Abstract
Defenses against bacterial infections involve activation of multiple systems of innate immunity, including complement, Toll-like receptors, and defensins. Reactions to chronic infections bring adaptive immune mechanisms into play as well, with the introduction of modulatory interactions between the two. In humans with chronic lung infections, the severity of inflammation and disease correlate with elevated levels of pathogen-specific immune complexes and complement activation. In mice with genetic deficiency in C5, or targeted deletion of the C5a receptor, Pseudomonas lung infections reveal a role for the C5a anaphylatoxin in disease severity. Deficient animals exhibit significantly reduced survival and clearance of infecting bacteria, simultaneous with greatly increased pulmonary influx of inflammatory cells. Among the actions of C5a on inflammatory cells mediated through the C5a receptor is a shift in the relative expression of Fcγ receptors to increase FcγRIII relative to FcγRII. This shift may significantly impact defenses against chronic infection, reflecting the cellular activation profiles of these IgG receptors. We addressed the role of FcγRIII in defense against Pseudomonas lung infection, and found that, like C5aR-deficient mice, animals with targeted deletion of FcγRIII are more susceptible to mortality upon infection and exhibit reduced clearance of the pathogen. Pseudomonas infection was associated with an increase in the FcγRIII/FcγRII ratio in wild-type mice, and the data support its role as an additional mechanism of host defense against bacterial infection.
Keywords: Fcγ, receptors, host defense, bacterial infection, Pseudomonas, pneumonia
CLINICAL RELEVANCE
The immunoglobulinG receptor, FcγRIII, is protective in a murine model of Pseudomonas aeruginosa lung infection. FcγRIII-deficient mice exhibit increased mortality after P. aeruginosa lung infection and are impaired in their ability to clear the organisms.
Chronic bacterial lung infections in humans are associated with the presence of pathogen-specific antibodies, and the severity of disease may correlate with levels of immunoglobulin (Ig)G containing immune complexes. In addition to promoting activation of complement to generate C5a anaphylatoxin and the membrane attack complex, these immune complexes engage Fcγ receptors expressed on myeloid cells, promoting phagocytosis and bacterial killing.
Fcγ receptors, selective for the Fc region of IgG, constitute a family of proteins with characteristic distribution on inflammatory cells and distinct activating or suppressing signaling properties (1). Like many other immune regulatory systems, the Fcγ receptors support both stimulatory (FcγRI, FcγRIII, and FcγRIV) and inhibitory functions (FcγRII) and their relative expression determines the net cellular response (2). The activating receptor, FcγRI, is important for promoting phagocytosis of bacterial pathogens, cytokine release, cellular cytotoxicity, and antigen presentation (3, 4). Mice deficient in FcγRIIb reveal its suppressive functions, as they exhibit increases in humoral responses, IgG-induced anaphylaxis, increased pulmonary immune complex injury, and IgG-mediated clearance of pathogens and tumor cells (5–9). Contrasting this, FcγRIII-deficient mice are protected from IgG-mediated injury, including autoimmune hemolytic anemia, thrombocytopenia, and immune complex injury (10, 11).
Regulation of Fcγ receptor expression is accomplished at least in part by the complement anaphylatoxin, C5a, and interaction with its primary receptor, the C5aR. This interaction triggers Gi protein–dependent signal transduction, and, in addition to its other cellular activation functions, sets the balance of inhibitory and stimulatory FcγRs toward FcγRIII (12). Our studies have demonstrated that activation of the C5aR is critical for mucosal defense against Pseudomonas aeruginosa lung infections (13). In its absence, or in animals lacking C5 (14), infected mice are more susceptible to mortality in spite of a massive influx of neutrophils. Mechanistically, this may at least in part reflect a defective modulation of FcγR expression. In addition, the recently characterized secondary C5a receptor, C5L2, which does not transduce signals through activation of G proteins used by the C5aR, appears to negatively modulate C5aR functions (15). A role for this receptor in FcγR expression has not been demonstrated. In wild-type mice, bacterial infection leads to significant increases in expression of the C5aR, without concomitant up-regulation of C5L2 (16). The relationship of the change in C5aR expression to the FcγRII/III balance is not established, nor is a distinct role for FcγRIII in defense against bacterial pathogens. The present study was undertaken to investigate the role of FcγRIII in the complex mechanisms of host defense against bacterial lung infections. We demonstrate a critical role for this component, potentially resulting from deficiency in the C5a-C5aR-FcγR axis.
