Abstract
The cytokine mRNA profiles of primary (arising from inhaled bacilli) and secondary (arising from hematogenous reseeding of the lung) granulomas from the lung lobes of bacillus Calmette-Guérin (BCG)-vaccinated and unimmunized guinea pigs challenged with virulent Mycobacterium tuberculosis by the pulmonary route were assessed in situ using laser capture microdissection (LCM) at 6 weeks after infection. The challenge dose chosen was so low that some lung lobes did not receive an implant from the airway. In unimmunized guinea pigs, some lobes contained either large, necrotic primary lesions or small, non-necrotic secondary lesions, or both. The lobes of BCG-vaccinated animals contained only non-necrotic primary tubercles, and no secondary lesions were visible. Real-time PCR analysis of the acquired RNA clearly demonstrated that primary tubercles from BCG-vaccinated guinea pigs were overwhelmed with mRNA from the anti-inflammatory cytokine, transforming growth factor (TGF)-β, with some IFN-γ and IL-12p40 mRNA. In contrast, primary lesions from unimmunized animals were dominated by proinflammatory TNF-α mRNA. The cytokine mRNA profile of secondary lesions from unimmunized animals was strikingly similar to the profile of primary lesions from BCG-vaccinated guinea pigs (i.e., a predominance of TGF-β mRNA with some IL-12p40 and IFN-γ mRNA), indicating that the lung lobes from which these lesions were retrieved had been naturally “vaccinated” by the time the bloodborne bacilli returned to the lung at 3 to 4 weeks after infection. Furthermore, cytokine mRNA analysis of splenic granulomas from nonvaccinated and vaccinated animals showed close resemblance to primary granulomas recovered from the lungs of the same animal, that is, high levels of TNF-α mRNA in unimmunized animals, and mostly TGF-β mRNA in BCG-vaccinated guinea pigs. Taken together, these data indicate that mycobacteria returning to the lungs of unimmunized guinea pigs 3 to 4 weeks after infection induce a local cytokine response that is fundamentally different from the response to inhaled bacilli and is reminiscent of the primary response in a vaccinated animal.
Keywords: guinea pig, vaccine, tuberculosis, granuloma, cytokine
CLINICAL RELEVANCE
The studies presented here extend our knowledge of granuloma formation and hematogenous reseeding in a highly relevant animal model, as well as add to our knowledge base of the mechanisms by which the bacillus Calmette-Guérin vaccine exerts its protective effects.
Disease caused by Mycobacterium tuberculosis (M.tb) can result either from the early progression of a primary granuloma that results from the inhalation of an infectious particle, or from the reactivation of a dormant granuloma that the patient may have carried for many years (1, 2). The precise location and nature of the so-called “reactivatable” granulomas remain controversial. However, there is strong evidence to suggest that secondary, bloodborne lesions in the apex or sub-apex of the lung, rather than the primary lesion, are the reactivating lesions responsible for disease manifestation. Single, calcified, primary lesions were found in various locations in the lungs of 105 patients with tuberculosis (TB) (3), whereas cavitary lesions in patients with reactivation TB had a propensity to be found in the apical regions of the lungs (2). In the highly biologically relevant guinea pig model of low-dose pulmonary TB, the virulent bacilli multiply extensively at the site of primary implantation and are transported very early (7–10 d after infection) to the draining (hilar or bronchotracheal) lymph nodes. From this point, the bacilli quickly escape into the bloodstream and are observed within a few days (14–21 d after infection) in the spleen. Several days later, bacilli appear for the first time in all lung lobes, including those that do not contain a primary lesion (4–7). When individual lung lobes were cultured by Ho and coworkers, the bacilli were detected from primary lesion–free lobes (i.e., lobes containing only secondary lesions) only after 22 days after infection (6). Since the arrival of bacteria in previously sterile lobes was detected after the bacilli were detected in the spleen, these data indirectly imply that secondary lesions result from bloodborne reseeding. Intrathoracic spread would have occurred earlier if it was an important route of bacterial dissemination to previously uninfected lobes. Thus, two different types of lesions exist in the lung after hematogenous dissemination: primary (arising from inhaled bacilli) and secondary (bloodborne). Lenaerts and colleagues (8), recently demonstrated that inflammation in secondary lung lesions resolved more completely after antimycobacterial therapy in guinea pigs infected under conditions similar to those used in the present study (8).
