Abstract
The purpose of this review is to summarize the neurobiological factors involved in the etiology of adolescent addiction and present evidence implicating various mechanisms in its development. Adolescents are at heightened risk for experimentation with substances, and early experimentation is associated with higher rates of SUD in adulthood. Both normative (e.g., immature frontal-limbic connections, immature frontal lobe development) and non-normative (e.g., lowered serotonergic function, abnormal hypothalamic-pituitary-adrenal axis function) neurobiological developmental factors can predispose adolescents to a heightened risk for SUD. In addition, a normative imbalance in the adolescent neurobiological motivational system may be caused by the relative underdevelopment of suppressive mechanisms when compared to stimulatory systems. These neurobiological liabilities may correspond to neurobehavioral impairments in decision-making, affiliation with deviant peers and externalizing behavior; these and other cognitive and behavioral traits converge with neurobiological factors to increase SUD risk. The progression to SUD acts as an amplifying feedback loop, where the development of SUD results in reciprocal impairments in neurobehavioral and neurobiological processes. A clearer understanding of adolescent neurobiology is a necessary step in the development of prevention and treatment interventions for adolescent SUD.
INTRODUCTION
The consequences of substance use disorders (SUD) are well publicized and involve substantial costs to society.1–3 Using data from the late 1990s, various government agencies have estimated that the annual cost of alcohol, drug, and nicotine use disorders was nearly five hundred billion dollars.4–6 In large part, the initiation of addictive substance use appears to be an adolescent phenomenon: nearly 60% of individuals who initiate drug use do so at or before 18 years of age,7 and the rates of initiation rise to roughly 80% for alcohol7 and cigarettes.8 Furthermore, it appears that the early use of certain substances (e.g., cigarettes, methamphetamine, inhalants, or marijuana) is associated with accelerated use of other substances,9,10 greater progression to SUD,11–14 and psychiatric comorbidity.13,15 The 2003 Youth Risk Behavior Survey stated that the use of alcohol, tobacco and illicit drugs by high school students markedly increased their likelihood of injury or death due to the four major causes of fatalities.16
Adolescence is a time of great neurobiological change.17 Evidence increasingly indicates that these changes impact the propensity of adolescents to experiment and experience persistent alterations from psychoactive substance use;18 substance use (and the consequent sequelae) in adolescence may correspond to accelerations in the development of SUDs in adulthood.19–21 Thus, prevention or early treatment holds great promise for limiting the costs, morbidity, and mortality associated with addiction. In order to develop more effective treatment interventions, it is essential to understand the pathophysiology of addiction in youth.
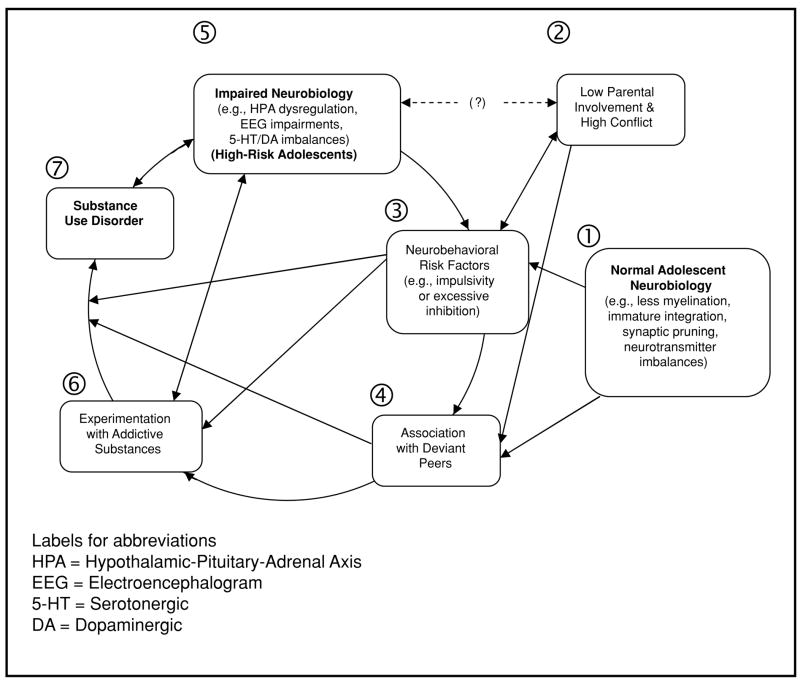
This review will present an integrated etiology of the development and entrenchment of addiction in adolescents. A conceptual summary is provided in Figure 1. Research findings have led some to posit that all adolescents have neurobiological risks stemming from immature connectivity22 and/or imbalances in the expression of the motivational learning system.18 Thus, the changing neurobiology of adolescence (#1 in Figure 1) may underlie the increase in high-risk behaviors and disinhibition (#3) associated with the development of SUD.23,24 Adolescents at high-risk (#5; e.g., children of probands with SUD) likely have neurobiological liabilities in serotonergic (5-HT), hypothalamic-pituitary-adrenal (HPA) axis and/or neurophysiological (e.g., P300) functioning above those of low-risk adolescents. These factors in high-risk youth may correspond to greater levels of conflict with parents and the formation of affiliative friendships with other high-risk youth (#2 and 4), both risk factors for SUD. Finally, following the initiation of psychoactive substance use (#6), adolescents appear to be more acutely and persistently affected than adults. One result appears to be a more rapid progression to SUD. The acute differences and persistent alterations may reflect neuroplastic changes that serve to entrench and accelerate use, resulting in greater neurobiological liability (#3) and SUD (#7).
FIGURE 1.
Etiology of SUD Development in Adolescents
This review will focus on factors associated with or leading to levels of substance use that would meet criteria for a diagnosis of abuse or dependence,25 rather than factors leading only to experimental use. Many substance users remain experimenters,26 and experimentation may be associated with outcomes that are no worse,27 or are even better,28 than outcomes in those who abstain. While the first step to addiction is experimentation,29 infrequent use is significantly different than heavy use. Factors common to all adolescents (and thus, present in experimenters) will be examined only to create the foundation on which dysfunctional traits accelerate levels of substance use. In addition, this review will focus on adolescents with a familial history of SUD; such individuals have a significantly greater incidence of SUD than individuals without a family history30,31 and are more likely to have dysfunctional neurobiological and neurobehavioral traits.32 Given the concentration of risk factors, high-risk adolescents are thought to be most likely to demonstrate pathways to SUD development.
THE DEVELOPMENTAL NEUROSCIENCE OF ADOLESCENCE: ANIMAL AND HUMAN STUDIES
Adolescence is perhaps the greatest time of neural growth, change, and maturation since infancy. The development of the executive functions (e.g., decision-making, self-monitoring, impulse control, delay of gratification) continues from childhood through adolescence,33–38 with completion as late as early adulthood.39–42 These neurocognitive traits correlate with prefrontal cortex (PFC) and anterior cingulate activity; the development of these traits appears dependent upon the maturation of PFC and limbic system interconnectivity. (The limbic system consists of diverse neural structures, including the cingulate, amygdala, and hippocampus, and serves to regulate emotional experience, memory, and motivational learning). Furthermore, the maturation of connections between the PFC, basal ganglia, and cerebellum also appear to be crucial for the development of higher cognitive functions.43
PRUNING AND MYELINATION
In large part, these neurocognitive changes occur during adolescence and depend on large-scale myelination and synaptic pruning (#1 in Figure 1). The deposition of white matter, or myelin, increases the speed of neural transmission. White matter allows for quicker processing and more concentrated circuits to respond to the rapid demands of the environment. Using magnetic resonance imaging (MRI), longitudinal studies of human brain development have found that white matter increases linearly from ages 4 to 20.44,45 In a study of men ranging in age from 19 to 76, Bartzokis et al.46 reported that frontal lobe myelination peaked in adult males at 44 years of age.
Synaptic pruning is the process by which excess connections (synapses) between neurons are removed. Rakic and et al.47 and Lictman et al.48 theorize that pruning is determined by synapse use: connections that are employed to respond to the environment are retained and strengthened, while those that are used less often are eliminated. Synapse elimination is believed to reduce the childhood pattern of processing, which requires greater metabolic activity and the recruitment of a wider array of structures.49–51 In addition, pruning appears to increase the efficiency of cognitive processing through the creation of dedicated neural networks.52 For instance, synaptic overproduction followed by selective pruning allows for maximum efficiency in associative memory functions.53,54
Rakic and colleagues47 have estimated that up to 30,000 synapses are pruned per second in the non-human primate adolescent brain. Thus, nearly one-half of the cortical synapses present before adolescence may be pruned during this period. Regional gray matter in humans tends to peak and decline, at least partially due to pruning, beginning from 12 to 20 years of age.44, 55–57 Using structural MRI, Sowell and colleagues58,59 found large-scale cortical and subcortical brain changes in late adolescence and early adulthood. Numerous other studies document that concomitant synaptic pruning and increased myelination occur in the human frontal cortex during adolescence.45,60–62 Referring back to Figure 1 (#1), it appears that normal adolescent neurobiology is characterized by lesser myelination, synaptic pruning, and integration than is found in adults; overall, these processes do not appear to culminate until early adulthood.63
DEVELOPMENT OF NEUROTRANSMITTER SYSTEMS DURING ADOLESCENCE
In addition to connective and structural changes in the central nervous system (CNS), adolescents undergo dramatic alterations in virtually all neurotransmitter systems (also within #1 of Figure 1). Most relevant to the development of SUD are the changes experienced in dopamine-related systems. Dopamine (DA) plays a central role in the mesolimbic neural pathway. This circuit originates in the ventral tegmental area (VTA) and projects to the nucleus accumbens (NAc) and various limbic structures.64,65 The mesostriatal release of DA occurs in response to a wide variety of environmental reinforcers, including water,66 food,67 and drugs of abuse.68–70 Increased striatal concentrations of DA are essential in assigning value to these reinforcing stimuli.71
Both animal and human studies indicate that DA receptors reach a density peak early in development and undergo elimination during adolescence.72–75 Synaptic pruning of DA receptors occurs in both the human and rat NAc in adolescence,74,76,77 although some studies report otherwise.78 In contrast, DA receptors in the PFC do not demonstrate significant pruning until late adolescence.74,77,79 DA fiber density increases in the PFC of adolescent rats80,81 and NAc of gerbils82 and DA inputs to the primate PFC peak in adolescence.83,84 Teicher and colleagues85 found age-related striatal differences in synaptic DA levels in rats; these were not seen in the NAc or medial PFC. Furthermore, increased DA synthesis has been observed in the striatum, NAc and PFC in adolescent rats.86 Finally, striatal DA turnover is higher in adolescent than adult rats, with smaller (non-significant) differences in the NAc and medial PFC.85 This study also found age-related differences in DA metabolism in rats.85 Finally, DA systems in the adolescent rat display significant regenerative plasticity following neurotoxin administration.87,88 In toto, these studies may indicate a functional increase in mesostriatal DA activity during adolescence,18,89 though the region and time of these increases seem to differ from species to species.
Adolescent developmental maturation is also seen in the cannabinoid, glutaminergic, gamma-aminobutyric acid (or GA-BAergic) and serotonergic (5-HT) systems. Cannabinoid systems regulate mesocortical90 and striatial DA systems,91–93 and reach functional maturity in rats during adolescence.94–96 Changes in the cannabinoid system may influence motivational learning, with preclinical studies highlighting the complex role of cannabinoid input on mesolimbic DA.97–99 The behavioral effects of agonists for a specific glutamate receptor, the N-methyl-D-aspartate (NMDA) receptor, appear to peak late in the pre-adolescent period in rats.100,101 This coincides with greater NMDA agonist sensitivity.102 NMDA receptor binding peaks differentially by subtype,103 and glutaminergic inputs to the PFC decrease slightly during adolescence.83,84
The nature of GABA and 5-HT alterations during adolescence are not as well established. GABA inhibits NAc activity and opposes the modulating excitatory effects of glutamate.104 GABA receptors achieve maturity in adolescence,105 with increased responsiveness of GABAergic systems linked to stress in animal models.106,107 GABAergic input to the PFC appears to decrease strongly through adolescence in humans,83,84 and, following a pre-adolescent peak, rat GABAergic neurons in the PFC decrease in size during adolescence.108 5-HT inhibits and opposes DA activity, particularly as DA relates to aggressive and impulsive behaviors.109–117 Thus, DA is thought to promote motivated behaviors, whereas 5-HT is conceptualized as a break upon mesostriatal promotion of appetitive behavior. NAc 5-HT turnover is up to four times lower in adolescent rats than in younger or older rats,118 and 5-HT1A receptor binding appears to decrease most dramatically in human males during adolescence.119 DA input to the PFC is up to three times greater than 5-HT input,120 and PFC concentrations of a DA precursor are much greater than those of a 5-HT precursor in pubertal rhesus monkeys.121 Finally, there is evidence that early adolescent rats undergo significant 5-HT synaptic pruning in the basal forebrain.122
In summary, many neurotransmitter systems demonstrate notable maturation during adolescence. This is captured in box 1 of Figure 1. DA systems display extensive pruning and plasticity with concurrent maturation of the cannabinoid, glutaminergic, and GABAergic systems during adolescence. The latter three systems all exert modulatory effects on mesolimbic DA, and it is probable that changes in these systems have consequences for the development of mesolimbic DA circuitry. Finally, 5-HT input may be underdeveloped when compared to DA NAc input during adolescence. The relevance of the adolescent DA to 5-HT ratio for behavior is explored in the next section.
IMPLICATIONS OF NEUROBIOLOGICAL CHANGES ON STIMULATORY AND SUPPRESSIVE PROCESSES
In examining the motivational learning system, the relevant circuits and neurotransmitters can be divided into stimulatory and suppressing aspects. The stimulatory substrates of this system encode for appetitive behaviors (e.g., drug-seeking), whereas the suppressive substrates encode for both regulatory and harm-avoidance behaviors (e.g., avoidance of drug use environment). This system is primarily composed of a neural network that loops from the PFC to the striatum and NAc (through the thalamus) and back to the PFC.123,124 This system receives affect-related input from limbic structures as well as information concerning biologically motivated drives (e.g., thirst) from the hypothalamus.71,125 In the motivational learning system, DA and glutamate are stimulatory neurotransmitters, 5-HT and GABA are suppressive, and the PFC functions as a regulatory and/or suppressive influence. As noted in box 1 of Figure 1, greater expression of the stimulatory over the suppressive aspects appears to be present during normal adolescent development.18
Chambers and colleagues18 posited that the release of NAc DA “is a principle neuromodulatory event implicated in the translation of encoded motivated drives into action, operating like a general ‘go’ signal” (p. 1045). The exact action of DA on motivational learning is unclear,71 although it appears to be related to novel stimuli and reward valence.126 In an animal model, Hoglund et al.113 found that the administration of a DA precursor increased aggression, an aspect of stimulatory expression. DA release modulates NAc firing in response to glutaminergic input from the PFC, limbic regions, and the hippocampus.127,128 DA also induces neuroplastic modifications in the NAc129,130 and has been associated with learning.131–133 Glutamatergic projections from the PFC and basolateral amygdala to the NAc core also appear to promote motivational learning.134
Conversely, 5-HT appears to play a primary suppressive role in the motivational system. 5-HT projections involved originate in the raphe nucleus in the midbrain and project to the PFC, NAc, hippocampus, and limbic regions.64 Taylor and Jentsch135 found that following five days of 3,4-Methylenedioxy-methamphetamine (MDMA) administration, which is toxic for 5-HT axonal projections,136,137 reward-related learning was impaired in rats. Unlike control rats, MDMA-administered rats performed conditioned behaviors for more than a week in the absence of cue for action.135 Other studies have correlated impulsive or aggressive behaviors with lowered levels of 5-HT turnover,109,112,115 and increases in 5-HT activity correlate with attenuated aggression and impulsivity in both humans and animals.109,138,139
The PFC appears to be crucial for the regulation of motivationally driven behaviors140–145 through a glutaminergic feedback loop with the NAc.146–148 PFC damage has been associated with impulsiveness, dysfunctional affect,149–152 and a higher risk for SUD.153–155 Dysfunctional PFC input to the NAc could disrupt the motivational loop by reducing suppressive options; persistent dysfunction could impair the neuroplastic shaping of the motivational circuit, entrenching maladaptive stimulatory responses.156 GABA also likely regulates motivationally driven responses through projections from the central amygdala to the VTA and through connections from the NAc core and ventral pallidum.134
The activation of the motivational circuit appears to differ between adolescent and adult humans. In a task in which participants could either win or avoid losing money, Bjork et al.157 found reduced activation in striatal and amygdalar structures during the anticipation of a gain in adolescents, relative to young adults. This implies differences in the ability of adolescents to use information to regulate motivational behavior. Ernst and collaborators158 compared the neural responses of adolescents and adults on a gambling task. Adolescents had stronger NAc responses (likely stimulatory) to outcomes than adults. This study also found attenuated amygdalar responses (likely suppressive), implying that adolescents process stimulatory feedback better than inhibitory feedback.158 Thus, both studies suggest that adolescents have impaired suppressive and enhanced stimulatory systems when compared to adults.
IMPLICATIONS FOR EXPERIMENTATION WITH ADDICTIVE SUBSTANCES AND DEVELOPMENT OF SUD
Given the evidence that DA, unlike 5-HT, may be close to a functional maximum,89,118,159 adolescence appears to be marked by greater influence for activating substrates. Experimentation with addictive substances is certainly the product of many influences, one of which is likely to be this motivational imbalance. Furthermore, DA release in the NAc could interact synergistically with adolescent plasticity to promote the reinforcement-related learning of drug cues and hence continued drug use.160 Glutamate-mediated learning also appears to influence behavioral adaptations to repeated drug use; for example, mice with mutated NMDA receptors did not develop conditioned place preference (CPP) or locomotor sensitization to cocaine administration.161 Greater NMDA agonist sensitivity in adolescence may indicate that motivated learning is altered, especially in relation to psychoactive substance use.