MATERIALS AND METHODS
Animals
All studies were performed according to institutional and National Institutes of Health guidelines for animal use and care. Experiments were performed using FcγRIII−/− mice on the C57BL/6 background (Jackson Labs, Bar Harbor, ME). Wild-type C57BL/6 mice were used as controls. Animals were housed under barrier conditions; age (6–10 wk old)- and sex-matched mice were used for all studies described.
Induction of Pneumonia
P. aeruginosa, strain PAO1, was kindly provided by Dr. Gerald Pier (Harvard Medical School). Bacteria in log phase growth were suspended in PBS containing 1% fetal calf serum (FCS) at OD650 = 0.5, and 2 × 106 colony-forming units (CFU) PsA was administered intranasally in 20 μl to anesthetized mice (ketamine, 90 mg/kg, and xylazine, 10 mg/kg, intramuscularly) for determination of survival. For all other experiments an inoculum of 5 × 106 CFU per animal was used, and mice were killed at 12 and 24 hours thereafter.
Quantitation of P. aeruginosa–Specific IgG
Enzyme-linked immunosorbent assay (ELISA) plates were coated with PsA at OD650 of 0.5 in 40 mM sodium phosphate, pH 7.0 by incubating overnight at 4°C. Plates were washed with PBS containing 0.05% Tween-20, and blocked with 1% bovine serum albumin (BSA) in PBS. Mouse serum, diluted 1:10 in PBS containing 1% BSA and 0.05% Tween-20, was serially diluted through four sequential 1:2 dilutions and plated in duplicate. After 90 minutes at 37°C, plates were washed and incubated for 90 minutes at 37°C with Fc-specific goat anti-mouse IgG conjugated with alkaline phosphatase (diluted 1:1,000). Reactivity was detected by measuring the OD405 developed with p-nitrophenyl phosphate, and the relative titer of anti-PsA IgG assessed from the dilution resulting in no further change in color development.
Analysis of Bacterial Clearance and Histopathology
At 12 or 24 hours after PsA infection, animals were killed and the lungs and spleens were harvested. Organs were homogenized in 1 ml of 1% proteose peptone at 0°C, and an aliquot suspended in 1 ml Dulbecco's modified Eagle's medium/F12 containing 10% FCS and 0.5% Triton X-100. Serial 10-fold dilutions were plated on MacConkey agar for overnight growth. For histologic examination, lungs were fixed in Bouin's solution. Paraffin sections were stained with hematoxylin and eosin and evaluated by light microscopy.
Measurement of Vascular Permeability
Vascular permeability 24 hours after infection was determined using Evans blue dye. Four hours before killing, mice were injected intraperitoneally with 200 μl 1% Evans blue dye in PBS. Bronchoalveolar lavage (BAL) and serum were collected and permeability changes evaluated by comparison of the ratio of OD600 in BAL to serum.
Analysis of BAL
Lungs were lavaged with PBS at 4°C, three times 0.8 ml, and total cell count was assessed (Beckman Coulter, Fullerton, CA). Differential cell counts were determined from Wright/Giemsa stained cytospins, counting 200 cells per slide.
In Vitro Assessment of Macrophage Phagocytosis
Resident peritoneal macrophages were harvested from uninfected wild-type and FcγRIII−/− mice by lavage with RPMI at 4°C three times 4 ml. After lysis of red blood cells, macrophage preparations were routinely greater than 95% pure. Cells (1 × 106) were incubated with 1 × 107 CFU PsA PA01 expressing green fluorescent protein (GFP) in the presence of 10% serum obtained from FcγRIII−/− or wild-type mice. After 1 hour at 37°C with gentle shaking the cells were washed, stained with DAPI, fixed with 4% paraformaldehyde, and counted by confocal microscopy. Cell-associated GFP-PsA was quantitated using ImagePro Software (Media Cybernetics, Bethesda, MD).