The timing of arrival in the lung of bloodborne M.tb coincides with the onset of an immune response (3–4 wk after infection) (7). In addition, bacillary replication in the secondary, bloodborne lesion is curtailed at peak levels approximately 10- to 100-fold lower than peak levels in the primary lesions of the same animal (6). These observations suggest that previously uninvolved portions of the lung are actually “vaccinated” by the primary infection (9). Therefore, our hypothesis was that the local immune response in primary and secondary lesions would differ significantly, with the latter reflecting a protective phenotype. Understanding the immunologic differences between the two lesions will help us better understand the process of reactivation and, thus, the management of the estimated 2 billion people at risk for reactivation TB.
Using laser capture microdissection (LCM), we have previously demonstrated that the cytokine profile of pulmonary granulomas taken from individual lung lobes of M.tb-infected guinea pigs were affected significantly by bacillus Calmette-Guérin (BCG) vaccination (10). Primary lesions microdissected from unimmunized guinea pigs were overwhelmed by the proinflammatory TNF-α mRNA at both 3 and 6 weeks after infection, indicating the struggle to control the mounting infection. The cytokine profile of granulomas from vaccinated guinea pigs shifted from Type 1 cytokine mRNA (IFN-γ and IL-12p40) at 3 weeks to a predominantly anti-inflammatory environment (transforming growth factor [TGF]-β mRNA) at 6 weeks. Some heterogeneity between granulomas in different lung lobes of the same animal was also observed (10).
In the current study, we examined the cytokine milieu of primary and secondary lesions of the lungs of nonvaccinated and BCG-vaccinated guinea pigs infected with a dose of virulent M.tb too low to result in the initial infection of all lung lobes. For comparison, the cytokine mRNA profiles of granulomas from the spleen were also studied. These results, for the first time, allow us to compare the immunological differences between primary and secondary lesions, view a map of the lobes in which these lesions exist, compare the cytokine profile of granulomas from the lung and spleen of the same animal, and evaluate the effect of BCG vaccination status on these variables in the guinea pig.
MATERIALS AND METHODS
Animals and Vaccination
Specific pathogen–free outbred Hartley strain guinea pigs (250–300 g) from Charles River Breeding Laboratories, Inc. (Willmington, MA) were housed individually in polycarbonate cages under conventional conditions and rested for at least 7 days. Half of the animals were vaccinated via intradermal injection in the left inguinal region with 0.1 ml (103 colony-forming units [CFU]) of viable Mycobacterium bovis BCG (Danish 1331 strain; Statens Seruminstitut) and allowed to rest for 6 weeks before aerosol infection. After virulent infection, the guinea pigs were housed in a BSL-3 containment facility. All protocols were approved by the Texas A&M University Laboratory Animal Care Committee.
Aerosol Infection
Nonvaccinated and BCG-vaccinated guinea pigs were infected at the same time with a nebulizer concentration of 2 × 104 CFU/ml of M.tb H37Rv (frozen, single cell suspension, ATCC 27294; American Type Culture Collection, Rockville, MD) via the respiratory route in an aerosol chamber constructed by the University of Wisconsin Engineering Shops (Madison, WI), as described previously (11). We have determined a precise mathematical relationship between the nebulizer concentration and inhaled bacilli (unpublished data). This infection level was expected to result in the inhalation and retention of approximately three to five infectious particles per animal.
Necropsy and Tissue Processing
Guinea pigs were killed at 6 weeks after aerosol infection by intraperitoneal injection of an overdose (100 mg/kg) of sodium pentobarbital (Sleepaway; Fort Dodge Laboratories, Inc., Fort Dodge, IA). The four main lung lobes (lower left [LL], upper left [UL], lower right [LR], and upper right [UR]) and spleen were excised aseptically. Each tissue was then cut into 10 equal pieces using a scalpel, placed into 10% buffered formalin for 1 hour at 4°C, and transferred into 70% EtOH. This process adequately sterilized the M.tb-infected tissues while preserving the RNA as determined by the Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA) (10). Eighteen to twenty-four hours later, tissues were paraffin-embedded, sections (5 μm) were cut at −30°C and immediately placed onto uncharged, “non-plus” slides (Fisher Scientific, Pittsburgh, PA). Cryostat blades, tools, surfaces, slides, and staining vessels were all pretreated with RNase Zap (Ambion, Austin, TX) before use. The glass slides were stored at less than 4°C until further processed. Slides were dewaxed in xylene, rehydrated, stained with hematoxylin and eosin (H&E), and dehydrated using Ambion's LCM Staining Kit.