As mentioned previously, 5-HT systems appear to be functioning at a lesser level than DA systems during adolescence. Furthermore, GABA may attenuate drug-seeking,162 but the effects of GABA appear to decrease through adolescence. This may indicate attenuated GABA-related regulation of appetitive drives. Finally, Bjork et al.157 and Ernst et al.158 provide neuroimaging evidence that adolescents process stimulatory information more strongly than inhibitory or regulatory information. As the integration of diverse neural structures is crucial for the regulation of motivated drives,22 adolescents are likely not equipped to exert maximal control over their appetitive urges. Thus, it appears that a heightened ratio of the stimulatory relative to the suppressive aspects of motivation may be normal for the adolescent phase of development. This imbalance may have explanatory power for the impulsivity (#2) and experimentation with addictive substances (#6) seen across adolescents.
BEHAVIORAL FACTORS RELATING TO SUBSTANCE ABUSE IN ADOLESCENTS
Neurobiological changes during adolescence contribute to three behavioral factors that relate to the development of SUD: increases in peer affiliation, decreased parental monitoring, and risk-taking (#2–4 in Figure 1).163 These occur across all adolescents and appear to be conserved across species.164–166 The transition to adulthood necessitates a shift from dependence on parents and family to peer networks for support. The transition to greater dependence on peers co-occurs with increased levels of parent-adolescent conflict,167,168 which also involves decreases in reported closeness and time that adolescents and parents spend together.169 Changes in the parent-child relationship appear to be increasingly influenced by age in youngsters, potentially due to gene-environment interactions.170 These changes may adversely impact parental monitoring. For even well-adjusted adolescents, this transition can be a time of heightened stress, as self-identity changes within the context of these shifting relationships. Finally, animal166 and human adolescents171,172 engage in a higher level of risk-taking behavior than during any other developmental period. Steinberg24 posits that risk-taking in adolescents is a normal developmental consequence of the need for greater stimulation due to decreased reward sensitivity.
The processes mentioned in the preceding paragraph are seen across adolescents; other processes involved in SUD development are present in adolescents at high-risk (#2 in Figure 1). These include impulsivity, labile emotions, questionable decision-making, and other dysfunctional neurobehavioral processes thought to be regulated by the frontal lobes. While some combination of these is a hallmark of adolescence, those at high-risk appear to have amplified versions of these traits. Internalizing psychopathology,173 externalizing behavior,172 and even alcohol use174 are present in children and pre-adolescents; furthermore, such childhood phenomena can predict the development of SUD.175,176 Thus, many of the risk factors for the development of SUD are present before the transition to adolescence occurs.177,178
DECISION-MAKING, DIFFICULT TEMPERAMENT, AND INTERNALIZING PSYCHOPATHOLOGY
The evidence for decision-making as a risk factor for SUD has been mixed. Executive functioning (EF), which subsumes decision-making, is a minor risk for SUD and is mediated by aggressive behavior179,180 and/or difficult temperament.179,181 Overman and associates182 found that heavy alcohol and substance use decreased performance on a gambling task among adolescents; greater polysubstance use led to increasing decrements in performance. Given the design, it is unknown whether these decision-making liabilities predated or were sequelae of substance use. In a prominent comment, Steinberg24 noted that many laboratory-based investigations of decision-making in adolescents may not be ecologically valid. Specifically, Gardner and Steinberg183 have found that adolescents make riskier decisions than adults on a computer driving simulation task, but only when participants were with peers and emotionally aroused. These are common situational variables when adolescents use addictive substances.24,183 Thus, the lack of compelling findings may be the result of measurement and design, and not the result of a weak relationship between EF and SUD.
Difficult temperament is defined as a set of traits that include negative affect, irritability, and problems with attention, persistence, and coping.181 While difficult temperament predicts SUD,184 the relationship is mediated by aggression and deviant-peer-association.179 Giancola and Parker185 posited that difficult temperament was often a step on the path to SUD, but not a necessary step. Internalizing psychopathology (e.g., depressive or anxiety disorders) may also serve as a risk factor for the development of SUD, as studies in adolescents consistently find that depression and substance use co-occur.186–189 While much of the evidence indicates that SUD precedes depressive diagnosis,190,191 there does appear to be a subgroup for whom depressive symptoms come first.192,193 Furthermore, Lopez and colleagues194 found that post-traumatic stress disorder appeared to predict SUD. Despite some negative results,195 it appears that a relationship between SUD and internalizing psychopathology exists. Aspects of this relationship, such as direction and strength of influence, however, cannot be stated given the current literature.196
EXTERNALIZING BEHAVIOR
Externalizing behavioral syndromes (e.g., conduct disorder) appear to be an unmediated causal risk for the development of SUD in adolescents. Genetic research has found a pathway for SUD development beginning with parental antisocial personality disorder (ASPD) and progressing through aggressive behavior and conduct disorder (CD) in the child, resulting in SUD.197,198 In an experiment that began with three-year-old children, a subset was classified as undercontrolled based on the presence of difficult temperament, impulsivity, hyperactivity, and aggressive, uncontrolled behavior. Under-controlled children were 2.9 times more likely to be diagnosed with ASPD, and undercontrolled males were 2.7 times more likely to have alcohol dependence at 21 years of age.199 In all, Robins200 concluded that extensive but insufficient evidence exists to justify the idea that CD and SUD have a reciprocal causal relationship. That said, externalizing behavior may be a non-specific risk for SUD: initiation of problem behavior before the age of 15 was a risk factor for diagnosis of SUD and/or ASPD at the age of 20.201
It is important to note that anxiety,202,203 depressive,145,202,204 and externalizing behavioral disorders205 share neurological circuits and neurobiological processes with SUD, which may speak to a common developmental mechanism. Fronto-limbic, HPA axis, DA, 5-HT, NE and/or GABA dysfunction are believed to play an important role in the development of internalizing, externalizing, and addictive disorders.18,202,203,205 While further examination of the comorbidity and common mechanisms of these disorders is beyond the scope of this review, it is crucial for the practicing clinician to be aware of issues in the combined assessment and treatment of other psychiatric disorders and SUD in adolescents).206 Treatment of the SUD and the comorbid psychiatric disorder(s) appears necessary to achieve the greatest reductions in SUD symptoms and to prevent relapse to substance use;207 furthermore, emerging evidence indicates that treatment of psychiatric disorders prior to the emergence of substance problems can prevent the development of SUD.208,209
ASSOCIATION WITH DEVIANT PEERS
As mentioned previously, adolescence is a time of social transition: while affiliation with healthy peers is important for development, increased interaction with deviant peers is a risk factor for SUD development (#3 in Figure 1). Adolescents tend to befriend peers with similar identities and interests,210 as demonstrated by a tendency for aggressive boys to affiliate with other aggressive boys.211 Friendships with deviant peers predict development of SUD among adolescents;212 these results have been replicated in adolescents without a familial SUD history213 and across gender and ethnicity.214–216 Ary and collaborators217,218 proposed that increasing family conflict in adolescence discouraged parental monitoring, which led to SUD through increased affiliation with deviant peers. Indeed, affiliation with deviant peers was a more powerful predictor of SUD than conduct problems in the index subject, particularly in females.215 The best evidence indicates that adolescent deviance and association with deviant peers amplify each other as risk factors.219,220
Researchers at the University of Pittsburgh have investigated a construct termed neurobehavioral disinhibition, which combines measures of externalizing behavior, EF, and affect into a single factor.221,222 It has been used to predict SUD at age 19222,223 and marijuana use at age 16,223 and it is believed to be highly heritable.224 Although the concept does not account for peer affiliation and needs further investigation, neurobehavioral disinhibition is a good example of the push to aggregate neurobehavioral risk factors that can inform primary prevention. Overall, it appears that the combination of deviant peer associations and externalizing behavior problems poses the greatest risk for the development of SUD, with difficult temperament and decision-making serving as less important risk factors. This is demonstrated by McGue and Iacono,201 though, it may be that these traits serve as non-specific risks for later diagnoses in the disinhibited spectrum rather than as specific risks for SUD.
TREATMENT IMPLICATIONS
As stated previously, best practice appears to be treatment of both the SUD and any co-occurring psychiatric disorders. It appears that selective serotonin reuptake inhibitors have efficacy in ameliorating both depressive and SUD symptoms.225–227 For those with externalizing disorders and SUD, various medications, including divalproate,228 fluoxetine,227 and bupropion,229 have shown efficacy in reducing SUD symptoms. The effects were weaker on externalizing symptoms. All of these studies were of a pilot nature, so larger-scale randomized controlled trials are needed to fully evaluate the effects of these medications in adolescents with SUD.207
For behavioral treatment, there is evidence that cognitive-behavioral interventions (both in group or individual settings),230 motivational enhancement,231 and family therapy232 have efficacy in adolescents with SUD. The evidence for cognitive-behavioral intervention in a group setting is notable, given earlier evidence that group interventions with adolescents may increase high-risk behaviors, including substance use.233 It is also notable that improvements in familial interaction appear to co-occur with a reduction in adolescent substance use for those in family therapy.232 Given the unique developmental transitions adolescents are undergoing, many of which specifically relate to familial relationships, family therapy could be an important component of any treatment regimen. Biglan and colleagues234 have written an informative book reviewing treatments for adolescents with multiple high-risk behaviors, including substance use; they note that many adolescents with SUD often have other externalizing problems and that such a multi-problem presentation requires multi-faceted treatment.234 Clinicians should assess patients with substance use problems for other high-risk behaviors (e.g., sexual risk behaviors) and act to treat the entire set of problem behaviors. Such treatment will likely necessitate both pharmacological and behavioral interventions.
THE NEUROBIOLOGY OF RISK FOR SUD IN HUMAN ADOLESCENTS
Neuroimaging: ERP, EEG, and fMRI
One of the best examined neurobiological risk markers (noted in #5 in Figure 1) for the development of SUDs is the P300 event-related potential (ERP). ERPs are EEG voltage changes that are temporally related to sensory, motor, or cognitive events. These electrical potentials represent the activity of large numbers of synchronous neuronal elements during information processing. There is considerable evidence that GABA and glutamate modulate, or perhaps directly cause, the expression of the P300 signal.235 The P300 ERP appears to correlate with the attention one devotes to a stimulus, and latency in attending to a stimulus.235–237
Begleiter and collaborators238 initiated interest in P300 ERPs as a marker for SUD risk by demonstrating that the P300 response was blunted in male children of alcoholics. The P300 signal appeared to be a useful predictor of later alcohol-related disorders239 and was found to be moderately heritable.240–242 A study of monozygotic twins also indicated that reduced P300 amplitudes predated SUD development, and persisted in twins both with and without SUD.243 The association of P300 abnormalities with SUD, however, may be a function of the relationship between P300 amplitude and non-specific disinhibition.244 Bauer and Hesselbrock245 found that P300 dysfunction might be better explained by comorbid CD, especially given the correlation between P300 signals and CD.246 Habeych and colleagues247 found that SUD was predicted both by P300 attenuations and trait disinhibition, with disinhibition mediating P300 signal and SUD. This is not to say that P300 decrements are without utility; instead, the P300 may serve as an endophenotype (or “downstream traits” between genes and the disorder248) to guide investigations into more specific risk factors for SUD. Furthermore, P300 signals have been used to predict treatment response in ADHD,249 so they could serve a similar function for SUD. Also, investigations that evaluated changes in P300 signals following treatment could lend insight into the mechanisms of interventions.
EEG studies have shown that right frontal activity during a gambling task, similar to the task mentioned above,182,250 corresponds with risky decision-making.251 Poor performance on these tasks may represent deficits in the participant’s ability to tie emotional state to the predicted outcome of a decision. Functional MRI (fMRI) studies of emotional regulation and processing, which are dysfunctional in individuals with negative affect, implicate bilateral activations of the PFC, the orbitofrontal cortex, and the right anterior cingulate in adolescents.252 Adults activated a different pattern of structures.253,254 Finally, on a go/no-go task (a measure of inhibition), substance-näive high-risk young adolescents had attenuated left frontal middle gyrus activity on fMRI when compared to low-risk adolescents. There was a trend toward less overall frontal activity in high-risk individuals.255
5-HT and DA Receptor Systems
Neurobiological measures of risk have focused on 5-HT functioning, prompted partially by the research mentioned earlier on the association of 5-HT function with appetitive behaviors.115,117 Often, these studies have used peripheral markers, including platelet 5-HT, whole blood 5-HT concentrations, and/or platelet MAO activity, as proxies for central 5-HT function. In an examination of children with an alcoholic parent, the level of the child’s externalizing behavior was inversely correlated with that child’s level of whole blood 5-HT.256,257 Askenazy et al.258 found that platelet 5-HT concentrations were higher in those with greater impulsivity, and Mezzich and colleagues259 reported that for adolescents with SUD, platelet MAO activity corresponded to difficult temperament. In addition, certain 5-HT transporter polymorphisms260 and 5-HT transporter gene combinations261 appear to increase the risk for SUD, especially alcohol-related problems.
However, studies exploring the relationship of 5-HT to the development of SUD often contain methodological issues.262 Results in animals indicate that 5-HT depletion is associated with motoric impulsivity, whereas the development of SUD in human adolescents may be associated instead with impulse choice or delayed discounting.250,263,264 These terms denote the selection of smaller, immediate rewards over larger, delayed rewards. In all, 5-HT dysfunction may be a non-specific marker for disinhibition and negative affect rather than a specific risk factor for the development of addiction. Indeed, Oreland hypothesized that platelet MAO (as a peripheral marker of central 5-HT) may serve as a non-specific marker for CNS dysfunction corresponding to disinhibited behavior.265,266 Nonetheless, disinhibition and negative affect are both risk factors for SUD, so 5-HT dysfunction remains relevant as a risk factor for SUD. The salience of the association between 5-HT and SUD is strengthened by data (mentioned previously) that treatment with fluoxetine can improve adolescent substance-related outcomes, with stronger effects on depressive symptoms.225–227 It is likely that some of the improvements seen are due to the normalization of 5-HT function. That said, selective serotonin reuptake inhibitors have mixed results in treating SUD alone in adults;267 the efficacy of these medications is seen primarily in comorbid presentations, which may indicate that reductions in depressive symptoms aid abstinence efforts.
DA-related genotypes also appear to influence the development of SUD. In examining high-risk adolescents, the presence of A1 allele for the D2 receptor (DRD2) was related to greater use of alcohol, tobacco, and illicit drugs.268 The A1 allele has been linked to reduced binding to D2 receptors and lowered D2 receptor availability.269–271 That said, measures of psychoticism and negative affect were also related to drug use outcomes in adolescents with the A1 allele, and no analyses were performed to assess mediation effects. Currently, it is unclear whether the DRD2 polymorphism serves as a specific risk factor for SUD development or whether, like 5-HT, it may be a non-specific risk factor of disinhibition and/or negative affect.
HPA Axis
Another major neurobiological measure corresponding to SUD is HPA axis function. The HPA axis is a neuroendocrine system that plays a significant role in the stress response, particularly through the downstream release of cortisol. Cortisol appears to have a permissive effect on the mesostriatal release of DA, much like drugs of abuse.272–274 Moss et al.275 found that male offspring of individuals with SUD had a blunted cortisol response to a stressor. These high-risk males also had higher levels of impulsivity and externalizing behavior. Pajer et al.276 replicated these results. Dawes and collaborators277 found that the correlation of blunted cortisol response and disinhibition was greater in adolescent females than in males, with cortisol response accounting for 24% of the variance in disinhibition. Findings from our laboratory indicate that HPA activity and SUD are related, as increased HPA activity was associated with development of SUD during the follow-up period. This was particularly true when individuals had comorbid depressive and/or anxiety disorders.193,278
These studies suggest that either HPA hyperactivity or hypoactivity can serve as a risk marker for SUD development. HPA dysfunction might mark either physiological under-reaction to a stressor, as in externalizing disorders, or overreaction to a stressor, often observed in adolescents with internalizing disorders. There is clear evidence that externalizing behavioral syndromes pose a risk for SUD development.175,279 Internalizing symptoms pose a risk for SUD as well,280 possibly through HPA hyperactivity.281 Thus, there may be two pathways to SUD involving HPA dysfunction: internalizing symptoms in tandem with HPA hyperactivity, or externalizing symptoms and HPA hypoactivity. While these may be etiological mechanisms, the limited results and speculative nature of this hypothesis highlight the need for further investigation. Concomitantly, investigations of medications with modulatory effects on the HPA system may be warranted in adolescents with SUD. Some antidepressants appear to regulate HPA expression,282,283 and their use could be efficacious in the prevention or treatment of SUD and in the treatment of important co-occurring disorders, including major depression.284 Also, the opioid antagonists naltrexone and nalmefene appear to increase hypothalamic tone,71,285 indicating potential utility in those with HPA hypoactivity and externalizing disorders.
THE CONSEQUENCES OF SUD ON THE ADOLESCENT BRAIN: ANIMAL FINDINGS
While an examination of the sequelae of substance use in adults is outside the scope of this review, the burgeoning literature concerning the neurobiology of substance use in adolescent animals is instructive. Alcohol, nicotine, and cocaine all appear to affect the adolescent brain in a distinct manner relative to their effect in adults (#6 in Figure 1). Less substantial literature supports a similar developmentally specific effect of cannabinoids,286–288 morphine,289 and MDMA.290 Many of these unique effects predispose adolescents to greater substance use than adults, and a subset persist into adulthood, implying permanent alterations in structure and/or function.
Alcohol
After alcohol administration, adolescent rats experience less disruption of motor function291,292 and less sedation293 than adults. Adolescent rats also experience attenuated acute alcohol withdrawal symptoms, including reductions in anxiety,294,295 hyperthermia,296 and social isolation.294 These manifestations may be a result of less GABA-mediated inhibition in response to alcohol.297,298 Linking human and animal findings, Schuckit and colleagues299 found that a lower self-reported response to alcohol in younger adolescents related to six-month drinking frequency and number of experienced alcohol problems. Compared to adult rats, adolescent rats display higher seizure thresholds and hypoactivity during withdrawal300–302 and appear to develop tolerance to alcohol more quickly.21 In addition, rats administered high doses of alcohol both during adolescence and adulthood demonstrate more severe impairments in working memory303 and greater self-administration following uncontrollable stress304 during adulthood than rats given heavy doses only in adulthood.