Production of Reactive Oxygen Species
ROS production by phagocytes was measured using dihydro-2′4,5,6,7,7′-hexafluorofluorescein (H2HFF) (FcOxyburst; Invitrogen, Carlsbad, CA). Peritoneal macrophages or bone marrow cells isolated from uninfected FcγRIII−/− or wild-type mice (5 × 106) in PBS containing 1% BSA were incubated with 10 ng/ml PMA in the presence or absence of the inhibitor DPI (10 μg/ml). Fluorescence intensity was determined by flow cytometry (FACSCalibur; BD Biosciences, Franklin Lakes, NJ).
Cytokine and Anaphylatoxin Analyses
The BAL content of TNF-α, IL-6, and C5a was determined by ELISA according to the manufacturer's instructions (R&D Systems, Minneapolis, MN).
FcγR Expression Analysis
Mice were infected intranasally with PsA as described above. Alternatively, C5a (200 ng per animal) in 50 μl PBS was administered intratracheally. Four hours later, lungs were harvested, processed for total RNA, and FcγR levels quantitated by real-time RT-PCR (Taq-Man) using published FcγR-specific primers (17).
Statistical Analyses
All data are expressed as the mean ± SEM. Survival curves were compared using Fisher's exact test. Comparison between groups was conducted using Student's t test, and differences were considered significant for P ⩽ 0.05.
RESULTS
FcγRIII Expression Confers Protection against Mortality Associated with P. aeruginosa Pneumonia
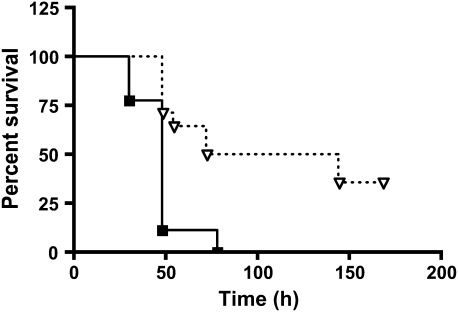
PsA lung infections cause severe bronchopneumonia, which previous studies have shown is controlled in part by functional expression of the complement C5a anaphylatoxin receptor (13). Mice deficient in either C5 or the C5aR exhibit exacerbated sequelae to PsA infection relative to wild-type animals, with increased mortality, decreased bacterial clearance, and an overabundance of infiltrating inflammatory cells (13, 14). In immune complex lung inflammation, C5a–C5aR interactions set the threshold for Fcγ receptor–mediated responses by modulating the relationship of stimulatory FcγRIII to inhibitory FcγRII (17). To understand whether the FcγRIII/FcγRII ratio also plays a role in host defense against bacterial infection, we studied the responses of FcγRIII-deficient mice compared with those of wild-type animals after lung infection with PsA. As shown by the data of Figure 1, FcγRIII-deficient mice are significantly more susceptible to mortality after intranasal infection with 5 × 106 CFU of PsA. All of the FcγRIII−/− mice died within 72 hours, whereas 50% of wild-type animals survived at least 8 days (n = 9–14 per group, P = 0.006).
Figure 1.
FcγRIII-deficient mice are more susceptible to mortality from Pseudomonas aeruginosa (PsA) lung infection. FcγRIII−/− (squares) and wild-type (triangles) mice were infected intranasally with 2 × 106 colony-forming units (CFU) PsA and monitored for survival (n = 9–14 mice per group, P = 0.002 for FcγRIII−/− versus wild-type animals).
FcγRIII Expression Contributes to Clearance of Pseudomonas
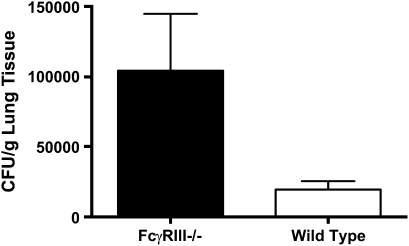
To determine whether expression of FcγRIII facilitates clearance of PsA infection, the number of viable bacteria recovered 12 or 24 hours after inoculation of 5 × 106 CFU was determined. As shown in Figure 2, almost 2-fold more bacteria were recovered from the lungs of FcγRIII-deficient mice compared with wild-type animals 24 hours after infection (1.04 ± 0.41 × 105 CFU/g lung tissue for FcγRIII−/− versus 1.96 ± 0.61 × 104 CFU/g lung tissue for wild-type mice, P = 0.022, n = 10–11 mice per group). A significant reduction in clearance was also observed at 12 hours (data not shown).
Figure 2.
