi: 10.1016/s0163-7258(00)00107-8. [DOI] [PubMed] [Google Scholar]
- 34.Nestler E. Molecular basis of long-term plasticity underlying addiction. Nat Rev Neurosci. 2001;2:119–28. doi: 10.1038/35053570. [DOI] [PubMed] [Google Scholar]
- 35.Harrison L, Kastin A, Zadina J. Opiate tolerance and dependence, receptors, G-proteins, and antiopiates. Peptides. 1998;19:1603–30. doi: 10.1016/s0196-9781(98)00126-0. [DOI] [PubMed] [Google Scholar]
- 36.Yang Y, Chen M, Loux TJ, Georgeson KE, Harmon CM. Molecular mechanism of the intracellular segments of the melanocortin-4 receptor for NDP-MSH signaling. Biochemistry. 2005;44:6971–79. doi: 10.1021/bi047521+. [DOI] [PubMed] [Google Scholar]
- 37.Hruby VJ. Conformational restrictions of biologically active peptides via amino acid side chain groups. Life Sci. 1982;31:189–92. doi: 10.1016/0024-3205(82)90578-1. [DOI] [PubMed] [Google Scholar]
- 38.Popov EM, Akhmedov NA, Makhmudova TA. Approach to studying the structure-activity relationship of native peptides. I. Structural organization of the opioid peptide Tyr-Glu-Gly-Phe-Met-Arg-Gly-Leu and its artificial analogs. Bioorganicheskaia khimiia. 1992;18:1454–63. [PubMed] [Google Scholar]
- 39.Kessler H. Conformational and Biological Activity of Cyclic Peptides. Angew Chem Int Ed End. 1982;21:512–23. [Google Scholar]
- 40.Sawyer TK. Peptidomimetic and Nonpeptide Drug Discovery, Impact of Structure-Based Drug Design. In: Veerapandian P, editor. Structure Based Drug Design, Disease, Targets, Techniques and Developments. Macel Dekker.; N. Y.: 1997. pp. 559–634. Chapter 23. [Google Scholar]
- 41.Hruby VJ. Designing peptide receptor agonists and antagonists. Nat Drug Des. 2002;1:847–58. doi: 10.1038/nrd939. [DOI] [PubMed] [Google Scholar]
- 42.Hirschmann R. Medicinal chemistry in the golden age biology, lessons from steroid and peptide research. Angew Chem Int Ed Eng. 1991;30:1278–1301. [Google Scholar]
- 43.Grieco P, Balse PM, Weinberg D, MacNeil T, Hruby VJ. D-Amino acid scan of γ-melanocyte-stimulating hormone, importance of Trp8 on human MC3 receptor selectivity. J Med Chem. 2000;43:4998–5002. doi: 10.1021/jm000211e. [DOI] [PubMed] [Google Scholar]
- 44.Bystrov VF, Okhanov VV, Miroshnikov AJ, Ovehinnikov YA. Solution spatial structure of apamin as derived from NMR study. FEBS Letter. 1980;119:113–7. doi: 10.1016/0014-5793(80)81010-6. [DOI] [PubMed] [Google Scholar]
- 45.Hruby VJ. The conformation of peptides in solution as determined by NMR spectroscopy and other physical methods. In: Weinstein B, editor. Chemistry and Biochemistry of Amino Acids, Peptides and Proteins. Marcel Dekker; New York, NY: 1974. p. pl-l88. [Google Scholar]
- 46.Marraud M, Aubry A. Crystal structures of peptides and modified peptides. Biopolymers. 1996;40:45–8. doi: 10.1002/(sici)1097-0282(1996)40:1<45::aid-bip3>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 47.Woody RW. Contributions of tryptophan side chains to the far-ultraviolet circular dichroism of proteins. Circ Dichroism. 1994;47:3. doi: 10.1007/BF00213575. [DOI] [PubMed] [Google Scholar]
- 48.Scheraga HA. Contribution of physical chemistry to an understanding of protein structure and function. Protein Science. 1992;1:691–3. doi: 10.1002/pro.5560010515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cone RD. Melanocortin system. Encyclopedia Biol Chem. 2004;2:617–20. [Google Scholar]