The effects of alcohol on the hippocampus305–311 in rats and humans, and on the cingulate312,313 in rats, are greater in adolescents than adults. These effects may be mediated by the NMDA receptor. Pyapali et al.307 found that NMDA-mediated long-term potentiation, which is associated with synapse formation,314 is more strongly inhibited in adolescent rats dosed with alcohol. Such functional suppression may result in impaired cortical restructuring.315–317 Furthermore, adolescent rats administered alcohol in a binge fashion evidenced alterations in 5-HT binding not found in rats that only binge as adults.318 Adolescent mice that are chronically administered alcohol have greater in vitro increases in VTA DA neuron firing in response to alcohol dosing and attenuated blunting of VTA DA firing following GABA administration.319
Nicotine
Young adolescent rats exposed to nicotine are more likely to self-administer as adults than nicotine-näive adult rats and they exhibit CPP for nicotine320,321 and self-administer nicotine more readily322 than do older rats.19 In addition, late adolescent rats develop tolerance to the inhibitory effects of low-dose nicotine on locomotion more strongly and more quickly than adult rats.321 Slawecki, Ehlers, and collaborators323–325 found altered ERP and EEG outputs following five days of nicotine dosing in adolescent rats; these persisted into adulthood. Most strikingly, Trauth et al.326,327 found increased expression of a set of genes (activated following cellular damage) in brain areas following nicotine administration in adolescent rats; the increased gene expression was most notable in the hippocampus.327 Nicotine dosing in adolescents produces immediate alterations in nicotinic, DA, and 5-HT transporter densities unlike those seen in adult rats.328 Finally, alterations from nicotine administration in adolescent rats that persist into adulthood have been seen in nicotinic,19,329 DA,330,331 and 5-HT systems.332,333
Cocaine and Other Stimulants
Many,334–336 but not all,337 studies have found that adolescent rats exhibit locomotor sensitization in response to cocaine dosing. Ehrlich and collaborators338 found that adolescents demonstrated greater increases in NAc levels of ΔFosB expression (implicated in neural plasticity) than adults following chronic cocaine dosing. Furthermore, Kosofsky and colleagues339 found that unique groups of genes were activated in the striatum following cocaine administration at different developmental periods. The genes involved have been implicated in regulation of the cell cycle and cell death,340,341 neural plasticity,342,343 and addiction.344,345
Research on other psychostimulants found that young adolescent rats did not develop sensitization to methylphenidate, unlike adults.336 Dosing with d-amphetamine did not produce CPP in adolescent mice, unlike adult mice,346 but adolescent dosing does increase sensitization in adulthood.347 Bolanos et al.348 administered methylphenidate to young adolescent rats and found disruptions in preference for reinforcers and in stress sensitivity in adulthood. Adriani and Laviola346 found that adolescent mice responded more often during the non-reinforced portion of a reinforcement schedule than did adults following d-amphetamine administration. Adolescent rats given methylphenidate did not differentiate well between a novel and familiar object,349 and late adolescent rats displayed learning deficits following methamphetamine administration.350 Finally, chronic administration of d-amphetamine in adolescent rats increased ΔFosB expression in the NAc and striatum, a result not seen in adult or pre-adolescent rats.338
Thus, the literature on the neurobiology of substance abuse in adolescent animals indicates that adolescents differ from adults in response to nearly all drugs of abuse; perhaps more importantly, adult animals evidence alterations in function following the administration of alcohol, nicotine, or stimulants during adolescence (all #6 in Figure 1). Furthermore, there is evidence that the use of nicotine351–353 or methylphenidate20 in adolescent animals alters adult responses to cocaine and other psychostimulants. Taken together, especially in light of the evidence for greater neural plasticity of adolescents (#1 in Figure 1), these results raise questions about the ability of psychoactive substances to alter development and increase substance use.
SUMMARY
Adolescence is a time of several transitions, particularly involving maturational changes in the CNS; foremost among these are synaptic pruning, myelination, and neurotransmitter system modifications. Overall, the CNS structural and functional evidence indicates that adolescents have greater neurobiologically based tendencies for risk-taking with attenuated suppressive and regulatory controls on behavior (as depicted in #1 in Figure 1). Adolescent transitions often feature strong affect and stress, and adolescents may be more adversely affected than adults by stressful and/or affect-laden situations due to functional neurobiological immaturity. Decreases in parental monitoring during adolescence (#2 in the figure) can allow for experimentation with substances and affiliative relationships between disinhibited peers. These friendships appear to create an amplifying feedback cycle that increases both SUD risk and disinhibition levels (#3). Disinhibiton and other less robust risk factors (e.g., EF liabilities and difficult temperament) are likely to be the expression of both normative (#1) and non-normative neurobiological changes in those at high-risk for SUD (#5).
The abnormal neurobiological markers of those at risk for SUD development seem to correspond most clearly to disinhibition and/or negative affect. While the evidence for an unmediated pathway from these traits to SUD is limited, these neurobiological markers can serve as useful endophenotypes associated with SUD. Once adolescents begin substance use (#5 and #6 in the figure), the evidence indicates that they are more vulnerable to the effects of many substances of abuse. Most likely, this vulnerability is mediated by the heightened neuroplasticity of adolescents and the effects of stress. Substance-induced neurobiological alterations likely strengthen drug-use behaviors. Neuroplasticity-mediated increases in drug use accelerate neurobehavioral dysfunction, which accelerates substance use, eventually resulting in SUD and continued maladaptive neurobiological alteration.
FUTURE DIRECTIONS
While there are concerns about the methods used to estimate the heritability of various types of SUD,354 these do not obscure the fact that genetic factors play a prominent role in their development.355,356 Stallings et al.357 found six genomic markers with high correspondence to SUD, which can be linked to various proteins and (eventually) functions for these products. Other research has highlighted DA261,358 and 5-HT268 gene-related polymorphisms linked to externalizing behavior and SUD; it seems that the identification of specific genes or polymorphisms could be useful to narrow the potential neurobiological targets for intervention.
Further investigation of the contributions of 5-HT, the HPA axis, and the frontal-striatal-thalamic neural circuit is also needed. While the results on 5-HT have been somewhat problematic, it appears to be one of the primary suppressive neurotransmitters. Given its role in behavioral inhibition and other psychiatric disorders (e.g., depressive disorders), there is evidence that it plays a role in drug use. Further investigation into the developmental course and effects of 5-HT (along with the glutaminergic, GABAergic, and cannabinoid) systems are required as well. Such research could result in targets for pharmacological intervention in a fashion similar to 5-HT in depressive disorders. Research into the interaction of the HPA axis and psychopathology is also very promising, not least because of the ability of researchers to quantify HPA reactivity in stress paradigms. There is some thought that medications with regulatory effects on HPA function (e.g., mifepristone) may help stress-sensitive adults with SUD; if true, attempts should be made to extend the concept to adolescents. Finally, neuroimaging findings that implicate frontal-striatal-thalamic dysfunction are useful in linking neurobehavioral findings to specific brain areas. Further clarification is needed on the contribution of the hippocampus, striatum and limbic structures to SUD.
In addition to 5-HT and the HPA axis, other pharmacological targets could include the glutaminergic (especially NMDA-related aspects), GABAergic or cannabinoid system. Neuromodulators such as enkephalin or dynorphin could also serve as targets, pending clarification of their roles in addiction. Despite a lack of efficacy for DA agents in adults,71 the significant developmental differences between adolescents and adults may indicate that DA agents could have differential efficacy in adolescents. In addition to pharmacological intervention, behavioral interventions need to be further studied and utilized. While there are many SUD-specific interventions, interventions targeting disinhibition may be most effective in combating both SUD and risky behavior. Such an intervention could improve the neurobehavioral skills of individuals at high-risk for substance use. There is some evidence that interventions can reduce disinhibited behavior,359,360 which calls for further investigation of programs aimed at risky behavior across domains.234
The concept of intervening on disinhibition is based on indications that there are few, if any, necessary or sufficient risk factors for the development of SUD. It appears that both multifinality and equifinality are at work in the development of SUD, clouding the causal picture: many different, separate paths seem to lead to SUD (equifinality) and the traits or paths involved lead to other disinhibited outcomes (multifinality) as well. It is essential that investigators continue to seek risk factors with greater specificity for the development of SUD. While current risk markers (e.g., P300, 5-HT, HPA dysfunction) are useful as warning signs of disinhibitory liabilities, the specific factors that lead to SUD are unknown. While such specific factors may not exist (or, more likely, may not be currently detectable), negative results are useful to rule out potential risk factors for the development of SUD.
The clearest area of investigative need is translational research, which bridges the findings concerning normal development, animal models of SUD, and disinhibition. While each area of research is compelling, the capability of investigators to link findings together remains problematic. Without translational research, the development of pharmacological and psychosocial interventions will be haphazard. The benefits of integrative research are compelling: targeted combinations of medication and psychosocial interventions that maximize the benefits to individual patients based on unique traits and risk patterns.
Acknowledgments
This work was supported, in part, by the National Institutes of Health grants DA11434 (Dr. Adinoff); DA14037, DA15131, DA17804, DA17805, MH62464, and MH68391(Dr. Rao); and DA007238 (Ismene Petrakis, MD, Yale University, West Haven, Conn.); the Sarah M. and Charles E. Seay Endowed Chair in Child Psychiatry at UT Southwestern Medical Center, Dallas, Tex. (Dr. Rao); and the VA North Texas Health Care System, Dallas, Tex.
References
- 1.Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Actual causes of death in the United States, 2000. JAMA. 2004;291:1238–1245. doi: 10.1001/jama.291.10.1238. [DOI] [PubMed] [Google Scholar]
- 2.Baigent MF. Physical complications of substance abuse: what the psychiatrist needs to know. Curr Opin Psychiatry. 2003;16:291–296. [Google Scholar]
- 3.Greenfield TK. Individual risk of alcohol-related disease and problems. In: Heather N, Peters TJ, Stockwell T, editors. International Handbook of Alcohol Dependence and Problems. New York: John Wiley & Sons Ltd; 2001. pp. 413–437. [Google Scholar]
- 4.Centers for Disease Control and Prevention. Annual smoking-attributable mortality, years of potential life lost, and economic costs—United States, 1995–1999. MMWR Surveill Summ. 2002;51:300–303. [PubMed] [Google Scholar]
- 5.Office of National Drug Control Policy. Publication No. NCJ-190636. Washington, DC: Executive Office of the President; 2001. The Economic Costs of Drug Abuse in the United States, 1992–1998. [Google Scholar]
- 6.Harwood H. Updating Estimates of the Economic Costs of Alcohol Abuse in the United States: Estimates, Update Methods, and Data. The Economic Costs of Alcohol and Drug Abuse in the United States 1992. Report prepared for the National Institute on Drug Abuse and the National Institute on Alcohol Abuse and Alcoholism, National Institutes of Health, Department of Health and Human Services. NIH Publication No. 98-4327; Rockville, Md: National Institutes of Health; 2000. [Google Scholar]
- 7.Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. College students and adults ages 19–45. NIH Publication No. 05-5728. II. Bethesda, Md: National Institute on Drug Abuse; 2005. Monitoring the Future national survey results on drug use, 1975–2004; p. 278. [Google Scholar]
- 8.Department of Health and Human Services. DHHS Publication No. CDC 89-8411. Washington, DC: U.S. Government Printing Office; 1994. Preventing tobacco use among young people: A report of the surgeon general. [Google Scholar]
- 9.McCambridge J, Strang J. Age of first use and ongoing patterns of legal and illegal drug use in a sample of young Londoners. Subst Use Misuse. 2005;40:313–319. doi: 10.1081/ja-200049333. [DOI] [PubMed] [Google Scholar]
- 10.Storr CL, Westergaard R, Anthony JC. Early onset inhalant use and risk for opiate initiation by young adulthood. Drug Alcohol Depend. 2005;78:253–261. doi: 10.1016/j.drugalcdep.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 11.Chen CY, O’Brien MS, Anthony JC. Who becomes cannabis dependent soon after onset of use? Epidemiological evidence from the United States: 2000–2001. Drug Alcohol Depend. 2005;79:11–22. doi: 10.1016/j.drugalcdep.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 12.Anthony JC, Petronis KR. Early-onset drug use and risk of later drug problems. Drug Alcohol Depend. 1995;40:9–15. doi: 10.1016/0376-8716(95)01194-3. [DOI] [PubMed] [Google Scholar]
- 13.Yen CF, Su YC. The associations of early-onset methamphetamine use with psychiatric morbidity among Taiwanese adolescents. Subst Use Misuse. 2006;41:35–44. doi: 10.1080/10826080500318616. [DOI] [PubMed] [Google Scholar]
- 14.Ridenour TA, Maldonado-Molina M, Compton WM, Spitznagel EL, Cottler LB. Factors associated with the transition from abuse to dependence among substance abusers: implications for a measure of addictive liability. Drug Alcohol Depend. 2005;80:1–14. doi: 10.1016/j.drugalcdep.2005.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Franken IHA, Hendriks VM. Early-onset of illicit substance use is associated with greater axis-II comorbidity, not with axis-I comorbidity. Drug Alcohol Depend. 2000;59:305–308. doi: 10.1016/s0376-8716(99)00132-5. [DOI] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention. Surveillance summaries. MMWR Surveill Summ. 2004 May 21, 2004;53(No SS2) [Google Scholar]
- 17.Giedd JN, Blumenthal J, Jeffries NO, et al. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- 18.Chambers RA, Taylor JR, Potenza MN. Developmental neurocircuitry of motivation in adolescence: a critical period of addiction vulnerability. Am J Psychiatry. 2003;160:1041–1052. doi: 10.1176/appi.ajp.160.6.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adriani W, Spijker S, Deroche-Gamonet V, et al. Evidence for enhanced neurobehavioral vulnerability to nicotine during periadolescence in rats. J Neurosci. 2003;23:4712–4716. doi: 10.1523/JNEUROSCI.23-11-04712.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schenk S, Izenwasser S. Pretreatment with methylphenidate sensitizes rats to the reinforcing effects of cocaine. Pharmacol Biochem Behav. 2002;72:651–657. doi: 10.1016/s0091-3057(02)00735-9. [DOI] [PubMed] [Google Scholar]