FcγRIII deficiency impairs clearance of pulmonary PsA infections. FcγRIII−/− and wild-type mice were infected intranasally with 5 × 106 CFU PsA. After 24 hours, animals were killed and lung homogenates assessed for bacterial content. Data are the mean CFU ± SEM per gram lung, P = 0.022 for n = 10–11 mice per group.
FcγRIII−/− Mice Exhibit Increased Inflammation after PsA Infection
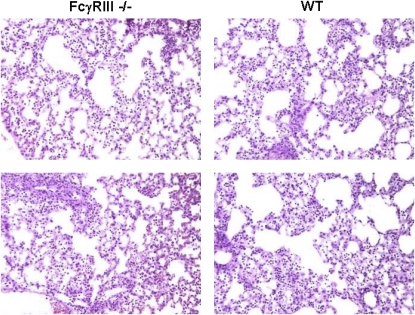
Histologic analysis of the lungs 24 hours after infection showed evidence of diffuse bronchopneumonia for both mouse strains, with no apparent difference between the two (Figure 3). Analysis of cellular infiltrates in BAL fluid, however, revealed a significant increase in cellularity, composed primarily of polymorphonuclear leukocytes (PMNs) and macrophages (Table 1). Both PMNs and macrophages were elevated by approximately 30% in the FcγRIII−/− mice with no significant difference in lymphocytes.
Figure 3.
Histologic appearance of lung tissues from FcγRIII−/− and wild-type mice 24 hours after PsA infection. Twenty-four hours after intranasal infection with 5 × 106 CFU PsA, mice were killed and lungs processed for histology. Both mouse strains exhibited diffuse inflammatory infiltrates. Sections are representative of three independent experiments, hematoxylin and eosin (original magnification: ×200).
TABLE 1.
FCγRIII-DEFICIENT MICE HAVE GREATER PULMONARY INFLUX OF LEUKOCYTES AFTER PSEUDOMONAS AERUGINOSA INFECTION
| Genotype | Total Cells (×106) | PMNs (×106) | Monocytes (×105) |
|---|---|---|---|
| FcγRIII-/- | 11.36 ± 1.22* | 10.28 ± 1.04* | 9.55 ± 1.76† |
| Wild type | 8.78 ± 1.95 | 7.96 ± 1.76 | 7.38 ± 1.53 |
FcγRIII and wild-type mice were infected with P. aeruginosa by intranasal inoculation. After 24 hours, animals were killed and bronchoalveolar lavage fluid assessed for inflammatory cell content. Results are the mean ± SEM for 9–11 mice per group.
P = 0.001.
P = 0.005.
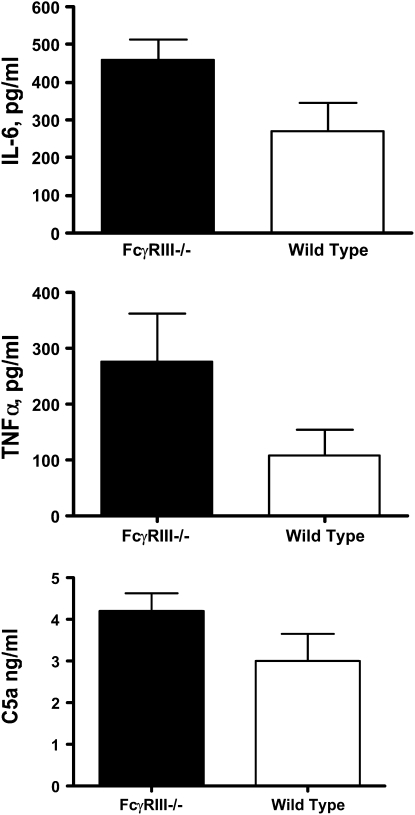
As shown in Figure 4, BAL levels of the proinflammatory cytokines, IL-6, and TNF-α were also elevated in PsA-infected FcγRIII−/− mice compared with wild-type animals (IL-6, 460 ± 52 pg/ml for FcγRIII−/− versus 270 ± 74 for wild-type mice, P = 0.025; TNF-α, 276 ± 86 pg/ml for FcγRIII−/− versus 108 ± 46 pg/ml for wild-type mice, P = 0.048, n = 9–11 mice per group). The extent of complement activation was determined by evaluating levels of the anaphylatoxin C5a/C5adesArg in BAL fluid, and was not significantly different for the two mouse strains (4.21 ± 0.41 ng/ml for FcγRIII−/− versus 3.0 ± 0.064 ng/ml for wild-type mice, P = 0.08, n = 9–11 mice per group).