- 50.Hruby VJ, Wilkes BC, Hadley ME, Al-Obeidi F, Sawyer TK, Staples DJ, de Vaux AE, Dym O, de Lauro Castrucci AM, Hintz MF, Riehm JR, Rao RR. α-Melanolropin, the minimum active sequence in the frog skin bioassay. J Med Chem. 1987;30:2126–30. doi: 10.1021/jm00394a033. [DOI] [PubMed] [Google Scholar]
- 51.de Lauro Castrucci AM, Hadley ME, Sawyer TK, Wilkes BC, Al-Obeidi F, Staples DJ, de Vaux AE, Dym O, Hintz MF, Riehm JP, Rao RR, Hruby VJ. α-Melanotropin, the minimal active sequence in the lizard skin bioassay. Gen Comp Endocrinol. 1989;73:157–63. doi: 10.1016/0016-6480(89)90066-x. [DOI] [PubMed] [Google Scholar]
- 52.Cai M, Mayorov AV, Cabello C, Stankova M, Trivedi D, Hruby VJ. Novel 3D pharmacophore of α-MSH/γ-MSH hybrids leads to selective human MCIR, MC3R analogues. J Med Chem. 2005;48:1839–48. doi: 10.1021/jm049579s. [DOI] [PubMed] [Google Scholar]
- 53.Hruby VJ, Lu D, Sharma SD, Castrucci A, De L, Kesterson RA, Al-Obeidi FA, Hadley ME, Cone RD. Cyclic lactam alpha-melanotropin analogues of Ac-Nlc4-c[Asp5, D-Phe7, Lys[10]alpha-MSH(4–10)-NH2 with bulky aromatic amino acids at position 7 show high antagonist potency and selectivity at specific melanocortin receptors. J Med Chem. 1995;38:3454–61. doi: 10.1021/jm00018a005. [DOI] [PubMed] [Google Scholar]
- 54.Balse-Srinivasan P, Grieco P, Cai M, Trivedi D, Hruby VJ. Structure-activity relationships of gamma-MSH analogues at the human melanocortin MC3, MC4, and MC5 receptors. Discovery of highly selective hMC3R, hMC4R, and hMC5R analogues. J Med Chem. 2003;46:4965–73. doi: 10.1021/jm030119t. [DOI] [PubMed] [Google Scholar]
- 55.Sawyer TK, Hruby VJ, Darman PS, Hadley ME. [4-half-cystine, 10-half-cystine]-α-Melanocyte stimulating hormone a cyclic α-melanotropin exhibiting superagonist biological activity. Proc Nat Acad Sci USA. 1982;79:1751–55. doi: 10.1073/pnas.79.6.1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Knittel JJ, Sawyer TK, Hruby VJ, Hadley ME. Structure-activity studies of highly potent [Cys4, Cys10]melanotropin analogues. J Med Chem. 1983;28:125–29. doi: 10.1021/jm00356a002. [DOI] [PubMed] [Google Scholar]
- 57.Al-Obeidi F, Hadley ME, Petiitt BM, Hruby VJ. Design of a new class of superpotent cyclic α-melanotropins based on quenched dynamic simulations. J Am Chem Soc. 1989;111:3413–16. [Google Scholar]
- 58.Al-Obeidi F, de Lauro Castrucci AM, Hadley ME, Hruby VJ. Potent and prolonged acting cyclic lactam analogues of α-melanotropin, design based on molecular dynamics. J Med Chem. 1989;32:2555–61. doi: 10.1021/jm00132a010. [DOI] [PubMed] [Google Scholar]
- 59.Hruby VJ, Cai M, Grieco P, Han G, Kavarana M, Trivedi D. Exploring the stereostructural requirements of peptide ligands for the melanocortin receptors. Ann NY Acad Sci. 2003;994:12–20. doi: 10.1111/j.1749-6632.2003.tb03157.x. [DOI] [PubMed] [Google Scholar]
- 60.Kavarana MJ, Trivedi D, Cai M, Ying J, Hammer M, Cabello C, Grieco P, Han G, Hruby VJ. Novel cyclic templates of a-MSH give highly selective and potent antagonists/agonists for human meianocortin-3/4 receptors. J Med Chem. 2002;45:2644–50. doi: 10.1021/jm020021z. [DOI] [PubMed] [Google Scholar]