- 21.Swartzwelder HS, Richardson RC, Markwiese-Foerch B, Wilson WA, Little PJ. Developmental differences in the acquisition of tolerance to ethanol. Alcohol. 1998;15:311–314. doi: 10.1016/s0741-8329(97)00135-3. [DOI] [PubMed] [Google Scholar]
- 22.Luna B, Sweeney JA. The emergence of collaborative brain function—fMRI studies of the development of response inhibition. Ann NY Acad Sci. 2004;1021:296–309. doi: 10.1196/annals.1308.035. [DOI] [PubMed] [Google Scholar]
- 23.Kelley AE, Schochet T, Landry CF. Risk taking and novelty seeking in adolescence: introduction to part I. Ann NY Acad Sci. 2004;1021:27–32. doi: 10.1196/annals.1308.003. [DOI] [PubMed] [Google Scholar]
- 24.Steinberg L. Risk taking in adolescence: what changes, and why? Ann NY Acad Sci. 2004;1021:51–58. doi: 10.1196/annals.1308.005. [DOI] [PubMed] [Google Scholar]
- 25.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed., text rev. Washington, DC: American Psychiatric Association; 2001. [Google Scholar]
- 26.Nace EP, Meyers AL, Rothberg JM, Maleson F. Addicted and nonaddicted drug users. A comparison of drug usage patterns. Arch Gen Psychiatry. 1975;32:77–80. doi: 10.1001/archpsyc.1975.01760190079009. [DOI] [PubMed] [Google Scholar]
- 27.Williams RJ, Zolner T, Bertrand LD, Davis R. Mental health status of infrequent adolescent substance users. J Child Adolesc Subst. 2004;14:41–60. [Google Scholar]
- 28.Shedler J, Block J. Adolescent drug use and psychological health. A longitudinal inquiry. Am Psychol. 1990;45:612–630. doi: 10.1037//0003-066x.45.5.612. [DOI] [PubMed] [Google Scholar]
- 29.Guilamo-Ramos V, Turrisi R, Jaccard J, Wood E, Gonzalez B. Progressing from light experimentation to heavy episodic drinking in early and middle adolescence. J Stud Alcohol. 2004;65:494–500. doi: 10.15288/jsa.2004.65.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dawson DA, Harford TC, Grant BF. Family history as a predictor of alcohol dependence. Alcohol Clin Exp Res. 1992;16:572–575. doi: 10.1111/j.1530-0277.1992.tb01419.x. [DOI] [PubMed] [Google Scholar]
- 31.Schuckit MA, Smith TL. A comparison of correlates of DSM-IV alcohol abuse or dependence among more than 400 sons of alcoholics and controls. Alcohol Clin Exp Res. 2001;25:1–8. [PubMed] [Google Scholar]
- 32.Martin CS, Earleywine M, Blackson TC, Vanyukov MM, Moss HB, Tarter RE. Aggressivity, inattention, hyperactivity, and impulsivity in boys at high and low risk for substance abuse. J Abnorm Child Psychol. 1994;22:177–203. doi: 10.1007/BF02167899. [DOI] [PubMed] [Google Scholar]
- 33.Leon-Carrion J, Garcia-Orza J, Perez-Santamaria FJ. Development of the inhibitory component of the executive functions in children and adolescents. Int J Neurosci. 2004;114:1291–1311. doi: 10.1080/00207450490476066. [DOI] [PubMed] [Google Scholar]
- 34.Brocki KC, Bohlin G. Executive functions in children aged 6 to 13: A dimensional and developmental study. Dev Neuropsychol. 2004;26:571–593. doi: 10.1207/s15326942dn2602_3. [DOI] [PubMed] [Google Scholar]
- 35.Anderson VA, Anderson P, Northam E, Jacobs R, Catroppa C. Development of executive functions through late childhood and adolescence in an Australian sample. Dev Neuropsychol. 2001;20:385– 406. doi: 10.1207/S15326942DN2001_5. [DOI] [PubMed] [Google Scholar]
- 36.Klenberg L, Korkman M, Lahti-Nuuttila P. Differential development of attention and executive functions in 3- to 12-year-old Finnish children. Dev Neuropsychol. 2001;20:407–428. doi: 10.1207/S15326942DN2001_6. [DOI] [PubMed] [Google Scholar]
- 37.Tamm L, Menon V, Reiss AL. Maturation of brain function associated with response inhibition. J Am Acad Child Adolesc Psychiatry. 2002;41:1231–1238. doi: 10.1097/00004583-200210000-00013. [DOI] [PubMed] [Google Scholar]
- 38.Levin HS, Culhane KA, Hartmann J, Evankovich K, Mattson AJ. Developmental changes in performance on tests of purported frontal lobe functioning. Dev Neuropsychol. 1991;7:377–395. [Google Scholar]
- 39.Iversen SD, Mishkin M. Perseverative interference in monkeys following selective lesions of the inferior prefrontal convexity. Exp Brain Res. 1970;11:376–386. doi: 10.1007/BF00237911. [DOI] [PubMed] [Google Scholar]
- 40.Hazeltine E, Poldrack R, Gabrieli JD. Neural activation during response competition. J Cogn Neurosci. 2000;12 (Suppl 2):118–129. doi: 10.1162/089892900563984. [DOI] [PubMed] [Google Scholar]
- 41.Ullsperger M, von Cramon DY. Neuroimaging of performance monitoring: Error detection and beyond. Cortex. 2004;40:593–604. doi: 10.1016/s0010-9452(08)70155-2. [DOI] [PubMed] [Google Scholar]
- 42.Fornito A, Yucel M, Wood S, et al. Individual differences in anterior cingulate/paracingulate morphology are related to executive functions in healthy males. Cereb Cortex. 2004;14:424–431. doi: 10.1093/cercor/bhh004. [DOI] [PubMed] [Google Scholar]
- 43.Heyder K, Suchan B, Daum I. Cortico-subcortical contributions to executive control. Acta Psychol. 2004;115:271–289. doi: 10.1016/j.actpsy.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 44.Giedd JN. Structural magnetic resonance imaging of the adolescent brain. Ann NY Acad Sci. 2004;1021:77–85. doi: 10.1196/annals.1308.009. [DOI] [PubMed] [Google Scholar]
- 45.Paus T, Zijdenbos A, Worsley K, et al. Structural maturation of neural pathways in children and adolescents: in vivo study. Science. 1999;283:1908–1911. doi: 10.1126/science.283.5409.1908. [DOI] [PubMed] [Google Scholar]
- 46.Bartzokis G, Beckson M, Lu PH, Nuechterlein KH, Edwards N, Mintz J. Age-related changes in frontal and temporal lobe volumes in men: A magnetic resonance imaging study. Arch Gen Psychiatry. 2001;58:461–465. doi: 10.1001/archpsyc.58.5.461. [DOI] [PubMed] [Google Scholar]
- 47.Rakic P, Bourgeois JP, Goldman-Rakic PS. Synaptic development of the cerebral cortex: implications for learning, memory, and mental illness. Prog Brain Res. 1994;102:227–243. doi: 10.1016/S0079-6123(08)60543-9. [DOI] [PubMed] [Google Scholar]
- 48.Lichtman JW, Colman H. Synapse elimination and indelible memory. Neuron. 2000;25:269–278. doi: 10.1016/s0896-6273(00)80893-4. [DOI] [PubMed] [Google Scholar]
- 49.Casey BJ, Giedd JN, Thomas KM. Structural and functional brain development and its relation to cognitive development. Biol Psychiatry. 2000;54:241–257. doi: 10.1016/s0301-0511(00)00058-2. [DOI] [PubMed] [Google Scholar]
- 50.Casey BJ, Thomas KM, Welsh TF, et al. A developmental fMRI study of prefrontal organization. Neuroimage. 1998;7:S512. [Google Scholar]
- 51.Casey BJ, Trainor RJ, Orendi JL, et al. A developmental functional MRI study of prefrontal activation during performance of a go–no-go task. J Cogn Neurosci. 1997;9:835–847. doi: 10.1162/jocn.1997.9.6.835. [DOI] [PubMed] [Google Scholar]
- 52.Luna B, Sweeney JA. The emergence of collaborative brain function—fMRI studies of the development of response inhibition. Ann NY Acad Sci. 2004;1021:296–309. doi: 10.1196/annals.1308.035. [DOI] [PubMed] [Google Scholar]
- 53.Chechik G, Meilijson I, Ruppin E. Synaptic pruning in development: A computational account. Neural Comput. 1998;10:1759–1777. doi: 10.1162/089976698300017124. [DOI] [PubMed] [Google Scholar]
- 54.Mimura K, Kimoto T, Okada M. Synapse efficiency diverges due to synaptic pruning following overgrowth. Phys Rev E Stat Nonlin Soft Matter Phys. 2003;68:031910. doi: 10.1103/PhysRevE.68.031910. [DOI] [PubMed] [Google Scholar]
- 55.Giedd JN, Blumenthal J, Jeffries NO, et al. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- 56.Durston S, Hulshoff Pol HE, Casey BJ, Giedd JN, Buitelaar JK, van Engeland H. Anatomical MRI of the developing human brain: what have we learned? J Am Acad Child Adolesc Psychiatry. 2001;40:1012–1020. doi: 10.1097/00004583-200109000-00009. [DOI] [PubMed] [Google Scholar]
- 57.Rajapakse JC, DeCarli C, McLaughlin A, et al. Cerebral magnetic resonance image segmentation using data fusion. J Comput Assist Tomogr. 1996;20:206–218. doi: 10.1097/00004728-199603000-00007. [DOI] [PubMed] [Google Scholar]
- 58.Sowell ER, Thompson PM, Tessner KD, Toga AW. Mapping continued brain growth and gray matter density reduction in dorsal frontal cortex: inverse relationships during postadolescent brain maturation. J Neurosci. 2001;21:8819–8829. doi: 10.1523/JNEUROSCI.21-22-08819.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sowell ER, Thompson PM, Holmes CJ, Jernigan TL, Toga AW. In vivo evidence for post-adolescent brain maturation in frontal and striatal regions. Nat Neurosci. 1999;2:859–861. doi: 10.1038/13154. [DOI] [PubMed] [Google Scholar]
- 60.Jernigan TL, Trauner DA, Hesselink JR, Tallal PA. Maturation of human cerebrum observed in vivo during adolescence. Brain. 1991;114:2037–2049. doi: 10.1093/brain/114.5.2037. [DOI] [PubMed] [Google Scholar]
- 61.Kanemura H, Aihara M, Aoki S, Araki T, Nakazawa S. Development of the prefrontal lobe in infants and children: A three-dimensional magnetic resonance volumetric study. Brain Dev. 2003;25:195–199. doi: 10.1016/s0387-7604(02)00214-0. [DOI] [PubMed] [Google Scholar]
- 62.Sowell ER, Thompson PM, Holmes CJ, Batth R, Jernigan TL, Toga AW. Localizing age-related changes in brain structure between childhood and adolescence using statistical parametric mapping. Neuroimage. 1999;9:587–597. doi: 10.1006/nimg.1999.0436. [DOI] [PubMed] [Google Scholar]
- 63.Gogtay N, Giedd JN, Lusk L, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci USA. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kalivas PW. Neurotransmitter regulation of dopamine neurons in the ventral tegmental area. Brain Res Brain Res Rev. 1993;18:75–113. doi: 10.1016/0165-0173(93)90008-n. [DOI] [PubMed] [Google Scholar]
- 65.Gardner EL. Brain reward mechanisms. In: Lowinson JH, Ruiz P, Millman RB, Langrod JG, editors. Substance Abuse: A Comprehensive Textbook. Baltimore, Md: Williams & Wilkins; 1997. [Google Scholar]
- 66.Carelli RM, Deadwyler SA. A comparison of nucleus accumbens neuronal firing patterns during cocaine self-administration and water reinforcement in rats. J Neurosci. 1994;14:7735–7746. doi: 10.1523/JNEUROSCI.14-12-07735.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schultz W, Apicella P, Ljungberg T. Responses of monkey dopamine neurons to reward and conditioned stimuli during successive steps of learning a delayed response task. J Neurosci. 1993;13:900–913. doi: 10.1523/JNEUROSCI.13-03-00900.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Carlezon WA, Jr, Wise RA. Rewarding actions of phencyclidine and related drugs in nucleus accumbens shell and frontal cortex. J Neurosci. 1996;16:3112–3122. doi: 10.1523/JNEUROSCI.16-09-03112.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Di Chiara G. A motivational learning hypothesis of the role of mesolimbic dopamine in compulsive drug use. J Psychopharmacol. 1998;12:54–67. doi: 10.1177/026988119801200108. [DOI] [PubMed] [Google Scholar]
- 70.Di Chiara G. Alcohol and dopamine. Alcohol Health Res World. 1997;21:108–114. [PMC free article] [PubMed] [Google Scholar]
- 71.Adinoff B. Neurobiologic processes in drug reward and addiction. Harv Rev Psychiatry. 2004;12:305–320. doi: 10.1080/10673220490910844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Palacios JM, Camps M, Cortes R, Probst A. Mapping dopamine receptors in the human brain. J Neural Transm Suppl. 1988;27:227–235. doi: 10.1007/978-3-7091-8954-2_20. [DOI] [PubMed] [Google Scholar]
- 73.Seeman P, Bzowej NH, Guan HC, et al. Human brain dopamine receptors in children and aging adults. Synapse. 1987;1:399–404. doi: 10.1002/syn.890010503. [DOI] [PubMed] [Google Scholar]
- 74.Tarazi FI, Tomasini EC, Baldessarini RJ. Postnatal development of dopamine D1-like receptors in rat cortical and striatolimbic brain regions: An autoradiographic study. Dev Neurosci. 1999;21:43–49. doi: 10.1159/000017365. [DOI] [PubMed] [Google Scholar]
- 75.Ennulat DJ, Andersen SL, Yang M, Teicher MH. Ontogeny of dopamine D1 receptor mRNA during the periadolescent period in rats. Paper presented at: Society for Neuroscience’s 30th Annual Meeting; November 4–9, 2000; New Orleans, La. [Google Scholar]
- 76.Montague DM, Lawler CP, Mailman RB, Gilmore JH. Developmental regulation of the dopamine D1 receptor in human caudate and putamen. Neuropsychopharmacology. 1999;21:641–649. doi: 10.1016/S0893-133X(99)00062-7. [DOI] [PubMed] [Google Scholar]
- 77.Tarazi FI, Tomasini EC, Baldessarini RJ. Postnatal development of dopamine D4-like receptors in rat forebrain regions: comparison with D2-like receptors. Brain Research: Developmental Brain Research. 1998;110:227–233. doi: 10.1016/s0165-3806(98)00111-4. [DOI] [PubMed] [Google Scholar]
- 78.Teicher MH, Andersen SL, Hostetter JC., Jr Evidence for dopamine receptor pruning between adolescence and adulthood in striatum but not nucleus accumbens. Brain Research: Developmental Brain Research. 1995;89:167–172. doi: 10.1016/0165-3806(95)00109-q. [DOI] [PubMed] [Google Scholar]
- 79.Andersen SL, Thompson AT, Rutstein M, Hostetter JC, Teicher MH. Dopamine receptor pruning in prefrontal cortex during the periadolescent period in rats. Synapse. 2000;37:167–169. doi: 10.1002/1098-2396(200008)37:2<167::AID-SYN11>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 80.Leslie CA, Robertson MW, Cutler AJ, Bennett JP., Jr Postnatal development of D1 dopamine receptors in the medial prefrontal cortex, striatum and nucleus accumbens of normal and neonatal 6-hydroxydopamine treated rats: A quantitative autoradiographic analysis. Brain Research: Developmental Brain Research. 1991;62:109–114. doi: 10.1016/0165-3806(91)90195-o. [DOI] [PubMed] [Google Scholar]
- 81.Kalsbeek A, Voorn P, Buijs RM, Pool CW, Uylings HB. Development of the dopaminergic innervation in the prefrontal cortex of the rat. J Comp Neurol. 1988;269:58–72. doi: 10.1002/cne.902690105. [DOI] [PubMed] [Google Scholar]
- 82.Lesting J, Neddens J, Teuchert-Noodt G. Ontogeny of the dopamine innervation in the nucleus accumbens of gerbils. Brain Res. 2005;1066:16–23. doi: 10.1016/j.brainres.2005.08.058. [DOI] [PubMed] [Google Scholar]
- 83.Lewis DA. Development of the prefrontal cortex during adolescence: Insights into vulnerable neural circuits in schizophrenia. Neuropsychopharmacology. 1997;16:385–398. doi: 10.1016/S0893-133X(96)00277-1. [DOI] [PubMed] [Google Scholar]
- 84.Spear LP. Neurobehavioral changes in adolescence. Current Directions in Psychological Science. 2000;9:111–114. [Google Scholar]
- 85.Teicher MH, Barber NI, Gelbard HA, et al. Developmental differences in acute nigrostriatal and mesocorticolimbic system response to haloperidol. Neuropsychopharmacology. 1993;9:147–156. doi: 10.1038/npp.1993.53. [DOI] [PubMed] [Google Scholar]
- 86.Andersen SL, Dumont NL, Teicher MH. Developmental differences in dopamine synthesis inhibition by (+/−)-7-OH-DPAT. Naunyn Schmiedebergs Arch Pharmacol. 1997;356:173–181. doi: 10.1007/pl00005038. [DOI] [PubMed] [Google Scholar]
- 87.Sandstrom MI, Bruno JP. Sensitivity to the motoric effects of a dopamine receptor antagonist differs as a function of age at the time of dopamine depletion. Dev Psychobiol. 1997;30:293–300. [PubMed] [Google Scholar]
- 88.Weihmuller FB, Bruno JP. Age-dependent plasticity in the dopaminergic control of sensorimotor development. Behav Brain Res. 1989;35:95–109. doi: 10.1016/s0166-4328(89)80110-x. [DOI] [PubMed] [Google Scholar]
- 89.Spear LP, Brake SC. Periadolescence: age-dependent behavior and psychopharmacological responsivity in rats. Dev Psychobiol. 1983;16:83–109. doi: 10.1002/dev.420160203. [DOI] [PubMed] [Google Scholar]
- 90.Bolla K, Ernst M, Kiehl K, et al. Prefrontal cortical dysfunction in abstinent cocaine abusers. J Neuropsychiatry Clin Neurosci. 2004;16:456–464. doi: 10.1176/appi.neuropsych.16.4.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Giuffrida A, Parsons LH, Kerr TM, Rodriguez de Fonseca F, Navarro M, Piomelli D. Dopamine activation of endogenous cannabinoid signaling in dorsal striatum. Nat Neurosci. 1999;2:358–363. doi: 10.1038/7268. [DOI] [PubMed] [Google Scholar]