Figure 4.
Lung cytokines are elevated in FcγRIII-deficient mice after PsA infection. The concentrations of IL-6, TNF-α, and C5a present in bronchoalveolar lavage (BAL) fluid 24 hours after PsA lung infection were assessed by enzyme-linked immunosorbent assay. Data are the mean ± SEM concentrations, P = 0.025 for IL-6, P = 0.048 for TNF-α, NS for C5a (n = 9–11 mice per group).
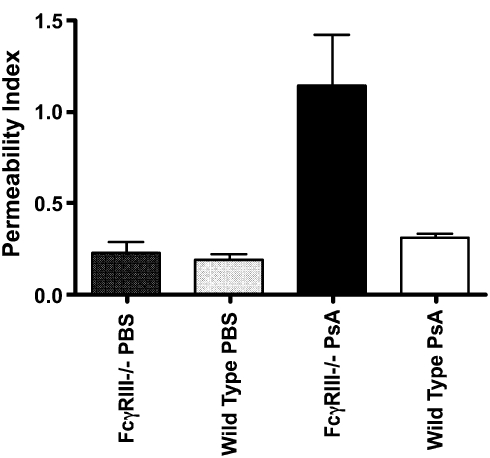
The change in permeability resulting from PsA infection was assessed from the extravasation of Evans blue dye (Figure 5). At 24 hours after infection, permeability in both FcγRIII−/− and wild-type mice mouse lungs was significantly elevated compared with uninfected controls (P < 0.05, n = 3–6 mice per group). Permeability in PsA-infected FcγRIII−/− mice was approximately 4-fold greater than for PsA-infected wild-type mice (P = 0.012, n = 5–6 mice per group).
Figure 5.
FcγRIII-deficient mice exhibit increased pulmonary vascular permeability after PsA lung infection. FcγRIII−/− and wild-type mice were infected intranasally with 5 × 106 CFU PsA or administered an equal volume of PBS. Permeability was assessed 24 hours later by extravasation of Evans blue dye into BAL fluid. Permeability index was determined from the dye content of BAL compared with plasma, P < 0.05 for infected versus PBS, P = 0.012 for PsA-infected FcγRIII versus wild-type, n = 3 mice per group for PBS controls, n = 5–6 per group for PsA-infected animals.
Role of Phagocytes in Defense against PsA Pneumonia
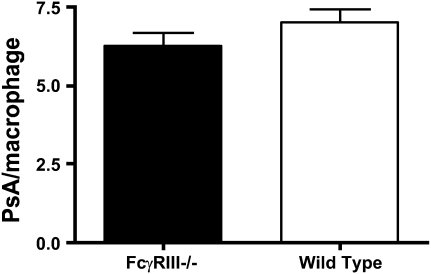
In light of the absence of FcγRIII on inflammatory cells in the knockout mice, we assessed their phagocytic ability in vitro. Peritoneal macrophages from uninfected FcγRIII−/− or wild-type mice were incubated with GFP-labeled PsA and the number of internalized bacteria evaluated by confocal microscopy. Cells from both mouse strains contained a similar number of engulfed bacteria (7.00 ± 0.43 per cell for wild-type and 6.29 ± 0.38 per cell for FcγRIII−/−) (Figure 6). We also compared the bactericidal competence of FcγRIII−/− phagocytes with those of wild-type mice by assessing their ability to produce reactive oxygen species. Cells from both mouse strains produced similar amounts of ROS after stimulation with phorbol myristate acetate (data not shown). These results indicate the lack of FcγRIII does not significantly alter the intrinsic inflammatory cell function.
Figure 6.
Phagocytosis of PsA by alveolar macrophages from FcγRIII−/− and wild-type mice. BAL macrophages were isolated 1 hour after intranasal inoculation with GFP-tagged PsA. Phagocytosis was determined by fluorescent microscopy as the number of bacteria per cell and was comparable for FcγRIII−/− and wild-type animals.