- 61.Grieco P, Lavecchia A, Cai M, Trivedi D, Weinberg D, MacNeil T, Van der Ploeg LHT, Hruby VJ. Structure-Activity Studies of the Melanocortin Peplides and Discovery of Potent and Selective Affinity Antagonists for the hMC3 and hMC4 Receptors. J Med Chem. 2002;45:5287–5294. doi: 10.1021/jm0202526. [DOI] [PubMed] [Google Scholar]
- 62.Grieco P, Cai M, Mayorov AV, Trivedi D, Hruby VJ. Structure-activity studies of new melanocortin peptides containing an aromatic amino acid at the N-terminal position. Peptides. 2006;27(2):472–481. doi: 10.1016/j.peptides.2005.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Balse-Srinivasan P, Grieco P, Cai M, Trivedi D, Hruby VJ. Structure-Activity Relationships of Novel Cyclic α-MSH/β-MSH Hybrid Analogues That Lead to Potent and Selective Ligands for the Human MC3R and Human MC5R. J Med Chum. 2003;46:3728–3733. doi: 10.1021/jm030111j. [DOI] [PubMed] [Google Scholar]
- 64.Cai M, Cai C, Mayorov AV, Xiong C, Cabello CM, Soloshonok VA, Swift JR, Trivedi D, Hruby VJ. Biological and conformational study of beta-substituted pralines in MT-II template, steric effects leading to human MC5 receptor selectivity. J Pep Res. 2004;63:116–31. doi: 10.1111/j.1399-3011.2003.00105.x. [DOI] [PubMed] [Google Scholar]
- 65.Wang W, Cai M, Xiong C, Zhang J, Trivedi D, Hruby VJ. Design and synthesis of novel χ2-constrained phenylalanine, naphthyialanine, and tryptophan analogs and their use in biologically active melanotropin peptides. Tetrahedron. 2002;58:7365–74. [Google Scholar]
- 66.Jackson PJ, McNulty JC, Yang YK, Thompson DA, Chai B, Gantz I, Barsh GS, Millhauser GL. Design, pharmacology, and NMR structure of a minimized cystine knot with agouti-related protein activity. Biochemistry. 2002;41:7565–72. doi: 10.1021/bi012000x. [DOI] [PubMed] [Google Scholar]
- 67.Ying J, Kõvër KE, Gu X, Han G, Trivedi DB, Kavarana MJ, Hruby VJ. Solution Structures of Cyclic Melanocortin Agonists and Antagonists by NMR. Biopolymers (peplideScience) 2003;71:696–716. doi: 10.1002/bip.10596. [DOI] [PubMed] [Google Scholar]
- 68.Grieco P, Han G, Weinberg D, MacNeil T, Van der Ploeg LHT, Hruby VJ. Design and Synthesis of Highly Potent and Selective Melanotropin Analogues of SHU9119 Modified at Position 6. Biochem Biophys Res Comm. 2002;292:1075–1080. doi: 10.1006/bbrc.2002.6739. [DOI] [PubMed] [Google Scholar]
- 69.Mayorov AV, Cai M, Chandler KB, Petrov RR, Van Scoy AR, Yu Z, Tanaka DK, Trivedi D, Hruby VJ. Development of cyclic γ-MSH analogues with selective hMC3R agonist and hMC3R/hMC5R antagonist activities. J Med Chem. 2006;49:1946–52. doi: 10.1021/jm0510326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mayorov AV, Cai M, Han S-Y, Tan B, Van Scoy AR, Dedek M, Palmer ES, Trivedi D, Hruby VJ. Novel cyclic lactam α-MSH analogs as potent and selective hMC3R agonists and antagonists, and hMC1R antagonists. Abstracts of Papers, 232nd ACS National Meeting; San Francisco, CA, United States. Sept. 10–14, 2006; MEDI-414. [Google Scholar]