- 92.Diana M, Melis M, Gessa GL. Increase in meso-prefrontal dopaminergic activity after stimulation of CB1 receptors by cannabinoids. Eur J Neurosci. 1998;10:2825–2830. doi: 10.1111/j.1460-9568.1998.00292.x. [DOI] [PubMed] [Google Scholar]
- 93.Melis M, Perra S, Muntoni AL, et al. Prefrontal cortex stimulation induces 2-arachidonoyl-glycerol-mediated suppression of excitation in dopamine neurons. J Neurosci. 2004;24:10707–10715. doi: 10.1523/JNEUROSCI.3502-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rodriguez de Fonseca F, Ramos JA, Bonnin A, Fernandez-Ruiz JJ. Presence of cannabinoid binding sites in the brain from early postnatal ages. Neuroreport. 1993;4:135–138. doi: 10.1097/00001756-199302000-00005. [DOI] [PubMed] [Google Scholar]
- 95.Fride E, Mechoulam R. Ontogenetic development of the response to anandamide and delta 9-tetrahydrocannabinol in mice. Brain Research: Developmental Brain Research. 1996;95:131–134. doi: 10.1016/0165-3806(96)00087-9. [DOI] [PubMed] [Google Scholar]
- 96.Fride E, Mechoulam R. Developmental aspects of anandamide: ontogeny of response and prenatal exposure. Psychoneuroendocrinology. 1996;21:157–172. doi: 10.1016/0306-4530(95)00039-9. [DOI] [PubMed] [Google Scholar]
- 97.Soria G, Mendizabal V, Tourino C, et al. Lack of CB1 cannabinoid receptor impairs cocaine self-administration. Neuropsychopharmacology. 2005;30:1670–1680. doi: 10.1038/sj.npp.1300707. [DOI] [PubMed] [Google Scholar]
- 98.Yamamoto T, Anggadiredja K, Hiranita T. New perspectives in the studies on endocannabinoid and cannabis: A role for the endocannabinoid-arachidonic acid pathway in drug reward and long-lasting relapse to drug taking. J Pharmacol Sci. 2004;96:382–388. doi: 10.1254/jphs.fmj04003x5. [DOI] [PubMed] [Google Scholar]
- 99.Cheer JF, Wassum KM, Heien ML, Phillips PE, Wightman RM. Cannabinoids enhance subsecond dopamine release in the nucleus accumbens of awake rats. J Neurosci. 2004;24:4393–4400. doi: 10.1523/JNEUROSCI.0529-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Frantz K, Van Hartesveldt C. Locomotion elicited by MK801 in developing and adult rats: Temporal, environmental, and gender effects. Eur J Pharmacol. 1999;369:145–157. doi: 10.1016/s0014-2999(99)00070-9. [DOI] [PubMed] [Google Scholar]
- 101.Frantz K, Van Hartesveldt C. The locomotor effects of MK801 in the nucleus accumbens of developing and adult rats. Eur J Pharmacol. 1999;368:125–135. doi: 10.1016/s0014-2999(99)00009-6. [DOI] [PubMed] [Google Scholar]
- 102.Subramaniam S, McGonigle P. Regional profile of developmental changes in the sensitivity of the N-methyl-D-aspartate receptor to polyamines. J Neurochem. 1994;62:1408–1415. doi: 10.1046/j.1471-4159.1994.62041408.x. [DOI] [PubMed] [Google Scholar]
- 103.Court JA, Perry EK, Johnson M, et al. Regional patterns of cholinergic and glutamate activity in the developing and aging human brain. Brain Research: Developmental Brain Research. 1993;74:73–82. doi: 10.1016/0165-3806(93)90085-o. [DOI] [PubMed] [Google Scholar]
- 104.Johnson BA. Recent advances in the development of treatments for alcohol and cocaine dependence: Focus on topiramate and other modulators of GABA or glutamate function. CNS Drugs. 2005;19:873–896. doi: 10.2165/00023210-200519100-00005. [DOI] [PubMed] [Google Scholar]
- 105.Nurse S, Lacaille JC. Late maturation of GABA(B) synaptic transmission in area CA1 of the rat hippocampus. Neuropharmacology. 1999;38:1733–1742. doi: 10.1016/s0028-3908(99)00122-7. [DOI] [PubMed] [Google Scholar]
- 106.Kellogg CK, Taylor MK, Rodriguez-Zafra M, Pleger GL. Altered stressor-induced changes in GABAA receptor function in the cerebral cortex of adult rats exposed in utero to diazepam. Pharmacol Biochem Behav. 1993;44:267–273. doi: 10.1016/0091-3057(93)90461-2. [DOI] [PubMed] [Google Scholar]
- 107.Kellogg CK. Early developmental modulation of GABAA receptor function. Influence on adaptive responses. Perspectives on Developmental Neurobiology. 1998;5:219–234. [PubMed] [Google Scholar]
- 108.Vincent SL, Pabreza L, Benes FM. Postnatal maturation of GABA-immunoreactive neurons of rat medial prefrontal cortex. J Comp Neurol. 1995;355:81–92. doi: 10.1002/cne.903550110. [DOI] [PubMed] [Google Scholar]
- 109.Goveas JS, Csernansky JG, Coccaro EF. Platelet serotonin content correlates inversely with life history of aggression in personality-disordered subjects. Psychiatry Res. 2004;126:23–32. doi: 10.1016/j.psychres.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 110.Spoont MR. Modulatory role of serotonin in neural information processing: implications for human psychopathology. Psychological Bulletin. 1992;112:330–350. doi: 10.1037/0033-2909.112.2.330. [DOI] [PubMed] [Google Scholar]
- 111.Robbins TW, Everitt BJ. Drug addiction: bad habits add up. Nature. 1999;398:567–570. doi: 10.1038/19208. [DOI] [PubMed] [Google Scholar]
- 112.Frankle WG, Lombardo I, New AS, et al. Brain serotonin transporter distribution in subjects with impulsive aggressivity: a positron emission study with [11C]McN 5652. Am J Psychiatry. 2005;162:915–923. doi: 10.1176/appi.ajp.162.5.915. [DOI] [PubMed] [Google Scholar]
- 113.Hoglund E, Korzan WJ, Watt MJ, et al. Effects of L-DOPA on aggressive behavior and central monoaminergic activity in the lizard Anolis carolinensis, using a new method for drug delivery. Behav Brain Res. 2005;156:53–64. doi: 10.1016/j.bbr.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 114.van der Vegt BJ, Lieuwes N, Cremers TI, de Boer SF, Koolhaas JM. Cerebrospinal fluid monoamine and metabolite concentrations and aggression in rats. Hormones & Behavior. 2003;44:199–208. doi: 10.1016/s0018-506x(03)00132-6. [DOI] [PubMed] [Google Scholar]
- 115.Ferrari PF, van Erp AM, Tornatzky W, Miczek KA. Accumbal dopamine and serotonin in anticipation of the next aggressive episode in rats. Eur J Neurosci. 2003;17:371–378. doi: 10.1046/j.1460-9568.2003.02447.x. [DOI] [PubMed] [Google Scholar]
- 116.Higley JD, Mehlman PT, Poland RE, et al. CSF testosterone and 5-HIAA correlate with different types of aggressive behaviors. Biol Psychiatry. 1996;40:1067–1082. doi: 10.1016/S0006-3223(95)00675-3. [DOI] [PubMed] [Google Scholar]
- 117.Mehlman PT, Higley JD, Faucher I, et al. Low Csf 5-Hiaa concentrations and severe aggression and impaired impulse control in nonhuman-primates. Am J Psychiatry. 1994;151:1485–1491. doi: 10.1176/ajp.151.10.1485. [DOI] [PubMed] [Google Scholar]
- 118.Teicher MH, Andersen SL. Limbic serotonin turnover plunges during puberty. Paper presented at: Meeting of the Society for Neuroscience; Miami Beach, Fla. 1999. [Google Scholar]
- 119.Dillon KA, Gross-Isseroff R, Israeli M, Biegon A. Autoradiographic analysis of serotonin 5-HT1A receptor binding in the human brain postmortem: Effects of age and alcohol. Brain Res. 1991;554:56–64. doi: 10.1016/0006-8993(91)90171-q. [DOI] [PubMed] [Google Scholar]
- 120.Lambe EK, Krimer LS, Goldman-Rakic PS. Differential postnatal development of catecholamine and serotonin inputs to identified neurons in prefrontal cortex of rhesus monkey. J Neurosci. 2000;20:8780–8787. doi: 10.1523/JNEUROSCI.20-23-08780.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Goldman-Rakic PS, Brown RM. Postnatal development of monoamine content and synthesis in the cerebral cortex of rhesus monkeys. Brain Res. 1982;256:339–349. doi: 10.1016/0165-3806(82)90146-8. [DOI] [PubMed] [Google Scholar]
- 122.Dinopoulos A, Dori I, Parnavelas JG. The serotonin innervation of the basal forebrain shows a transient phase during development. Brain Research: Developmental Brain Research. 1997;99:38–52. doi: 10.1016/s0165-3806(96)00198-8. [DOI] [PubMed] [Google Scholar]
- 123.Kalivas PW, Churchill L, Romanides A. Involvement of the pallidalthalamocortical circuit in adaptive behavior. Ann NY Acad Sci. 1999;877:64–70. doi: 10.1111/j.1749-6632.1999.tb09261.x. [DOI] [PubMed] [Google Scholar]
- 124.Jentsch JD, Roth RH, Taylor JR. Role for dopamine in the behavioral functions of the prefrontal corticostriatal system: implications for mental disorders and psychotropic drug action. Prog Brain Res. 2000;126:433–453. doi: 10.1016/S0079-6123(00)26028-7. [DOI] [PubMed] [Google Scholar]
- 125.Swanson LW. Cerebral hemisphere regulation of motivated behavior. Brain Res. 2000;886:113–164. doi: 10.1016/s0006-8993(00)02905-x. [DOI] [PubMed] [Google Scholar]
- 126.Schultz W, Tremblay L, Hollerman JR. Reward processing in primate orbitofrontal cortex and basal ganglia. Cereb Cortex. 2000;10:272–284. doi: 10.1093/cercor/10.3.272. [DOI] [PubMed] [Google Scholar]
- 127.Finch DM. Neurophysiology of converging synaptic inputs from the rat prefrontal cortex, amygdala, midline thalamus, and hippocampal formation onto single neurons of the caudate/putamen and nucleus accumbens. Hippocampus. 1996;6:495–512. doi: 10.1002/(SICI)1098-1063(1996)6:5<495::AID-HIPO3>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 128.Lavin A, Grace AA. Modulation of dorsal thalamic cell activity by the ventral pallidum: Its role in the regulation of thalamocortical activity by the basal ganglia. Synapse. 1994;18:104–127. doi: 10.1002/syn.890180205. [DOI] [PubMed] [Google Scholar]
- 129.Nestler EJ, Barrot M, Self DW. DeltaFosB: A sustained molecular switch for addiction. Proc Natl Acad Sci USA. 2001;98:11042–11046. doi: 10.1073/pnas.191352698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hyman SE, Malenka RC. Addiction and the brain: the neurobiology of compulsion and its persistence. Nat Rev Neurosci. 2001;2:695–703. doi: 10.1038/35094560. [DOI] [PubMed] [Google Scholar]
- 131.Gurden H, Tassin JP, Jay TM. Integrity of the mesocortical dopaminergic system is necessary for complete expression of in vivo hippocampal-prefrontal cortex long-term potentiation. Neuroscience. 1999;94:1019–1027. doi: 10.1016/s0306-4522(99)00395-4. [DOI] [PubMed] [Google Scholar]
- 132.Robinson TE, Kolb B. Alterations in the morphology of dendrites and dendritic spines in the nucleus accumbens and prefrontal cortex following repeated treatment with amphetamine or cocaine. Eur J Neurosci. 1999;11:1598–1604. doi: 10.1046/j.1460-9568.1999.00576.x. [DOI] [PubMed] [Google Scholar]
- 133.Mulder AB, Arts MP, Lopes da Silva FH. Short- and long-term plasticity of the hippocampus to nucleus accumbens and prefrontal cortex pathways in the rat, in vivo. Eur J Neurosci. 1997;9:1603–1611. doi: 10.1111/j.1460-9568.1997.tb01518.x. [DOI] [PubMed] [Google Scholar]
- 134.Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- 135.Taylor JR, Jentsch JD. Repeated intermittent administration of psychomotor stimulant drugs alters the acquisition of Pavlovian approach behavior in rats: differential effects of cocaine, d-amphetamine and 3,4-methylenedioxymethamphetamine (“ecstasy”) Biol Psychiatry. 2001;50:137–143. doi: 10.1016/s0006-3223(01)01106-4. [DOI] [PubMed] [Google Scholar]
- 136.Ricaurte G, Bryan G, Strauss L, Seiden L, Schuster C. Hallucinogenic amphetamine selectively destroys brain serotonin nerve terminals. Science. 1985;229:986–988. doi: 10.1126/science.4023719. [DOI] [PubMed] [Google Scholar]
- 137.Schmidt CJ. Neurotoxicity of the psychedelic amphetamine, methylene-dioxymethamphetamine. J Pharmacol Exp Ther. 1987;240:1–7. [PubMed] [Google Scholar]
- 138.Knyshevski I, Ricci LA, McCann TE, Melloni RH., Jr Serotonin type-1A receptors modulate adolescent, cocaine-induced offensive aggression in hamsters. Physiol Behav. 2005;85:167–176. doi: 10.1016/j.physbeh.2005.03.020. [DOI] [PubMed] [Google Scholar]
- 139.Hollander E, Rosen J. Impulsivity. J Psychopharmacol. 2000;14:S39–S44. doi: 10.1177/02698811000142S106. [DOI] [PubMed] [Google Scholar]
- 140.Matthews SC, Simmons AN, Arce E, Paulus MP. Dissociation of inhibition from error processing using a parametric inhibitory task during functional magnetic resonance imaging. Neuroreport. 2005;16:755–760. doi: 10.1097/00001756-200505120-00020. [DOI] [PubMed] [Google Scholar]
- 141.Aron AR, Poldrack RA. The cognitive neuroscience of response inhibition: Relevance for genetic research in attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57:1285–1292. doi: 10.1016/j.biopsych.2004.10.026. [DOI] [PubMed] [Google Scholar]
- 142.Harrison BJ, Shaw M, Yucel M, et al. Functional connectivity during Stroop task performance. Neuroimage. 2005;24:181–191. doi: 10.1016/j.neuroimage.2004.08.033. [DOI] [PubMed] [Google Scholar]
- 143.Herrmann MJ, Plichta MM, Ehlis AC, Fallgatter AJ. Optical topography during a Go-NoGo task assessed with multi-channel near-infrared spectroscopy. Behav Brain Res. 2005;160:135–140. doi: 10.1016/j.bbr.2004.11.032. [DOI] [PubMed] [Google Scholar]
- 144.Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- 145.Ernst M, Pine DS, Hardin M. Triadic model of the neurobiology of motivated behavior in adolescence. Psychol Med. 2005;36:299–312. doi: 10.1017/S0033291705005891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kalivas PW. Glutamate systems in cocaine addiction. Curr Opin Pharmacol. 2004;4:23–29. doi: 10.1016/j.coph.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 147.Karreman M, Westerink BH, Moghaddam B. Excitatory amino acid receptors in the ventral tegmental area regulate dopamine release in the ventral striatum. J Neurochem. 1996;67:601–607. doi: 10.1046/j.1471-4159.1996.67020601.x. [DOI] [PubMed] [Google Scholar]
- 148.Tzschentke TM, Schmidt WJ. Glutamatergic mechanisms in addiction. Mol Psychiatry. 2003;8:373–382. doi: 10.1038/sj.mp.4001269. [DOI] [PubMed] [Google Scholar]
- 149.Berlin HA, Rolls ET, Kischka U. Impulsivity, time perception, emotion and reinforcement sensitivity in patients with orbitofrontal cortex lesions. Brain. 2004;127:1108–1126. doi: 10.1093/brain/awh135. [DOI] [PubMed] [Google Scholar]
- 150.Spinella M. Neurobehavioral correlates of impulsivity: Evidence of prefrontal involvement. Int J Neurosci. 2004;114:95–104. doi: 10.1080/00207450490249347. [DOI] [PubMed] [Google Scholar]
- 151.Shamay-Tsoory SG, Tomer R, Berger BD, Goldsher D, Aharon-Peretz J. Impaired “affective theory of mind” is associated with right ventromedial prefrontal damage. Cogn Behav Neurol. 2005;18:55–67. doi: 10.1097/01.wnn.0000152228.90129.99. [DOI] [PubMed] [Google Scholar]
- 152.Max JE, Levin HS, Landis J, et al. Predictors of personality change due to traumatic brain injury in children and adolescents in the first six months after injury. J Am Acad Child Adolesc Psychiatry. 2005;44:434–442. doi: 10.1097/01.chi.0000156280.66240.61. [DOI] [PubMed] [Google Scholar]
- 153.Franklin TR, Acton PD, Maldjian JA, et al. Decreased gray matter concentration in the insular, orbitofrontal, cingulate, and temporal cortices of cocaine patients. Biol Psychiatry. 2002;51:134–142. doi: 10.1016/s0006-3223(01)01269-0. [DOI] [PubMed] [Google Scholar]
- 154.Fuchs RA, Evans KA, Ledford CC, et al. The role of the dorsomedial prefrontal cortex, basolateral amygdala, and dorsal hippocampus in contextual reinstatement of cocaine seeking in rats. Neuropsychopharmacology. 2005;30:296–309. doi: 10.1038/sj.npp.1300579. [DOI] [PubMed] [Google Scholar]
- 155.Kalivas PW, Volkow N, Seamans J. Unmanageable motivation in addiction: A pathology in prefrontal-accumbens glutamate transmission. Neuron. 2005;45:647–650. doi: 10.1016/j.neuron.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 156.Robinson TE, Kolb B. Structural plasticity associated with exposure to drugs of abuse. Neuropharmacology. 2004;47 (Suppl 1):33–46. doi: 10.1016/j.neuropharm.2004.06.025. [DOI] [PubMed] [Google Scholar]
- 157.Bjork JM, Knutson B, Fong GW, Caggiano DM, Bennett SM, Hommer DW. Incentive-elicited brain activation in adolescents: similarities and differences from young adults. J Neurosci. 2004;24:1793–1802. doi: 10.1523/JNEUROSCI.4862-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Ernst M, Nelson EE, Jazbec S, et al. Amygdala and nucleus accumbens in responses to receipt and omission of gains in adults and adolescents. Neuroimage. 2005;25:1279–1291. doi: 10.1016/j.neuroimage.2004.12.038. [DOI] [PubMed] [Google Scholar]