Pseudomonas Infection Alters the FcγRIII/FcγRII Ratio via a C5a/C5aR-Dependent Mechanism
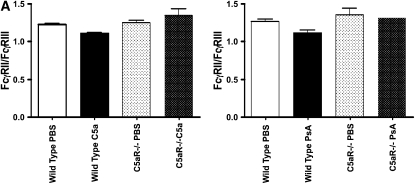
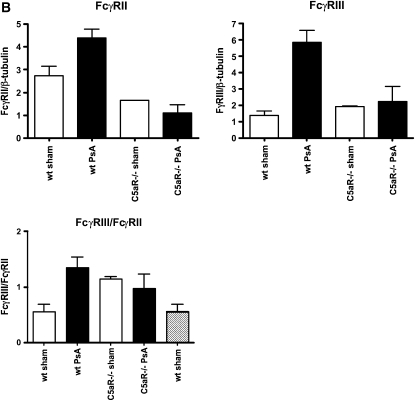
Activation of FcγR-expressing cells by ligation of C5a and C5a receptor has been shown to modulate the relative cell surface levels of FcγRII and FcγRIII. In models of immune complex injury, this activation determines the subsequent inflammatory response (12, 17). As activation of complement and generation of C5a is one response to bacterial infection, we evaluated alterations in the relative expression of FcγRII and FcγRIII in PsA-infected mouse lungs. Wild-type and C5aR-deficient mice were infected intranasally with PsA, and 4 hours later the lung mRNA levels of FcγRII and FcγRIII were analyzed by RT-PCR. As shown in Figure 7, PsA infection in wild-type mice revealed a significant increase in the FcγRIII/FcγRII ratio, but no change was observed in C5aR-deficient animals (P = 0.04 for PsA infected versus uninfected wild-type mice, n = 4 per group). The change in FcγRIII/FcγRII ratio was similar after intratracheal administration of recombinant C5a (P = 0.009 for wild-type mice with versus without C5a administration, n = 4 per group). Thus generation of C5a by infecting PsA regulates FcγR expression levels as it does in immune complex injury.
Figure 7.
Lung expression of FcγRII relative to FcγRIII is reduced following PsA infection and by C5a administration. (A) Recombinant C5a (200 ng/mouse) or PBS was administered intratracheally to wild-type or C5a receptor–deficient mice and the expression of FcγRII and FcγRIII assessed 4 hours later by quantitative PCR. (B) Wild-type or C5a receptor–deficient mice were infected intranasally with 5 × 106 CFU PsA, and 24 hours later lung expression of FcγRII and FcγRIII was assessed by quantitative PCR. Data are expressed as the ratio of FcγRII to FcγRIII, P = 0.01 for C5a-treated versus PBS control, P = 0.04 for PsA-infected versus PBS control wild-type mice, NS for C5aR−/− animals, n = 4 mice per group.
Presence and Induction of Anti-PsA Antibodies
Since ligation of FcγRIII requires the presence of immune complexes, we questioned whether PsA-reactive antibodies were detectable in uninfected mice. Naïve mice of both mouse strains were found to exhibit low but detectable levels of anti-PsA reactive IgGs, but no difference was detected in either the total or subclass distribution between the two. Within 24 hours of PsA infection, both FcγRIII−/− and wild-type mice exhibited increases in the titers of PsA-specific IgG1 and IgG2b, but not IgG2a (n = 7–8 mice per group).
DISCUSSION
In this investigation of mechanisms of host defense against P. aeruginosa lung infections, we found that mice lacking FcγRIII have significantly reduced survival and bacterial clearance compared with wild-type animals. Despite the increased mortality, FcγRIII−/− mice exhibit elevated numbers of inflammatory cells in BAL fluid. They also exhibited enhanced pulmonary vascular permeability and increased levels of IL-6 and TNF-α in BAL fluid compared with PsA infected wild-type mice. Comparison of levels of the C5a anaphylatoxin generated in the two strains indicated no significant difference in complement activation.
In probing the mechanism of FcγRIII-mediated protection against PsA infection, we assessed the presence of pathogen-specific IgG and found detectable levels in both mouse strains before infection, with no apparent difference in the total levels or the IgG subclass distribution. Within 24 hours of infection, the levels of PsA-specific IgG increased in both mouse strains, supporting a role for an immune complex–mediated component in the PsA lung infections, and suggestive of a scenario similar to that found in chronic bacterial lung infections in humans.