- 71.Ballet S, Mayorov AV, Cai M, Tymecka D, Chandler KB, Palmer ES, Van Rompaey K, Misicka A, Tourwë D, Hruby VJ. Novel selective human melanocortin-3 receptor ligands, use of the 4-amino-l,2,4,5-tetrahydro-2-benzazepin-3-one (Aba) scaffold. Bioorg Med Chem Lett. 2007;71:2492–2498. doi: 10.1016/j.bmcl.2007.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bednarek MA, MacNeil T, Kalyani RN, Tang R, Van der Ploeg LHT, Weinberg DH. Selective, High Affinity Peptide Antagonists of α-Melanotropin Action at Human Melanocortin Receptor 4, Their Synthesis and Biological Evaluation in vitro. J Med Chem. 2001;44:3665–3672. doi: 10.1021/jm010165y. [DOI] [PubMed] [Google Scholar]
- 73.Sebhat IK, Martin WJ, Ye Z, Barakat K, Mosley RT, Johnston DBR, Bakshi R, Palucki B, Weinberg DH, MacNeil T, Kalyani RN, Tang R, Steams RA, Miller RR, Tamvakopoulos C, Strack AM, McGowan E, Cashen DE, Drisko JE, Hom GJ, Howard AD, Maclntyre DE, Van Der Ploeg LHT, Patchett AA, Nargund RP. Design and Pharmacology of N-[(3R)-1,2,3,4-Tetrahydroisoquinolinium-3-ylcarbonyl]-( 1 R)-1 –(4-chlorobenzyl)-2-[4-cyclohexy]-4-( 1H— 1,2,4-triazol-1-ylmethyl)piperidin-1 -yl]-2-oxoelhylamine (1) a Potent, Selective, Melanocortin Subtype-4 Receptor Agonist. J Med Chem. 2002;45:4589–4593. doi: 10.1021/jm025539h. [DOI] [PubMed] [Google Scholar]
- 74.Dyck B, Parker J, Phillips T, Carter L, Murphy B, Summers R, Hermann J, Baker T, Cismowski M, Saunders J, Goodfellow V. Aryl Piperazinc Melanocortin MC4 Receptor Agonists. Bioorg Med Chem Lett. 2003;13:3793–3796. doi: 10.1016/s0960-894x(03)00796-0. [DOI] [PubMed] [Google Scholar]
- 75.Mutulis F, Yahorava S, Mutule K, Yahorau A, Liepinsh E, Kopantshuk S, Veiksina S, Tars K, Belyakov S, Mishnev A, Rinken A, Wikberg JES. New Substituted Piperazines as Ligands for Melanocortin Receptors. Correlation lo the X-ray Structure of “THIQ”. J Med Chem. 2004;47:4613–4626. doi: 10.1021/jm0311285. [DOI] [PubMed] [Google Scholar]
- 76.Richardson TI, Omstein PL, Briner K, Fisher MJ, Backer RT, Biggers CK, Clay MP, Emmerson PJ, Hertel LW, Hsiung HM, Husain S, Kahl SD, Lee JA, Lindstrom TD, Martinelli MJ, Mayer JP, Mullaney JT, O’Brien TP, Pawlak JM, Revell KD, Shah J, Zgombick JM, Herr RJ, Melekhov A, Sampson PB, King CR. Synthesis and Structure-Activity Relationships of Novel Arylpiperazines as Potent and Selective Agonists of the Melanocortin Subtype-4 Receptor. J Med Chem. 2004;47:744–755. doi: 10.1021/jm0304109. [DOI] [PubMed] [Google Scholar]
- 77.Herpin TF, Yu G, Carlson KE, Morton GC, Wu X, Kang L, Tuerdi H, Khanna A, Tokarski JS, Lawrence RM, Macor JE. Discovery of Tyrosine-Based Potent and Selective Melanocortin-1 Receptor Small-Molecule Agonists with Ants-inflammatory Properties. J Med Chem. 2003;46:1123–1126. doi: 10.1021/jm025600i. [DOI] [PubMed] [Google Scholar]
- 78.Palucki BL, Park MX, Nargund RP, Ye Z, Sebhat IK, Pollard PG, Kalyani RN, Tang R, MacNeil T, Weinberg DH, Vongs A, Rosenblum CI, Doss GA, Miller RR, Steams RA, Peng Q, Tamvakopoulos C, McGowan E, Martin WJ, Metzger JM, Shepherd CA, Strack AM, MacIntyre DE, Van der Ploeg LHT, Patchett AA. Discovery of (2S)-N-[(IR)-2-14-cyclohexyl-4-[[(1,1-dimethyl)-amino] carbonyl]-1-piperidinyl]-1-[(4-fluorophenyl)methyl]-2-oxoethyl]-4-methyl-2-piperazinecarboxamide (MB243), a potent and selective melanocortin subtype-4 receptor agonist. Bioorg Med Chem Lett. 2005;15:171–175. doi: 10.1016/j.bmcl.2004.10.020. [DOI] [PubMed] [Google Scholar]