- 159.Tarazi FI, Tomasini EC, Baldessarini RJ. Postnatal development of dopamine and serotonin transporters in rat caudate-putamen and nucleus accumbens septi. Neurosci Lett. 1998;254:21–24. doi: 10.1016/s0304-3940(98)00644-2. [DOI] [PubMed] [Google Scholar]
- 160.Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Research: Brain Research Reviews. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- 161.Heusner CL, Palmiter RD. Expression of mutant NMDA receptors in dopamine D1 receptor-containing cells prevents cocaine sensitization and decreases cocaine preference. J Neurosci. 2005;25:6651–6657. doi: 10.1523/JNEUROSCI.1474-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Di Canio P, Everitt BJ. Contribution of the ventral tegmental area to cocaine-seeking maintained by a drug conditioned stimulus in rats. Eur J Neurosci. 2004;19:1661–1667. doi: 10.1111/j.1460-9568.2004.03232.x. [DOI] [PubMed] [Google Scholar]
- 163.Steinberg LD. Adolescence. 7. New York: McGraw-Hill; 2005. [Google Scholar]
- 164.Primus RJ, Kellogg CK. Pubertal-related changes influence the development of environment-related social-interaction in the male rat. Dev Psychobiol. 1989;22:633–643. doi: 10.1002/dev.420220608. [DOI] [PubMed] [Google Scholar]
- 165.Weisfeld GE, Berger JM. Some features of human adolescence viewed in evolutionary perspective. Human Development. 1983;26:121–133. [Google Scholar]
- 166.Kraemer HC, Horvat JR, Doering C, McGinnis PR. Male chimpanzee development focusing on adolescence: integration of behavioral with physiological changes. Primates. 1982;23:393–405. [Google Scholar]
- 167.Spear LP. Adolescent brain development and animal models. Ann NY Acad Sci. 2004;1021:23–26. doi: 10.1196/annals.1308.002. [DOI] [PubMed] [Google Scholar]
- 168.Steinberg L. Reciprocal relation between parent child distance and pubertal maturation. Dev Psychol. 1988;24:122–128. [Google Scholar]
- 169.Larson R, Richards MH. Daily companionship in late childhood and early adolescence—changing developmental contexts. Child Dev. 1991;62:284–300. doi: 10.1111/j.1467-8624.1991.tb01531.x. [DOI] [PubMed] [Google Scholar]
- 170.McGue M, Elkins I, Walden B, Iacono WG. Perceptions of the parent-adolescent relationship: a longitudinal investigation. Dev Psychol. 2005;41:971–984. doi: 10.1037/0012-1649.41.6.971. [DOI] [PubMed] [Google Scholar]
- 171.Arnett J. Reckless behavior in adolescence: a developmental perspective. Developmental Review. 1992;12:339–373. [Google Scholar]
- 172.Moffitt TE. Adolescence-limited and life-course-persistent antisocial behavior: a developmental taxonomy. Psychol Rev. 1993;100:674–701. [PubMed] [Google Scholar]
- 173.Zahn-Waxler C, Klimes-Dougan B, Slattery MJ. Internalizing problems of childhood and adolescence: prospects, pitfalls, and progress in understanding the development of anxiety and depression. Dev Psychopathol. 2000;12:443–466. [PubMed] [Google Scholar]
- 174.Donovan JE, Leech SL, Zucker RA, et al. Really underage drinkers: alcohol use among elementary students. Alcohol Clin Exp Res. 2004;28:341–349. doi: 10.1097/01.alc.0000113922.77569.4e. [DOI] [PubMed] [Google Scholar]
- 175.Caspi A, Moffitt TE, Newman DL, Silva PA. Behavioral observations at age three years predict adult psychiatric disorders. Longitudinal evidence from a birth cohort. Arch Gen Psychiatry. 1996;53:1033–1039. doi: 10.1001/archpsyc.1996.01830110071009. [DOI] [PubMed] [Google Scholar]
- 176.Clark DB, Cornelius JR, Kirisci L, Tarter RE. Childhood risk categories for adolescent substance involvement: a general liability typology. Drug Alcohol Depend. 2005;77:13–21. doi: 10.1016/j.drugalcdep.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 177.Clark DB. The natural history of adolescent alcohol use disorders. Addiction. 2004;99(Suppl 2):5–22. doi: 10.1111/j.1360-0443.2004.00851.x. [DOI] [PubMed] [Google Scholar]
- 178.Simkin DR. Adolescent substance use disorders and comorbidity. Pediatr Clin North Am. 2002;49:463–477. doi: 10.1016/s0031-3955(01)00014-1. [DOI] [PubMed] [Google Scholar]
- 179.Shoal GD, Giancola PR. Executive cognitive functioning, negative affectivity, and drug use in adolescent boys with and without a family history of a substance use disorder. J Child Adolesc Subst. 2001;10:111–121. [Google Scholar]
- 180.Nigg JT, Glass JM, Wong MM, et al. Neuropsychological executive functioning in children at elevated risk for alcoholism: Findings in early adolescence. J Abnorm Psychol. 2004;113:302–314. doi: 10.1037/0021-843X.113.2.302. [DOI] [PubMed] [Google Scholar]
- 181.Giancola PR, Mezzich AC. Executive functioning, temperament, and drug use involvement in adolescent females with a substance use disorder. J Child Psychol Psychiatry. 2003;44:857–866. doi: 10.1111/1469-7610.00170. [DOI] [PubMed] [Google Scholar]
- 182.Overman WH, Frassrand K, Ansel S, Trawalter S, Bies B, Redmond A. Performance on the IOWA card task by adolescents and adults. Neuropsychologia. 2004;42:1838–1851. doi: 10.1016/j.neuropsychologia.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 183.Gardner M, Steinberg L. Peer influence on risk taking, risk preference, and risky decision making in adolescence and adulthood: an experimental study. Dev Psychol. 2005;41:625–635. doi: 10.1037/0012-1649.41.4.625. [DOI] [PubMed] [Google Scholar]
- 184.Neighbors BD, Clark DB, Donovan JE, Brody GH. Difficult temperament, parental relationships, and adolescent alcohol use disorder symptoms. J Child Adoles Subst. 2000;10:69–86. [Google Scholar]
- 185.Giancola PR, Parker AM. A six-year prospective study of pathways toward drug use in adolescent boys with and without a family history of a substance use disorder. J Stud Alcohol. 2001;62:166–178. doi: 10.15288/jsa.2001.62.166. [DOI] [PubMed] [Google Scholar]
- 186.Neighbors B, Kempton T, Forehand R. Co-occurrence of substance abuse with conduct, anxiety, and depression disorders in juvenile delinquents. Addict Behav. 1992;17:379–386. doi: 10.1016/0306-4603(92)90043-u. [DOI] [PubMed] [Google Scholar]
- 187.Turner AP, Larimer ME, Sarason IG, Trupin EW. Identifying a negative mood subtype in incarcerated adolescents: relationship to substance use. Addict Behav. 2005;30:1442–1448. doi: 10.1016/j.addbeh.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 188.Torikka A, Kaltiala-Heino R, Rimpela A, Rimpela M, Rantanen P. Depression, drinking, and substance use among 14- to 16-year-old Finnish adolescents. Nord J Psychiatry. 2001;55:351–357. doi: 10.1080/080394801317080864. [DOI] [PubMed] [Google Scholar]
- 189.Galambos NL, Leadbeater BJ, Barker ET. Gender differences in and risk factors for depression in adolescence: a four-year longitudinal study. International Journal of Behavioral Development. 2004;28:16–25. [Google Scholar]
- 190.Silberg J, Rutter M, D’Onofrio B, Eaves L. Genetic and environmental risk factors in adolescent substance use. J Child Psychol Psychiatry. 2003;44:664–676. doi: 10.1111/1469-7610.00153. [DOI] [PubMed] [Google Scholar]
- 191.Armstrong TD, Costello EJ. Community studies on adolescent substance use, abuse, or dependence and psychiatric comorbidity. J Consult Clin Psychol. 2002;70:1224–1239. doi: 10.1037//0022-006x.70.6.1224. [DOI] [PubMed] [Google Scholar]
- 192.Lewinsohn PM, Rohde P, Klein DN, Seeley JR. Natural course of adolescent major depressive disorder: I. Continuity into young adulthood. J Am Acad Child Adolesc Psychiatry. 1999;38:56–63. doi: 10.1097/00004583-199901000-00020. [DOI] [PubMed] [Google Scholar]
- 193.Rao U, Ryan ND, Dahl RE, et al. Factors associated with the development of substance use disorder in depressed adolescents. J Am Acad Child Adolesc Psychiatry. 1999;38:1109–1117. doi: 10.1097/00004583-199909000-00014. [DOI] [PubMed] [Google Scholar]
- 194.Lopez B, Turner RJ, Saavedra LM. Anxiety and risk for substance dependence among late adolescents/young adults. J Anxiety Disord. 2005;19:275–294. doi: 10.1016/j.janxdis.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 195.Hallfors DD, Waller MW, Bauer D, Ford CA, Halpern CT. Which comes first in adolescence—sex and drugs or depression? Am J Prev Med. 2005;29:163–170. doi: 10.1016/j.amepre.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 196.Rao U. Links between depression and substance abuse in adolescents: neurobiological mechanisms. Am J Prev Med. 2006;31:161–174. doi: 10.1016/j.amepre.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 197.Cadoret RJ, Yates WR, Troughton E, Woodworth G, Stewart MA. Adoption study demonstrating two genetic pathways to drug abuse. Arch Gen Psychiatry. 1995;52:42–52. doi: 10.1001/archpsyc.1995.03950130042005. [DOI] [PubMed] [Google Scholar]
- 198.Rose RJ, Dick DM, Viken RJ, Pulkkinen L, Kaprio J. Genetic and environmental effects on conduct disorder and alcohol dependence symptoms and their covariation at age 14. Alcohol Clin Exp Res. 2004;28:1541–1548. doi: 10.1097/01.alc.0000141822.36776.55. [DOI] [PubMed] [Google Scholar]
- 199.Caspi A, Moffitt TE, Newman DL, Silva PA. Behavioral observations at age three years predict adult psychiatric disorders. Longitudinal evidence from a birth cohort. Arch Gen Psychiatry. 1996;53:1033–1039. doi: 10.1001/archpsyc.1996.01830110071009. [DOI] [PubMed] [Google Scholar]
- 200.Robins LN. The intimate connection between antisocial personality and substance abuse. Soc Psychiatry Psychiatr Epidemiol. 1998;33:393–399. doi: 10.1007/s001270050071. [DOI] [PubMed] [Google Scholar]
- 201.McGue M, Iacono WG. The association of early adolescent problem behavior with adult psychopathology. Am J Psychiatry. 2005;162:1118–1124. doi: 10.1176/appi.ajp.162.6.1118. [DOI] [PubMed] [Google Scholar]
- 202.Brady KT, Verduin ML. Pharmacotherapy of comorbid mood, anxiety, and substance use disorders. Subst Use Misuse. 2005;40:2021–2041. 2043–2048. doi: 10.1080/10826080500294924. [DOI] [PubMed] [Google Scholar]
- 203.Krystal JH, D’Souza DC, Sanacora G, Goddard AW, Charney DS. Current perspectives on the pathophysiology of schizophrenia, depression, and anxiety disorders. Med Clin North Am. 2001;85:559–577. doi: 10.1016/s0025-7125(05)70329-1. [DOI] [PubMed] [Google Scholar]
- 204.Zalsman G, Oquendo MA, Greenhill L, et al. Neurobiology of depression in children and adolescents. Child Adolesc Psychiatr Clin N Am. 2006;15:vii–viii. 843–868. doi: 10.1016/j.chc.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 205.Lyoo IK, Lee HK, Jung JH, Noam GG, Renshaw PF. White matter hyperintensities on magnetic resonance imaging of the brain in children with psychiatric disorders. Compr Psychiatry. 2002;43:361–368. doi: 10.1053/comp.2002.34636. [DOI] [PubMed] [Google Scholar]
- 206.Deas D. Adolescent substance abuse and psychiatric comorbidities. J Clin Psychiatry. 2006;67 (Suppl 7):18–23. [PubMed] [Google Scholar]
- 207.Waxmonsky JG, Wilens TE. Pharmacotherapy of adolescent substance use disorders: a review of the literature. J Child Adolesc Psychopharmacol. 2005;15:810–825. doi: 10.1089/cap.2005.15.810. [DOI] [PubMed] [Google Scholar]
- 208.Wilens TE, Biederman J, Milberger S, et al. Is bipolar disorder a risk for cigarette smoking in ADHD youth? Am J Addict. 2000;9:187–195. doi: 10.1080/10550490050148017. [DOI] [PubMed] [Google Scholar]
- 209.Wilens TE, Faraone SV, Biederman J, Gunawardene S. Does stimulant therapy of attention-deficit/hyperactivity disorder beget later substance abuse? A meta-analytic review of the literature. Pediatrics. 2003;111:179–185. doi: 10.1542/peds.111.1.179. [DOI] [PubMed] [Google Scholar]
- 210.Akers JF, Jones RM, Coyl DD. Adolescent friendship pairs: Similarities in identity status development behaviors, attitudes, and intentions. J Adolesc Res. 1998;13:178–201. [Google Scholar]
- 211.Poulin F, Boivin M. The role of proactive and reactive aggression in the formation and development of boys’ friendships. Dev Psychol. 2000;36:233–240. doi: 10.1037//0012-1649.36.2.233. [DOI] [PubMed] [Google Scholar]
- 212.Fergusson DM, Swain-Campbell NR, Horwood LJ. Deviant peer affiliations, crime and substance use: a fixed effects regression analysis. J Abnorm Child Psychol. 2002;30:419–430. doi: 10.1023/a:1015774125952. [DOI] [PubMed] [Google Scholar]
- 213.Li F, Barrera M, Jr, Hops H, Fisher KJ. The longitudinal influence of peers on the development of alcohol use in late adolescence: a growth mixture analysis. J Behav Med. 2002;25:293–315. doi: 10.1023/a:1015336929122. [DOI] [PubMed] [Google Scholar]
- 214.Barnes GM, Welte JW, Hoffman JH, Dintcheff BA. Shared predictors of youthful gambling, substance use, and delinquency. Psychol Addict Behav. 2005;19:165–174. doi: 10.1037/0893-164X.19.2.165. [DOI] [PubMed] [Google Scholar]
- 215.Moss HB, Bonicatto S, Kirisci L, Girardelli AM, Murrelle L. Substance abuse and associated psychosocial problems among Argentina adolescents: sex heterogeneity and familial transmission. Drug Alcohol Depend. 1998;52:221–230. doi: 10.1016/s0376-8716(98)00099-4. [DOI] [PubMed] [Google Scholar]
- 216.Stormshak EA, Comeau CA, Shepard SA. The relative contribution of sibling deviance and peer deviance in the prediction of substance use across middle childhood. J Abnorm Child Psychol. 2004;32:635–649. doi: 10.1023/b:jacp.0000047212.49463.c7. [DOI] [PubMed] [Google Scholar]
- 217.Ary DV, Duncan TE, Biglan A, Metzler CW, Noell JW, Smolkowski K. Development of adolescent problem behavior. J Abnorm Child Psychol. 1999;27:141–150. doi: 10.1023/a:1021963531607. [DOI] [PubMed] [Google Scholar]
- 218.Ary DV, Duncan TE, Duncan SC, Hops H. Adolescent problem behavior: the influence of parents and peers. Behav Res Ther. 1999;37:217–230. doi: 10.1016/s0005-7967(98)00133-8. [DOI] [PubMed] [Google Scholar]
- 219.Moss HB, Lynch KG, Hardie TL. Affiliation with deviant peers among children of substance dependent fathers from pre-adolescence into adolescence: associations with problem behaviors. Drug Alcohol Depend. 2003;71:117–125. doi: 10.1016/s0376-8716(03)00073-5. [DOI] [PubMed] [Google Scholar]
- 220.Nurco DN, Blatchley RJ, Hanlon TE, O’Grady KE. Early deviance and related risk factors in the children of narcotic addicts. Am J Drug Alcohol Abuse. 1999;25:25–45. doi: 10.1081/ada-100101844. [DOI] [PubMed] [Google Scholar]
- 221.Tarter RE. Etiology of adolescent substance abuse: a developmental perspective. Am J Addict. 2002;11:171–191. doi: 10.1080/10550490290087965. [DOI] [PubMed] [Google Scholar]
- 222.Tarter RE, Kirisci L, Mezzich A, et al. Neurobehavioral disinhibition in childhood predicts early age at onset of substance use disorder. Am J Psychiatry. 2003;160:1078–1085. doi: 10.1176/appi.ajp.160.6.1078. [DOI] [PubMed] [Google Scholar]
- 223.Kirisci L, Tarter RE, Vanyukov M, Reynolds M, Habeych M. Relation between cognitive distortions and neurobehavior disinhibition on the development of substance use during adolescence and substance use disorder by young adulthood: a prospective study. Drug Alcohol Depend. 2004;76:125–133. doi: 10.1016/j.drugalcdep.2004.04.015. [DOI] [PubMed] [Google Scholar]
- 224.Tarter RE, Kirisci L, Habeych M, Reynolds M, Vanyukov M. Neurobehavior disinhibition in childhood predisposes boys to substance use disorder by young adulthood: direct and mediated etiologic pathways. Drug Alcohol Depend. 2004;73:121–132. doi: 10.1016/j.drugalcdep.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 225.Cornelius JR, Bukstein OG, Birmaher B, et al. Fluoxetine in adolescents with major depression and an alcohol use disorder: an open-label trial. Addict Behav. 2001;26:735–739. doi: 10.1016/s0306-4603(00)00152-0. [DOI] [PubMed] [Google Scholar]
- 226.Cornelius JR, Bukstein OG, Salloum IM, Kelly TM, Wood DS, Clark DB. Fluoxetine in depressed AUD adolescents: a one-year follow-up evaluation. J Child Adolesc Psychopharmacol. 2004;14:33–38. doi: 10.1089/104454604773840463. [DOI] [PubMed] [Google Scholar]
- 227.Riggs PD, Mikulich SK, Coffman LM, Crowley TJ. Fluoxetine in drug-dependent delinquents with major depression: an open trial. J Child Adolesc Psychopharmacol. 1997;7:87–95. doi: 10.1089/cap.1997.7.87. [DOI] [PubMed] [Google Scholar]