Previous studies have shown that the relative expression of FcγRIIb and FcγRIII is important for determining the nature of responses in immune complex–mediated inflammation, such that increased FcγRIII promotes inflammation and decreased FcγRIII promotes tolerance (17). These studies have also demonstrated a link between complement activation–mediated generation of C5a, triggering of the C5aR, and increases in the ratio of FcγRIII to FcγRIIb (17). Indeed, C5aR-deficient mice, present a phenotype similar to FcγRIII-deficient animals with respect to pulmonary infection with PsA (13). In our original report of this phenotype we did not ascribe a mechanism to this finding, and the relationship of the C5a receptor to Fcγ receptor expression was not known. Similarities in the phenotype of C5aR−/− and FcγRIII−/− mice with respect to PsA lung infection, in concert with an appreciation of C5a-mediated regulation of Fcγ receptor balance provide a compelling explanation for the complex interactions involved. Thus, deficiency in any component is likely sufficient to set the net response awry and lead to an impairment in host defense. Incomplete overlap of each of the components, however, may be reflected by distinctions among mouse strains in their responses to PsA infection. In particular, FcγRIII−/− mice exhibited an approximately 30% increase in the influx of inflammatory cells, whereas C5aR−/− animals experienced a massive (∼ 300%) infiltration of neutrophils into the lungs (13).
Consistent with previous reports, intratracheal administration of exogenous C5a led to an increase in the ratio of FcγRIII to FcγRII. In C5aR-deficient mice, this shift was not observed. PsA lung infection resulted in a similar change in the FcγRIII to FcγRII ratio in wild type mice, but not in C5aR−/− animals. Thus, in both FcγRIII- and C5aR-deficient mice, increased susceptibility to PsA pneumonia may be related in part to an inability to appropriately regulate Fcγ receptor expression.
The extent to which interaction between FcγRIII and pre-existing anti-PsA–specific antibodies contribute to the phenotype is not clear. We observed increases in PsA-reactive IgG1 and IgG2b after infection. Studies have suggested that the inflammatory potential of immune complexes mediated by distinct IgG subclasses is related to their binding affinities for specific Fcγ receptors (18). Other studies have demonstrated that patients with cystic fibrosis who have chronic PsA lung infections exhibit a correlation between lung function and levels of specific IgG subclasses (19–21). Additional studies are needed to define the relationship between immune complexes, Fcγ receptors, complement, and the host response to bacterial pathogens.
This work was supported by NIH HL51366 (to C.G.).
Originally Published in Press as DOI: 10.1165/rcmb.2007-0309OC on November 1, 2007
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Takai T. Fc receptors and their role in immune regulation and autoimmunity. J Clin Immunol 2005;25:1–18. [DOI] [PubMed] [Google Scholar]
- 2.Ravetch JV, Bolland S. IgG Fc receptors. Annu Rev Immunol 2001;19:275–290. [DOI] [PubMed] [Google Scholar]
- 3.Ioan-Facsinay A, de Kimpe SJ, Hellwig SM, van Lent PL, Hofhuis FM, van Ojik HH, Sedlik C, da Silveira SA, Gerber J, de Jong YF, et al. FcgammaRI (CD64) contributes substantially to severity of arthritis, hypersensitivity responses, and protection from bacterial infection. Immunity 2002;16:391–402. [DOI] [PubMed] [Google Scholar]
- 4.Barnes N, Gavin AL, Tan PS, Mottram P, Koentgen F, Hogarth PM. FcgammaRI-deficient mice show multiple alterations to inflammatory and immune responses. Immunity 2002;16:379–389. [DOI] [PubMed] [Google Scholar]