- 79.Pogozheva ID, Chai B, Lomize AL, Fong TM, Weinberg DH, Nargund RP, Mulholland MW, Gantz I, Mosberg HI. Interactions of Human Melanocortin 4 Receptor with Nonpeptide and Peptide Agonists. Biochemistry. 2005;44:11329–11341. doi: 10.1021/bi0501840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fotsch C, Smith DM, Adams JA, Cheetham J, Croghan M, Doherty EM, Hale C, Jarosinski MA, Kelly MG, Norman MH, Tamayo NA, Xi N, Baumgartner JW. Design of a New Peptidomimetic Agonist for the Melanocortin Receptors Based on the Solution Structure of the Peptide Ligand, Ac-Nle-cyclo[Asp-Pro-DPhe-Arg-Trp-Lys]-NH2. Bioorg, Med Chem Lett. 2003;13:2337–2340. doi: 10.1016/s0960-894x(03)00412-8. [DOI] [PubMed] [Google Scholar]
- 81.Cain JP, Mayorov AV, Cai M, Wang H, Tan B, Chandler K, Lee YS, Petrov RR, Trivedi D, Hruby VJ. Design, synthesis, and biological evaluation of a new class of small molecule peptide mimetics targeting the melanocortin receptors. Bioorg Med Chem Lett. 2006;16:5462–67. doi: 10.1016/j.bmcl.2006.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ujjainwalla F, Warner D, Walsh TF, Wyvratt MJ, Zhou C, Yang L, Kalyani RN, MacNeil T, Van der Ploeg LHT, Rosenblum CI, Tang R, Vongs A, Weinberg DH, Goulet MT. Design and Syntheses of Melanocortin Subtype-4 Receptor Agonists and Evolution of the Pyridazinone Archetype. Bioorg Med ChemLett. 2003;13:4431–4435. doi: 10.1016/j.bmcl.2003.09.026. [DOI] [PubMed] [Google Scholar]
- 83.Ghose AK, Herbertz T, Salvino JM, Mallamo J. PI Knowledge-based chemoinformalic approaches to drug discovery. Drug Discov Today. 2006;11:1107–1114. doi: 10.1016/j.drudis.2006.10.012. [DOI] [PubMed] [Google Scholar]
- 84.Sheridan RP, Kearsley SK. Why do we need so many chemical similarity search methods. Drug Discov, Today. 2002;7:903–911. doi: 10.1016/s1359-6446(02)02411-x. [DOI] [PubMed] [Google Scholar]
- 85.Balakin KV, Lang SA, Skorenko AV, Tkachenko SE, Ivashchenko AA, Savchuk NP. Structure-Based versus property-based approaches in the design of G-Protein-coupled receptor-targeted libraries. J Chem Inf Compul Aci. 2003;43:1553–1562. doi: 10.1021/ci034114g. [DOI] [PubMed] [Google Scholar]
- 86.Vos TJ, Caracoti A, Che JL, Dai M, Farrer CA, Forsyth NE, Drabic SV, Horlick RA, Lamppu D, Yowe DL, Balani S, Li P, Zeng H, Joseph IB, Rodriguez LE, Maguire MP, Patane MA, Claiborne CF. Identification of 2-[2-12-(5-bromo-2- methoxyphenyl)-ethyl]-3-fluorophenyl]-4,5-dihydro-IH-imidazole (MLOQ253764), a small molecule melanocortin 4 receptor antagonist that effectively reduces tumor-induced weight loss in a mouse model. J Med Chem. 2004;47:1602–1604. doi: 10.1021/jm034244g. [DOI] [PubMed] [Google Scholar]
- 87.Pontillo J, Tran JA, Fleck BA, Marinkovic D, Arellano M, Tucci FC, Lanier M, Nelson J, Parker J, Saunders J, Murphy B, Foster AC, Chen C. Piperazinebenzylamines as potent and selective antagonists of the human melanocortin-4 receptor. Bioorg Med Chem, Lett. 2004;14:5605–5609. doi: 10.1016/j.bmcl.2004.08.055. [DOI] [PubMed] [Google Scholar]