- 228.Donovan SJ, Susser ES, Nunes EV. Divalproex sodium for use with conduct-disordered adolescent marijuana users. Am J Addict. 1996;5:181–181. [Google Scholar]
- 229.Riggs PD, Leon SL, Mikulich SK, Pottle LC. An open trial of bupropion for ADHD in adolescents with substance use disorders and conduct disorder. J Am Acad Child Adolesc Psychiatry. 1998;37:1271–1278. doi: 10.1097/00004583-199812000-00010. [DOI] [PubMed] [Google Scholar]
- 230.Waldron HB, Kaminer Y. On the learning curve: the emerging evidence supporting cognitive-behavioral therapies for adolescent substance abuse. Addiction. 2004;99:93–105. doi: 10.1111/j.1360-0443.2004.00857.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Tevyaw TO, Monti PM. Motivational enhancement and other brief interventions for adolescent substance abuse: foundations, applications and evaluations. Addiction. 2004;99:63–75. doi: 10.1111/j.1360-0443.2004.00855.x. [DOI] [PubMed] [Google Scholar]
- 232.Liddle HA. Family-based therapies for adolescent alcohol and drug use: Research contributions and future research needs. Addiction. 2004;99:76–92. doi: 10.1111/j.1360-0443.2004.00856.x. [DOI] [PubMed] [Google Scholar]
- 233.Dishion TJ, McCord J, Poulin F. When interventions harm. Peer groups and problem behavior. Am Psychol. 1999;54:755–764. doi: 10.1037//0003-066x.54.9.755. [DOI] [PubMed] [Google Scholar]
- 234.Biglan A, Brennan PA, Foster SL, Holder HD. Helping Adolescents at Risk: Prevention of Multiple Problem Behaviors. New York: Guilford Press; 2004. [Google Scholar]
- 235.Frodl-Bauch T, Bottlender R, Hegerl U. Neurochemical substrates and neuroanatomical generators of the event-related P300. Neuropsychobiology. 1999;40:86–94. doi: 10.1159/000026603. [DOI] [PubMed] [Google Scholar]
- 236.Polich J, Herbst KL. P300 as a clinical assay: rationale, evaluation, and findings. Int J Psychophysiol. 2000;38:3–19. doi: 10.1016/s0167-8760(00)00127-6. [DOI] [PubMed] [Google Scholar]
- 237.Soltani M, Knight RT. Neural origins of the P300. Crit Rev Neurobiol. 2000;14:199–224. [PubMed] [Google Scholar]
- 238.Begleiter H, Porjesz B, Bihari B, Kissin B. Event-related brain potentials in boys at risk for alcoholism. Science. 1984;225:1493–1496. doi: 10.1126/science.6474187. [DOI] [PubMed] [Google Scholar]
- 239.Iacono WG, Carlson SR, Malone SM. Identifying a multivariate endophenotype for substance use disorders using psychophysiological measures. Int J Psychophysiol. 2000;38:81–96. doi: 10.1016/s0167-8760(00)00132-x. [DOI] [PubMed] [Google Scholar]
- 240.Anokhin AP, Heath AC, Myers E. Genetics, prefrontal cortex, and cognitive control: a twin study of event-related brain potentials in a response inhibition task. Neurosci Lett. 2004;368:314–318. doi: 10.1016/j.neulet.2004.07.036. [DOI] [PubMed] [Google Scholar]
- 241.Almasy L, Porjesz B, Blangero J, et al. Heritability of event-related brain potentials in families with a history of alcoholism. Am J Med Genet. 1999;88:383–390. [PubMed] [Google Scholar]
- 242.O’Connor S, Morzorati S, Christian JC, Li TK. Heritable features of the auditory oddball event-related potential: peaks, latencies, morphology and topography. Electroencephalography and Clinical Neurophysiology. 1994;92:115–125. doi: 10.1016/0168-5597(94)90052-3. [DOI] [PubMed] [Google Scholar]
- 243.Carlson SR, Iacono WG, McGue M. P300 amplitude in nonalcoholic adolescent twin pairs who become discordant for alcoholism as adults. Psychophysiology. 2004;41:841–844. doi: 10.1111/j.1469-8986.2004.00238.x. [DOI] [PubMed] [Google Scholar]
- 244.Iacono WG, Carlson SR, Malone SM, McGue M. P3 event-related potential amplitude and the risk for disinhibitory disorders in adolescent boys. Arch Gen Psychiatry. 2002;59:750–757. doi: 10.1001/archpsyc.59.8.750. [DOI] [PubMed] [Google Scholar]
- 245.Bauer LO, Hesselbrock VM. P300 decrements in teenagers with conduct problems: implications for substance abuse risk and brain development. Biol Psychiatry. 1999;46:263–272. doi: 10.1016/s0006-3223(98)00335-7. [DOI] [PubMed] [Google Scholar]
- 246.Costa L, Bauer L, Kuperman S, et al. Frontal P300 decrements, alcohol dependence, and antisocial personality disorder. Biol Psychiatry. 2000;47:1064–1071. doi: 10.1016/s0006-3223(99)00317-0. [DOI] [PubMed] [Google Scholar]
- 247.Habeych ME, Charles PJ, Sclabassi RJ, Kirisci L, Tarter RE. Direct and mediated associations between P300 amplitude in childhood and substance use disorders outcome in young adulthood. Biol Psychiatry. 2005;57:76–82. doi: 10.1016/j.biopsych.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 248.Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- 249.Sangal RB, Sangal JM. Attention-deficit/hyperactivity disorder: use of cognitive evoked potential (P300) to predict treatment response. Clin Neurophysiol. 2006;117:1996–2006. doi: 10.1016/j.clinph.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 250.Ernst M, Grant SJ, London ED, Contoreggi CS, Kimes AS, Spurgeon L. Decision making in adolescents with behavior disorders and adults with substance abuse. Am J Psychiatry. 2003;160:33–40. doi: 10.1176/appi.ajp.160.1.33. [DOI] [PubMed] [Google Scholar]
- 251.Schutter DJ, de Haan EH, van Honk J. Anterior asymetrical alpha activity predicts Iowa gambling performance: Distinctly but reversed. Neuropsychologia. 2004;42:939–943. doi: 10.1016/j.neuropsychologia.2003.11.014. [DOI] [PubMed] [Google Scholar]
- 252.Levesque J, Joanette Y, Mensour B, et al. Neural basis of emotional self-regulation in childhood. Neuroscience. 2004;129:361–369. doi: 10.1016/j.neuroscience.2004.07.032. [DOI] [PubMed] [Google Scholar]
- 253.Levesque J, Eugene F, Joanette Y, et al. Neural circuitry underlying voluntary suppression of sadness. Biol Psychiatry. 2003;53:502–510. doi: 10.1016/s0006-3223(02)01817-6. [DOI] [PubMed] [Google Scholar]
- 254.Ochsner KN, Bunge SA, Gross JJ, Gabrieli JD. Rethinking feelings: An FMRI study of the cognitive regulation of emotion. J Cogn Neurosci. 2002;14:1215–1229. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- 255.Schweinsburg AD, Paulus MP, Barlett VC, et al. An FMRI study of response inhibition in youths with a family history of alcoholism. Ann NY Acad Sci. 2004;1021:391–394. doi: 10.1196/annals.1308.050. [DOI] [PubMed] [Google Scholar]
- 256.Twitchell GR, Hanna GL, Cook EH, Fitzgerald HE, Little KY, Zucker RA. Overt behavior problems and serotonergic function in middle childhood among male and female offspring of alcoholic fathers. Alcohol Clin Exp Res. 1998;22:1340–1348. [PubMed] [Google Scholar]
- 257.Twitchell GR, Hanna GL, Cook EH, Fitzgerald HE, Zucker RA. Serotonergic function, behavioral disinhibition, and negative affect in children of alcoholics: the moderating effects of puberty. Alcohol Clin Exp Res. 2000;24:972–979. [PubMed] [Google Scholar]
- 258.Askenazy F, Caci H, Myquel M, Darcourt G, Lecrubier Y. Relationship between impulsivity and platelet serotonin content in adolescents. Psychiatry Res. 2000;94:19–28. doi: 10.1016/s0165-1781(00)00124-4. [DOI] [PubMed] [Google Scholar]
- 259.Mezzich AC, Tarter RE, Moss HB, Yao JK, Hsieh YC, Kirisci L. Platelet monoamine-oxidase activity and temperament and personality in adolescent female substance-abusers. Personality and Individual Differences. 1994;16:417–424. [Google Scholar]
- 260.Twitchell GR, Hanna GL, Cook EH, Stoltenberg SF, Fitzgerald HE, Zucker RA. Serotonin transporter promoter polymorphism genotype is associated with behavioral disinhibition and negative affect in children of alcoholics. Alcohol Clin Exp Res. 2001;25:953–959. [PubMed] [Google Scholar]
- 261.Nilsson KW, Sjoberg RL, Damberg M, et al. Role of the serotonin transporter gene and family function in adolescent alcohol consumption. Alcohol Clin Exp Res. 2005;29:564–570. doi: 10.1097/01.alc.0000159112.98941.b0. [DOI] [PubMed] [Google Scholar]
- 262.Berman ME, Tracy JI, Coccaro EF. The serotonin hypothesis of aggression revisited. Clin Psychol Rev. 1997;17:651–665. doi: 10.1016/s0272-7358(97)00039-1. [DOI] [PubMed] [Google Scholar]
- 263.Winstanley CA, Theobald DE, Dalley JW, Glennon JC, Robbins TW. 5-HT2A and 5-HT2C receptor antagonists have opposing effects on a measure of impulsivity: interactions with global 5-HT depletion. Psychopharmacology. 2004;176:376–385. doi: 10.1007/s00213-004-1884-9. [DOI] [PubMed] [Google Scholar]
- 264.Winstanley CA, Dalley JW, Theobald DE, Robbins TW. Fractionating impulsivity: contrasting effects of central 5-HT depletion on different measures of impulsive behavior. Neuropsychopharmacology. 2004;29:1331–1343. doi: 10.1038/sj.npp.1300434. [DOI] [PubMed] [Google Scholar]
- 265.Oreland L. Platelet monoamine oxidase, personality and alcoholism: the rise, fall and resurrection. Neurotoxicology. 2004;25:79–89. doi: 10.1016/S0161-813X(03)00115-3. [DOI] [PubMed] [Google Scholar]
- 266.Oreland L, Hallman J, Damberg M. Platelet MAO and personality—function and dysfunction. Curr Med Chem. 2004;11:2007–2016. doi: 10.2174/0929867043364838. [DOI] [PubMed] [Google Scholar]
- 267.Naranjo CA, Knoke DM. The role of selective serotonin reuptake inhibitors in reducing alcohol consumption. J Clin Psychiatry. 2001;62 (Suppl 20):18–25. [PubMed] [Google Scholar]
- 268.Conner BT, Noble EP, Berman SM, et al. DRD2 genotypes and substance use in adolescent children of alcoholics. Drug Alcohol Depend. 2005;79:379–387. doi: 10.1016/j.drugalcdep.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 269.Thompson J, Thomas N, Singleton A, et al. D2 dopamine receptor gene (DRD2) TaqI polymorphism: reduced dopamine D2 receptor binding in the human striatum associated with the A1 allele. Pharmacogenetics. 1997;7:479–484. doi: 10.1097/00008571-199712000-00006. [DOI] [PubMed] [Google Scholar]
- 270.Jonsson EG, Nothen MM, Grunhage F, et al. Polymorphisms in the dopamine D2 receptor gene and their relationships to striatal dopamine receptor density of healthy volunteers. Mol Psychiatry. 1999;4:290–296. doi: 10.1038/sj.mp.4000532. [DOI] [PubMed] [Google Scholar]
- 271.Pohjalainen T, Rinne JO, Nagren K, et al. The A1 allele of the human D2 dopamine receptor gene predicts low D2 receptor availability in healthy volunteers. Mol Psychiatry. 1998;3:256–260. doi: 10.1038/sj.mp.4000350. [DOI] [PubMed] [Google Scholar]
- 272.Barrot M, Marinelli M, Abrous DN, Rouge-Pont F, Le Moal M, Piazza PV. The dopaminergic hyper-responsiveness of the shell of the nucleus accumbens is hormone-dependent. Eur J Neurosci. 2000;12:973–979. doi: 10.1046/j.1460-9568.2000.00996.x. [DOI] [PubMed] [Google Scholar]
- 273.Marinelli M, Aouizerate B, Barrot M, Le Moal M, Piazza PV. Dopamine-dependent responses to morphine depend on glucocorticoid receptors. Proc Natl Acad Sci USA. 1998;95:7742–7747. doi: 10.1073/pnas.95.13.7742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Saal D, Dong Y, Bonci A, Malenka RC. Drugs of abuse and stress trigger a common synaptic adaptation in dopamine neurons. Neuron. 2003;37:577–582. doi: 10.1016/s0896-6273(03)00021-7. [DOI] [PubMed] [Google Scholar]
- 275.Moss HB, Vanyukov MM, Martin CS. Salivary cortisol responses and the risk for substance abuse in prepubertal boys. Biol Psychiatry. 1995;38:547–555. doi: 10.1016/0006-3223(94)00382-D. [DOI] [PubMed] [Google Scholar]
- 276.Pajer K, Gardner W, Kirillova GP, Vanyukov MM. Sex differences in cortisol level and neurobehavioral disinhibition in children of substance abusers. J Child Adoles Subst. 2001;10:65–76. [Google Scholar]
- 277.Dawes MA, Dorn LD, Moss HB, et al. Hormonal and behavioral homeostasis in boys at risk for substance abuse. Drug Alcohol Depend. 1999;55:165–176. doi: 10.1016/s0376-8716(99)00003-4. [DOI] [PubMed] [Google Scholar]
- 278.Rao U, Hammen C, Poland RE. Relationships among depression, cigarette smoking and HPA activity in adolescents. Annual Meeting of the International Society for Research in Child and Adolescent Psychopathology; Sydney, Australia. 2003. [Google Scholar]
- 279.Cadoret RJ, Yates WR, Troughton E, Woodworth G, Stewart MA. Adoption study demonstrating two genetic pathways to drug abuse. Arch Gen Psychiatry. 1995;52:42–52. doi: 10.1001/archpsyc.1995.03950130042005. [DOI] [PubMed] [Google Scholar]
- 280.Rao U. Links between depression and substance abuse in adolescents: neurobiological mechanisms. Am J Prev Med. 2006;31(6 Suppl 1):S161–74. doi: 10.1016/j.amepre.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 281.Kagan J, Reznick JS, Snidman N. Biological bases of childhood shyness. Science. 1988;240:167–171. doi: 10.1126/science.3353713. [DOI] [PubMed] [Google Scholar]
- 282.Barden N. Implication of the hypothalamic-pituitary-adrenal axis in the physiopathology of depression. J Psychiatry Neurosci. 2004;29:185–193. [PMC free article] [PubMed] [Google Scholar]
- 283.Reul JM, Stec I, Soder M, Holsboer F. Chronic treatment of rats with the antidepressant amitriptyline attenuates the activity of the hypothalamic-pituitary-adrenocortical system. Endocrinology. 1993;133:312–320. doi: 10.1210/endo.133.1.8391426. [DOI] [PubMed] [Google Scholar]
- 284.Antonijevic IA. Depressive disorders—is it time to endorse different pathophysiologies? Psychoneuroendocrinology. 2006;31:1–15. doi: 10.1016/j.psyneuen.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 285.Adinoff B, Junghanns K, Kiefer F, Krishnan-Sarin S. Suppression of the HPA axis stress-response: implications for relapse. Alcohol Clin Exp Res. 2005;29:1351–1355. doi: 10.1097/01.ALC.0000176356.97620.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Biscaia M, Marin S, Fernandez B, et al. Chronic treatment with CP 55,940 during the peri-adolescent period differentially affects the behavioural responses of male and female rats in adulthood. Psychopharmacology (Berl) 2003;170:301–308. doi: 10.1007/s00213-003-1550-7. [DOI] [PubMed] [Google Scholar]
- 287.O’Shea M, Singh ME, McGregor IS, Mallet PE. Chronic cannabinoid exposure produces lasting memory impairment and increased anxiety in adolescent but not adult rats. J Psychopharmacol. 2004;18:502–508. doi: 10.1177/026988110401800407. [DOI] [PubMed] [Google Scholar]
- 288.Viveros MP, Llorente R, Moreno E, Marco EM. Behavioural and neuroendocrine effects of cannabinoids in critical developmental periods. Behav Pharmacol. 2005;16:353–362. doi: 10.1097/00008877-200509000-00007. [DOI] [PubMed] [Google Scholar]
- 289.White DA, Holtzman SG. Periadolescent morphine exposure alters subsequent behavioral sensitivity to morphine in adult rats. Eur J Pharmacol. 2005;528:119–123. doi: 10.1016/j.ejphar.2005.10.026. [DOI] [PubMed] [Google Scholar]
- 290.Piper BJ, Meyer JS. Memory deficit and reduced anxiety in young adult rats given repeated intermittent MDMA treatment during the periadolescent period. Pharmacol Biochem Behav. 2004;79:723–731. doi: 10.1016/j.pbb.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 291.White AM, Truesdale MC, Bae JG, et al. Differential effects of ethanol on motor coordination in adolescent and adult rats. Pharmacol Biochem Behav. 2002;73:673–677. doi: 10.1016/s0091-3057(02)00860-2. [DOI] [PubMed] [Google Scholar]
- 292.Little PJ, Kuhn CM, Wilson WA, Swartzwelder HS. Differential effects of ethanol in adolescent and adult rats. Alcohol Clin Exp Res. 1996;20:1346–1351. doi: 10.1111/j.1530-0277.1996.tb01133.x. [DOI] [PubMed] [Google Scholar]
- 293.Silveri MM, Spear LP. Decreased sensitivity to the hypnotic effects of ethanol early in ontogeny. Alcohol Clin Exp Res. 1998;22:670–676. doi: 10.1111/j.1530-0277.1998.tb04310.x. [DOI] [PubMed] [Google Scholar]
- 294.Varlinskaya EI, Spear LP. Acute ethanol withdrawal (hangover) and social behavior in adolescent and adult male and female Sprague-Dawley rats. Alcohol Clin Exp Res. 2004;28:40–50. doi: 10.1097/01.ALC.0000108655.51087.DF. [DOI] [PubMed] [Google Scholar]
- 295.Doremus TL, Brunell SC, Varlinskaya EI, Spear LP. Anxiogenic effects during withdrawal from acute ethanol in adolescent and adult rats. Pharmacol Biochem Behav. 2003;75:411–418. doi: 10.1016/s0091-3057(03)00134-5. [DOI] [PubMed] [Google Scholar]
- 296.Brasser SM, Spear NE. Physiological and behavioral effects of acute ethanol hangover in juvenile, adolescent, and adult rats. Behav Neurosci. 2002;116:305–320. doi: 10.1037//0735-7044.116.2.305. [DOI] [PubMed] [Google Scholar]