- 5.Clatworthy MR, Smith KG. FcgammaRIIb balances efficient pathogen clearance and the cytokine-mediated consequences of sepsis. J Exp Med 2004;199:717–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clynes R, Maizes JS, Guinamard R, Ono M, Takai T, Ravetch JV. Modulation of immune complex-induced inflammation in vivo by the coordinate expression of activation and inhibitory Fc receptors. J Exp Med 1999;189:179–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ujike A, Ishikawa Y, Ono M, Yuasa T, Yoshino T, Fukumoto M, Ravetch JV, Takai T. Modulation of immunoglobulin (Ig)E-mediated systemic anaphylaxis by low-affinity Fc receptors for IgG. J Exp Med 1999;189:1573–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takai T, Ono M, Hikida M, Ohmori H, Ravetch JV. Augmented humoral and anaphylactic responses in Fc gamma RII-deficient mice. Nature 1996;379:346–349. [DOI] [PubMed] [Google Scholar]
- 9.Dijstelbloem HM, van de Winkel JG, Kallenberg CG. Inflammation in autoimmunity: receptors for IgG revisited. Trends Immunol 2001;22:510–516. [DOI] [PubMed] [Google Scholar]
- 10.Hazenbos WL, Gessner JE, Hofhuis FM, Kuipers H, Meyer D, Heijnen IA, Schmidt RE, Sandor M, Capel PJ, Daeron M, et al. Impaired IgG-dependent anaphylaxis and Arthus reaction in Fc gamma RIII (CD16) deficient mice. Immunity 1996;5:181–188. [DOI] [PubMed] [Google Scholar]
- 11.Meyer D, Schiller C, Westermann J, Izui S, Hazenbos WL, Verbeek JS, Schmidt RE, Gessner JE. FcgammaRIII (CD16)-deficient mice show IgG isotype-dependent protection to experimental autoimmune hemolytic anemia. Blood 1998;92:3997–4002. [PubMed] [Google Scholar]
- 12.Skokowa J, Ali SR, Felda O, Kumar V, Konrad S, Shushakova N, Schmidt RE, Piekorz RP, Nurnberg B, Spicher K, et al. Macrophages induce the inflammatory response in the pulmonary Arthus reaction through G alpha i2 activation that controls C5aR and Fc receptor cooperation. J Immunol 2005;174:3041–3050. [DOI] [PubMed] [Google Scholar]
- 13.Hopken UE, Lu B, Gerard NP, Gerard C. The C5a chemoattractant receptor mediates mucosal defence to infection. Nature 1996;383:86–89. [DOI] [PubMed] [Google Scholar]
- 14.Larsen GL, Mitchell BC, Harper TB, Henson PM. The pulmonary response of C5 sufficient and deficient mice to Pseudomonas aeruginosa. Am Rev Respir Dis 1982;126:306–311. [DOI] [PubMed] [Google Scholar]
- 15.Gerard NP, Lu B, Liu P, Craig S, Fujiwara Y, Okinaga S, Gerard C. An anti-inflammatory function for the complement anaphylatoxin C5a-binding protein, C5L2. J Biol Chem 2005;280:39677–39680. [DOI] [PubMed] [Google Scholar]
- 16.Okinaga S, Slattery D, Humbles A, Zsengeller Z, Morteau O, Kinrade MB, Brodbeck RM, Krause JE, Choe HR, Gerard NP, et al. C5L2, a nonsignaling C5A binding protein. Biochemistry 2003;42:9406–9415. [DOI] [PubMed] [Google Scholar]
- 17.Shushakova N, Skokowa J, Schulman J, Baumann U, Zwirner J, Schmidt RE, Gessner JE. C5a anaphylatoxin is a major regulator of activating versus inhibitory FcgammaRs in immune complex-induced lung disease. J Clin Invest 2002;110:1823–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nimmerjahn F, Bruhns P, Horiuchi K, Ravetch JV. FcgammaRIV: a novel FcR with distinct IgG subclass specificity. Immunity 2005;23:41–51. [DOI] [PubMed] [Google Scholar]
- 19.Cowan RG, Winnie GB. Anti-Pseudomonas aeruginosa IgG subclass titers in patients with cystic fibrosis: correlations with pulmonary function, neutrophil chemotaxis, and phagocytosis. J Clin Immunol 1993;13:359–370. [DOI] [PubMed] [Google Scholar]
- 20.Garside JP, Kerrin DP, Brownlee KG, Gooi HC, Taylor JM, Conway SP. Low gammaglobulin subclass 2 levels in paediatric cystic fibrosis patients followed over a 2-year period. Pediatr Pulmonol 2007;42:125–130. [DOI] [PubMed] [Google Scholar]
- 21.Garside JP, Kerrin DP, Brownlee KG, Gooi HC, Taylor JM, Conway SP. Immunoglobulin and IgG subclass levels in a regional pediatric cystic fibrosis clinic. Pediatr Pulmonol 2005;39:135–140. [DOI] [PubMed] [Google Scholar]