- 88.Tucci FC, White NS, Markison S, Joppa M, Tran JA, Fleck BA, Madan A, Dyck BP, Parker J, Pontillo J, Arellano LM, Marinkovic D, Jiang W, Chen CW, Gogas KR, Goodfellow VS, Saunders J, Foster AC, Chen C. Potent and orally active non-peptide antagonists of the human melanocortin-4 receptor based on a series of trans-2-disubstituted cyclohexylpiperazines. Bioorg Med Chem Lett. 2005;15:4389–4395. doi: 10.1016/j.bmcl.2005.06.071. [DOI] [PubMed] [Google Scholar]
- 89.Prusis P, Frändberg PA, Muceniece R, Kalvinsh I, Wikberg JES. A three dimensional model for the interaction of MSH with the melanocortin-1 receptor. Biochem Biophy, Res Commun. 1995;210:205–10. doi: 10.1006/bbrc.1995.1647. [DOI] [PubMed] [Google Scholar]
- 90.Chen M, Aprahamian CJ, Celik A, Georgeson KE, Garvey WT, Harmon CM, Yang Y. Molecular characterization of human melanocortin-3 receptor ligand-receptor interaction. Biochemistry. 2006;45:1128–37. doi: 10.1021/bi0521792. [DOI] [PubMed] [Google Scholar]
- 91.Yang YK, Fong TM, Dickinson CJ, Mao C, Li JY, Tota MR, Mosley R, Van Der Ploeg LHT, Gantz I. Molecular determinants of ligand binding to the human melanocortin-4 receptor. Biochemistry. 2000;39:14900–11. doi: 10.1021/bi001684q. [DOI] [PubMed] [Google Scholar]
- 92.Yang Y, Dickinson C, Haskell-Luevano C, Gantz I. Molecular basis for the interaction of [Nle4, D-Phe7]melanocyte stimulating hormone with the human melanocortin-1 receptor. J Biol Chem. 1997;272:23000–10. doi: 10.1074/jbc.272.37.23000. [DOI] [PubMed] [Google Scholar]
- 93.Tao YX, Segaloff DL. Functional characterization of melanocortin-3 receptor variants identify a loss-of-function mutation involving an aminoacid critical for G protein-coupled receptor activation. J clin Endocrinol Metab. 2004;89:3936–42. doi: 10.1210/jc.2004-0367. [DOI] [PubMed] [Google Scholar]
- 94.Ellacott KLJ, Cone RD. The Central Melanocortin System and the Integration of Short- and Long-term Regulators of Energy Homeostasis. Rec Prog Horm Res. 2004;59:395–408. doi: 10.1210/rp.59.1.395. [DOI] [PubMed] [Google Scholar]
- 95.Krude H, Biebermann H, Luck W, Horn R, Brabant G, Gruters A. Severe early-onset obesity, adrenal insufficiency and red hair pigmentation caused by POMC mutations in humans. Nat Genet. 1998;19:155–7. doi: 10.1038/509. [DOI] [PubMed] [Google Scholar]
- 96.Clement K, Vaisse C, Lahlou N, Cabrol S, Pelloux V, Cassuto D, Gourmelen M, Dina C, Chambaz J, Lacorte JM, Basdevant A, Bougneres P, Lebouc Y, Froguel P, Guy-Grand B. A mutation in the human leptin receptor gene causes obesity and pituitary dysfunction. Nature. 1998;392:398–401. doi: 10.1038/32911. [DOI] [PubMed] [Google Scholar]
- 97.Cowley MA, Pronchuk N, Fan W, Dinulescu DM, Colmers WF, Cone RD. Integration of NPY, AGRP, and melanocortin signals in the hypothalamic paraventicular nucleus, evidence of a cellular basis for the Adipostat. Neuron. 1999;24:155–63. doi: 10.1016/s0896-6273(00)80829-6. [DOI] [PubMed] [Google Scholar]
- 98.Ollmann MM, Wilson BD, Yang Y, Kerns JA, Chen Y, Gantz J, Barsh GS. Antagonism of central melanocortin receptors in vitro and in vivo by agouti-related protein. Science. 1997;278:137. doi: 10.1126/science.278.5335.135. [DOI] [PubMed] [Google Scholar]
- 99.Fong TM, Mao C, MacNeil C, Kalyani R, Smith T, Weinberg D, Tota MR, Van der Ploeg LH. ART (protein product of agouti-related transcript) as an antagonist of MC-3 and MC-4 receptors. Biochem Biophys, Res Commun. 1997;237:629–31. doi: 10.1006/bbrc.1997.7200. [DOI] [PubMed] [Google Scholar]