- 297.Li Q, Wilson WA, Swartzwelder HS. Developmental differences in the sensitivity of spontaneous and miniature IPSCs to ethanol. Alcohol Clin Exp Res. 2006;30:119–126. doi: 10.1111/j.1530-0277.2006.00006.x. [DOI] [PubMed] [Google Scholar]
- 298.Li Q, Wilson WA, Swartzwelder HS. Developmental differences in the sensitivity of hippocampal GABAA receptor-mediated IPSCS to ethanol. Alcohol Clin Exp Res. 2003;27:2017–2022. doi: 10.1097/01.ALC.0000108390.62394.71. [DOI] [PubMed] [Google Scholar]
- 299.Schuckit MA, Smith TL, Beltran I, Waylen A, Horwood J, Davis JM. Performance of a self-report measure of the level of response to alcohol in 12- to 13-year-old adolescents. J Stud Alcohol. 2005;66:452–458. doi: 10.15288/jsa.2005.66.452. [DOI] [PubMed] [Google Scholar]
- 300.Slawecki CJ, Ehlers CL. Enhanced prepulse inhibition following adolescent ethanol exposure in Sprague-Dawley rats. Alcohol Clin Exp Res. 2005;29:1829–1836. doi: 10.1097/01.alc.0000183024.47167.27. [DOI] [PubMed] [Google Scholar]
- 301.Acheson SK, Richardson R, Swartzwelder HS. Developmental changes in seizure susceptibility during ethanol withdrawal. Alcohol. 1999;18:23–26. doi: 10.1016/s0741-8329(98)00063-9. [DOI] [PubMed] [Google Scholar]
- 302.Slawecki CJ, Roth J. Comparison of the onset of hypoactivity and anxiety-like behavior during alcohol withdrawal in adolescent and adult rats. Alcohol Clin Exp Res. 2004;28:598–607. doi: 10.1097/01.alc.0000122767.69206.1b. [DOI] [PubMed] [Google Scholar]
- 303.White AM, Ghia AJ, Levin ED, Swartzwelder HS. Binge pattern ethanol exposure in adolescent and adult rats: differential impact on subsequent responsiveness to ethanol. Alcohol Clin Exp Res. 2000;24:1251–1256. [PubMed] [Google Scholar]
- 304.Siegmund S, Vengeliene V, Singer MV, Spanagel R. Influence of age at drinking onset on long-term ethanol self-administration with deprivation and stress phases. Alcohol Clin Exp Res. 2005;29:1139–1145. doi: 10.1097/01.alc.0000171928.40418.46. [DOI] [PubMed] [Google Scholar]
- 305.Swartzwelder HS, Wilson WA, Tayyeb MI. Age-dependent inhibition of long-term potentiation by ethanol in immature versus mature hippocampus. Alcohol Clin Exp Res. 1995;19:1480–1485. doi: 10.1111/j.1530-0277.1995.tb01011.x. [DOI] [PubMed] [Google Scholar]
- 306.Swartzwelder HS, Wilson WA, Tayyeb MI. Differential sensitivity of NMDA receptor-mediated synaptic potentials to ethanol in immature versus mature hippocampus. Alcohol Clin Exp Res. 1995;19:320–323. doi: 10.1111/j.1530-0277.1995.tb01509.x. [DOI] [PubMed] [Google Scholar]
- 307.Pyapali GK, Turner DA, Wilson WA, Swartzwelder HS. Age and dose-dependent effects of ethanol on the induction of hippocampal long-term potentiation. Alcohol. 1999;19:107–111. doi: 10.1016/s0741-8329(99)00021-x. [DOI] [PubMed] [Google Scholar]
- 308.De Bellis MD, Clark DB, Beers SR, et al. Hippocampal volume in adolescent-onset alcohol use disorders. Am J Psychiatry. 2000;157:737–744. doi: 10.1176/appi.ajp.157.5.737. [DOI] [PubMed] [Google Scholar]
- 309.White AM, Swartzwelder HS. Hippocampal function during adolescence: a unique target of ethanol effects. Ann NY Acad Sci. 2004;1021:206–220. doi: 10.1196/annals.1308.026. [DOI] [PubMed] [Google Scholar]
- 310.Yttri EA, Burk JA, Hunt PS. Intermittent ethanol exposure in adolescent rats: dose-dependent impairments in trace conditioning. Alcohol Clin Exp Res. 2004;28:1433–1436. doi: 10.1097/01.alc.0000147657.51745.a7. [DOI] [PubMed] [Google Scholar]
- 311.Slawecki CJ, Betancourt M, Cole M, Ehlers CL. Periadolescent alcohol exposure has lasting effects on adult neurophysiological function in rats. Brain Research: Developmental Brain Research. 2001;128:63–72. doi: 10.1016/s0165-3806(01)00150-x. [DOI] [PubMed] [Google Scholar]
- 312.Li Q, Wilson WA, Swartzwelder HS. Differential effect of ethanol on NMDA EPSCs in pyramidal cells in the posterior cingulate cortex of juvenile and adult rats. J Neurophysiol. 2002;87:705–711. doi: 10.1152/jn.00433.2001. [DOI] [PubMed] [Google Scholar]
- 313.Crews FT, Braun CJ, Hoplight B, Switzer RC, III, Knapp DJ. Binge ethanol consumption causes differential brain damage in young adolescent rats compared with adult rats. Alcohol Clin Exp Res. 2000;24:1712–1723. [PubMed] [Google Scholar]
- 314.Toni N, Buchs PA, Nikonenko I, Bron CR, Muller D. LTP promotes formation of multiple spine synapses between a single axon terminal and a dendrite. Nature. 1999;402:421–425. doi: 10.1038/46574. [DOI] [PubMed] [Google Scholar]
- 315.Dudek SM, Bear MF. A biochemical correlate of the critical period for synaptic modification in the visual cortex. Science. 1989;246:673–675. doi: 10.1126/science.2573152. [DOI] [PubMed] [Google Scholar]
- 316.Zhang LI, Tao HW, Poo M. Visual input induces long-term potentiation of developing retinotectal synapses. Nat Neurosci. 2000;3:708–715. doi: 10.1038/76665. [DOI] [PubMed] [Google Scholar]
- 317.Schlaggar BL, Fox K, O’Leary DD. Postsynaptic control of plasticity in developing somatosensory cortex. Nature. 1993;364:623–626. doi: 10.1038/364623a0. [DOI] [PubMed] [Google Scholar]
- 318.Monti PM, Miranda R, Jr, Nixon K, et al. Adolescence: booze, brains, and behavior. Alcohol Clin Exp Res. 2005;29:207–220. doi: 10.1097/01.alc.0000153551.11000.f3. [DOI] [PubMed] [Google Scholar]
- 319.Brodie MS. Increased ethanol excitation of dopaminergic neurons of the ventral tegmental area after chronic ethanol treatment. Alcohol Clin Exp Res. 2002;26:1024–1030. doi: 10.1097/01.ALC.0000021336.33310.6B. [DOI] [PubMed] [Google Scholar]
- 320.Vastola BJ, Douglas LA, Varlinskaya EI, Spear LP. Nicotine-induced conditioned place preference in adolescent and adult rats. Physiol Behav. 2002;77:107–114. doi: 10.1016/s0031-9384(02)00818-1. [DOI] [PubMed] [Google Scholar]
- 321.Belluzzi JD, Lee AG, Oliff HS, Leslie FM. Age-dependent effects of nicotine on locomotor activity and conditioned place preference in rats. Psychopharmacology (Berl) 2004;174:389–395. doi: 10.1007/s00213-003-1758-6. [DOI] [PubMed] [Google Scholar]
- 322.Adriani W, Macri S, Pacifici R, Laviola G. Peculiar vulnerability to nicotine oral self-administration in mice during early adolescence. Neuropsychopharmacology. 2002;27:212–224. doi: 10.1016/S0893-133X(02)00295-6. [DOI] [PubMed] [Google Scholar]
- 323.Slawecki CJ, Ehlers CL. Lasting effects of adolescent nicotine exposure on the electroencephalogram, event related potentials, and locomotor activity in the rat. Brain Research: Developmental Brain Research. 2002;138:15–25. doi: 10.1016/s0165-3806(02)00455-8. [DOI] [PubMed] [Google Scholar]
- 324.Slawecki CJ, Ehlers CL. The effects of corticotropin-releasing factor on the cortical EEG are reduced following adolescent nicotine exposure. Neuropeptides. 2003;37:66–73. doi: 10.1016/s0143-4179(03)00006-4. [DOI] [PubMed] [Google Scholar]
- 325.Slawecki CJ, Thorsell A, Ehlers CL. Long-term neurobehavioral effects of alcohol or nicotine exposure in adolescent animal models. Ann NY Acad Sci. 2004;1021:448–452. doi: 10.1196/annals.1308.062. [DOI] [PubMed] [Google Scholar]
- 326.Trauth JA, Seidler FJ, Slotkin TA. An animal model of adolescent nicotine exposure: Effects on gene expression and macromolecular constituents in rat brain regions. Brain Res. 2000;867:29–39. doi: 10.1016/s0006-8993(00)02208-3. [DOI] [PubMed] [Google Scholar]
- 327.Trauth JA, McCook EC, Seidler FJ, Slotkin TA. Modeling adolescent nicotine exposure: Effects on cholinergic systems in rat brain regions. Brain Res. 2000;873:18–25. doi: 10.1016/s0006-8993(00)02465-3. [DOI] [PubMed] [Google Scholar]
- 328.Collins SL, Wade D, Ledon J, Izenwasser S. Neurochemical alterations produced by daily nicotine exposure in periadolescent vs. adult male rats. Eur J Pharmacol. 2004;502:75–85. doi: 10.1016/j.ejphar.2004.08.039. [DOI] [PubMed] [Google Scholar]
- 329.Trauth JA, Seidler FJ, McCook EC, Slotkin TA. Adolescent nicotine exposure causes persistent upregulation of nicotinic cholinergic receptors in rat brain regions. Brain Res. 1999;851:9–19. doi: 10.1016/s0006-8993(99)01994-0. [DOI] [PubMed] [Google Scholar]
- 330.Trauth JA, Seidler FJ, Ali SF, Slotkin TA. Adolescent nicotine exposure produces immediate and long-term changes in CNS noradrenergic and dopaminergic function. Brain Res. 2001;892:269–280. doi: 10.1016/s0006-8993(00)03227-3. [DOI] [PubMed] [Google Scholar]
- 331.McDonald CG, Dailey VK, Bergstrom HC, et al. Periadolescent nicotine administration produces enduring changes in dendritic morphology of medium spiny neurons from nucleus accumbens. Neurosci Lett. 2005;385:163–167. doi: 10.1016/j.neulet.2005.05.041. [DOI] [PubMed] [Google Scholar]
- 332.Xu Z, Seidler FJ, Ali SF, Slikker W, Jr, Slotkin TA. Fetal and adolescent nicotine administration: effects on CNS serotonergic systems. Brain Res. 2001;914:166–178. doi: 10.1016/s0006-8993(01)02797-4. [DOI] [PubMed] [Google Scholar]
- 333.Xu Z, Seidler FJ, Cousins MM, Slikker W, Jr, Slotkin TA. Adolescent nicotine administration alters serotonin receptors and cell signaling mediated through adenylyl cyclase. Brain Res. 2002;951:280–292. doi: 10.1016/s0006-8993(02)03174-8. [DOI] [PubMed] [Google Scholar]
- 334.Schramm-Sapyta NL, Pratt AR, Winder DG. Effects of periadolescent versus adult cocaine exposure on cocaine conditioned place preference and motor sensitization in mice. Psychopharmacology (Berl) 2004;173:41–48. doi: 10.1007/s00213-003-1696-3. [DOI] [PubMed] [Google Scholar]
- 335.Caster JM, Walker QD, Kuhn CM. Enhanced behavioral response to repeated-dose cocaine in adolescent rats. Psychopharmacology (Berl) 2005;183:218–225. doi: 10.1007/s00213-005-0159-4. [DOI] [PubMed] [Google Scholar]
- 336.Niculescu M, Ehrlich ME, Unterwald EM. Age-specific behavioral responses to psychostimulants in mice. Pharmacol Biochem Behav. 2005;82:280–288. doi: 10.1016/j.pbb.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 337.Collins SL, Izenwasser S. Cocaine differentially alters behavior and neurochemistry in periadolescent versus adult rats. Brain Research: Developmental Brain Research. 2002;138:27–34. doi: 10.1016/s0165-3806(02)00471-6. [DOI] [PubMed] [Google Scholar]
- 338.Ehrlich ME, Sommer J, Canas E, Unterwald EM. Periadolescent mice show enhanced DeltaFosB upregulation in response to cocaine and amphetamine. J Neurosci. 2002;22:9155–9159. doi: 10.1523/JNEUROSCI.22-21-09155.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Kosofsky BE, Genova LM, Hyman SE. Postnatal age defines specificity of immediate early gene induction by cocaine in developing rat brain. J Comp Neurol. 1995;351:27–40. doi: 10.1002/cne.903510104. [DOI] [PubMed] [Google Scholar]
- 340.Karin M, Gallagher E. From JNK to pay dirt: jun kinases, their biochemistry, physiology and clinical importance. IUBMB Life. 2005;57:283–295. doi: 10.1080/15216540500097111. [DOI] [PubMed] [Google Scholar]
- 341.Silva RM, Kuan CY, Rakic P, Burke RE. Mixed lineage kinase-c-jun N-terminal kinase signaling pathway: a new therapeutic target in Parkinson’s disease. Mov Disord. 2005;20:653–664. doi: 10.1002/mds.20390. [DOI] [PubMed] [Google Scholar]
- 342.Tischmeyer W, Grimm R. Activation of immediate early genes and memory formation. Cell Mol Life Sci. 1999;55:564–574. doi: 10.1007/s000180050315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.McClung CA, Ulery PG, Perrotti LI, Zachariou V, Berton O, Nestler EJ. DeltaFosB: a molecular switch for long-term adaptation in the brain. Brain Research: Molecular Brain Research. 2004;132:146–154. doi: 10.1016/j.molbrainres.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 344.Konkle AT, Bielajew C. Tracing the neuroanatomical profiles of reward pathways with markers of neuronal activation. Rev Neurosci. 2004;15:383–414. doi: 10.1515/revneuro.2004.15.6.383. [DOI] [PubMed] [Google Scholar]
- 345.Nestler EJ. Molecular mechanisms of drug addiction. Neuropharmacology. 2004;47 (Suppl 1):24–32. doi: 10.1016/j.neuropharm.2004.06.031. [DOI] [PubMed] [Google Scholar]
- 346.Adriani W, Laviola G. Elevated levels of impulsivity and reduced place conditioning with d-amphetamine: two behavioral features of adolescence in mice. Behav Neurosci. 2003;117:695–703. doi: 10.1037/0735-7044.117.4.695. [DOI] [PubMed] [Google Scholar]
- 347.McPherson CS, Lawrence AJ. Exposure to amphetamine in rats during periadolescence establishes behavioural and extrastriatal neural sensitization in adulthood. Int J Neuropsychopharmacol. 2005:1–16. doi: 10.1017/S1461145705005845. [DOI] [PubMed] [Google Scholar]
- 348.Bolanos CA, Barrot M, Berton O, Wallace-Black D, Nestler EJ. Methylphenidate treatment during pre- and periadolescence alters behavioral responses to emotional stimuli at adulthood. Biol Psychiatry. 2003;54:1317–1329. doi: 10.1016/s0006-3223(03)00570-5. [DOI] [PubMed] [Google Scholar]
- 349.Heyser CJ, Pelletier M, Ferris JS. The effects of methylphenidate on novel object exploration in weanling and periadolescent rats. Ann NY Acad Sci. 2004;1021:465–469. doi: 10.1196/annals.1308.066. [DOI] [PubMed] [Google Scholar]
- 350.Vorhees CV, Reed TM, Morford LL, et al. Periadolescent rats (P41–50) exhibit increased susceptibility to D-methamphetamine-induced long-term spatial and sequential learning deficits compared to juvenile (P21–30 or P31–40) or adult rats (P51–60) Neurotoxicol Teratol. 2005;27:117–134. doi: 10.1016/j.ntt.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 351.Collins SL, Izenwasser S. Chronic nicotine differentially alters cocaine-induced locomotor activity in adolescent vs. adult male and female rats. Neuropharmacology. 2004;46:349–362. doi: 10.1016/j.neuropharm.2003.09.024. [DOI] [PubMed] [Google Scholar]
- 352.McMillen BA, Davis BJ, Williams HL, Soderstrom K. Periadolescent nicotine exposure causes heterologous sensitization to cocaine reinforcement. Eur J Pharmacol. 2005;509:161–164. doi: 10.1016/j.ejphar.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 353.Collins SL, Montano R, Izenwasser S. Nicotine treatment produces persistent increases in amphetamine-stimulated locomotor activity in periadolescent male but not female or adult male rats. Brain Research: Developmental Brain Research. 2004;153:175–187. doi: 10.1016/j.devbrainres.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 354.Partridge T. Are genetically informed designs genetically informative? Comment on McGue, Elkins, Walden, and Iacono (2005) and quantitative behavioral genetics. Dev Psychol. 2005;41:985–988. doi: 10.1037/0012-1649.41.6.985. discussion 993–987. [DOI] [PubMed] [Google Scholar]
- 355.Vanyukov MM, Kirisci L, Tarter RE, et al. Liability to substance use disorders: 2. A measurement approach. Neurosci Biobehav Rev. 2003;27:517–526. doi: 10.1016/j.neubiorev.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 356.Vanyukov MM, Tarter RE, Kirisci L, Kirillova GP, Maher BS, Clark DB. Liability to substance use disorders: 1. Common mechanisms and manifestations. Neurosci Biobehav Rev. 2003;27:507–515. doi: 10.1016/j.neubiorev.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 357.Stallings MC, Corley RP, Hewitt JK, et al. A genome-wide search for quantitative trait loci influencing substance dependence vulnerability in adolescence. Drug Alcohol Depend. 2003;70:295–307. doi: 10.1016/s0376-8716(03)00031-0. [DOI] [PubMed] [Google Scholar]
- 358.Twitchell GR, Hanna GL, Cook EH, Stoltenberg SF, Fitzgerald HE, Zucker RA. Serotonin transporter promoter polymorphism genotype is associated with behavioral disinhibition and negative affect in children of alcoholics. Alcohol Clin Exp Res. 2001;25:953–959. [PubMed] [Google Scholar]
- 359.Friedman AS, Terras A, Glassman K. Multimodel substance use intervention program for male delinquents. J Child Adolesc Subst. 2002;11:43–65. [Google Scholar]
- 360.Mason WA, Kosterman R, Hawkins JD, Haggerty KP, Spoth RL. Reducing adolescents’ growth in substance use and delinquency: Randomized trial effects of a parent-training prevention intervention. Prev Sci. 2003;4:203–212. doi: 10.1023/a:1024653923780. [DOI] [PubMed] [Google Scholar]