- 100.Gantz 1, Konda Y, Shimoto Y, Miwa H, Munzert G, Watson SJ, Del Valle V, Yamada T. Molecular cloning of a novel melanocortin receptor. J Biol Chem. 1993;268:8246–50. [PubMed] [Google Scholar]
- 101.Roselli-Rehfuss L, Mountjoy KG, Robbins LS, Mortrud MT, Low MJ, Tatro JB, Entwistle ML, Simerly RB, Cone RD. Identification of a receptor for gamma melanotropin and other proopiomelanocortin peptides in the hypothalamus and limbric system. Proc Natl Acad Sci. 1993;90:8856–60. doi: 10.1073/pnas.90.19.8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mountjoy KG, Mortrud MT, Low MJ, Simerly RB, Cone RD. Localization of the melanocortin-4 receptor (MC4-R) in neuroendocrine and autonomic control circuits in the brain. Mol Endocrinol. 1994;8:1298–1308. doi: 10.1210/mend.8.10.7854347. [DOI] [PubMed] [Google Scholar]
- 103.Fan W, Boston BA, Kesterson RA, Hruby VJ, Cone RD. Role of melanocortinergic neurons in feeding and the agouti obesity syndrome. Nature. 1997;385:165–8. doi: 10.1038/385165a0. [DOI] [PubMed] [Google Scholar]
- 104.Raffin-Samson ML, Bertherat J. Mc3 and Mc4 receptors, complementary role in weight control. Eur J Emdocrinol. 2001;144:207–8. doi: 10.1530/eje.0.1440207. [DOI] [PubMed] [Google Scholar]
- 105.Getting SJ, Gibbs L, Clark AJL, Flower RJ, Perretti M. POMC gene derived peptides activate melanocortin type 3 receptor on murine macrophages, suppress cytokine release and inhibit neutrophil migration in acute experimental inflammation. J Immunol. 1999;162:7446–53. [PubMed] [Google Scholar]
- 106.Abbott CR, Rossi M, Kim M, Al Ahmed SH, Taylor GM, Ghatei MA, Smith DM, Bloom SR. Investigation of the melanocyte stimulating hormones on food intake. Lack of evidence to support a role for the melanocortin-3-receptor. Brain Res. 2000;869:203–10. doi: 10.1016/s0006-8993(00)02386-6. [DOI] [PubMed] [Google Scholar]
- 107.Versteeg DHG, Bergen PV, Adan RAH, DeWildt DJ. Melanocortins and cardiovascular regulation. Eur J Pharmacol. 1998;360:1–14. doi: 10.1016/s0014-2999(98)00615-3. [DOI] [PubMed] [Google Scholar]
- 108.Li SJ, Varga K, Archer P, Hruby VJ, Sharma SD, Kesterson RA, Cone RD, Kunos G. Melanocortin Antagonists Define Two Distinct Pathways of Cardiovascular Control by α- and γ-Melanocyte-Stimulating Hormones. J Neurosci. 1996;16:5182–88. doi: 10.1523/JNEUROSCI.16-16-05182.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kim MS, Russell SH, Morgan DGA, Abbott CR, Al Ahmed SH, Hay DL, Ghatei MA, Smith DM, Bloom SR. Effects of melanocortin receptor ligands on thyrotropin-releasing hormone release evidence for the differential roles of melanocortin 3,4 receptors. J Neuroendocrinol. 2002;14:276–82. doi: 10.1046/j.1365-2826.2002.00769.x. [DOI] [PubMed] [Google Scholar]
- 110.Marks DL, Hruby VJ, Brookhart G, Cone RD. The regulation of food intake by selective stimulation of the type 3 melanocortin receptor (MC3R) Peptides. 2006;27:259–264. doi: 10.1016/j.peptides.2005.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Marks DL, Cone RD. Central melanocortins and the regulation of weight during acute and chronic disease. Recent Prog Horm Res. 2001;56:359–75. doi: 10.1210/rp.56.1.359. [DOI] [PubMed] [Google Scholar]