Laser Capture Microdissection
LCM was performed immediately on a PixCell IIe microscope (Arcturus Engineering, Mountain View, CA) using a pulse power of 40 to 50 mW, a 15- to 30-μm laser sport diameter, pulse duration of 650 μs to 2.0 ms, and a target voltage of 200 mW. Granulomatous lesions were microscopically identified and the same portion of the granuloma was isolated on Capsure Macro LCM caps (Arcturus). Cells adjacent to the laser-targeted cells that were co-isolated onto the cap were removed by pressing the cap surface onto a PrepStrip (Arcturus). The transparent cap film was carefully peeled from the cap using forceps and placed in an Eppendorf tube containing 110 μl Proteinase K solution. Three to four capfuls microdissected from the one to three granulomas on three slides were pooled to obtain sufficient amounts of RNA and stored at −20°C until further processing. In the same samples, nongranulomatous sections were collected as controls. In nonvaccinated guinea pigs, 1° and 2° granulomas were characterized as large (with the presence of necrosis) and small (with the absence of necrosis), respectively. All samples of cytokine mRNA from secondary lesions were captured in the UL lung lobes, which contained no primary lesions. This ensured that the samples truly represented secondary granulomas. In BCG-vaccinated guinea pigs, the appearance of primary lung lesions were homogenous and noted as small and non-necrotic (10, 12).
Total RNA Isolation and Real-Time PCR
Total RNA was isolated using the Optimum FFPE RNA Isolation Kit (Asuragen Diagnostics, Austin, TX) according to the manufacturer's protocol. Reverse transcription, performed by using TaqMan reverse transcription reagents, and real-time PCR, performed by using Power SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA), were performed as previously described (10, 13, 14). The real-time primer sequences for the guinea pig IL-12p40, TNF-α, TGF-β1, IFN-γ, and hypoxanthine-guanine phosphoribosyltransferase (HPRT) were published previously (13, 14). Guinea pig IL-10 primers (forward: CCTTACTGGCCGGGGTCAA; reverse: GCTGATCCTGTGTTTGGAAGAAAG) were designed by Primer Express Software (Applied Biosystems) and checked for sequence homology against known genes using a BLAST search (http://www.ncbi.nlm.nih.gov/blast). Guinea pig inducible nitric oxide synthase (iNOS) primer sequences (forward: GCAGCAGCGGCTTCACA; reverse: ACATCCAAACAGGAGCGTCAT) were published previously (15). As a positive control, the validity of the real-time PCR assay was confirmed by detecting iNOS mRNA in the kidneys of noninfected guinea pigs injected intraperitoneally with LPS (4 mg/kg) for 6 hours.
All data were normalized to HPRT mRNA expression and then normalized to the values derived from nongranulomatous microdissected cells. For relative percentages of cytokines expressed in Figures 3–6, the sum of the five mRNA cytokine relative induction values for each group was used to calculate the proportion of transcripts represented by each individual cytokine mRNA as we reported previously (10).
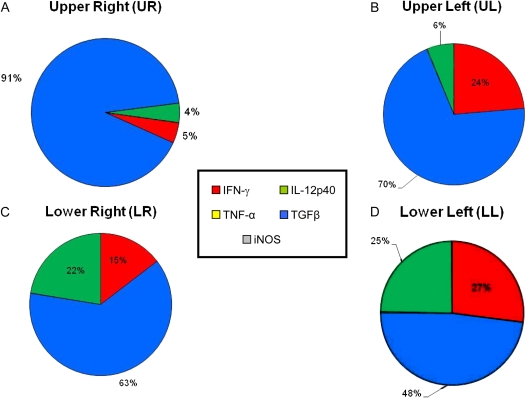
Figure 3.
Comprehensive charts mapping the relative contributions of cytokine mRNA expression to primary granulomas from the lung lobes of BCG-vaccinated guinea pigs. The sum of the relative expression levels of IL-12p40 mRNA (green), TNF-α mRNA (yellow), TGF-β mRNA (blue), IFN-γ mRNA (red), and iNOS mRNA (gray) in the primary granulomas microdissected from BCG-vaccinated guinea pigs from Figure 2 was used to calculate the relative proportion of transcripts represented by each cytokine.
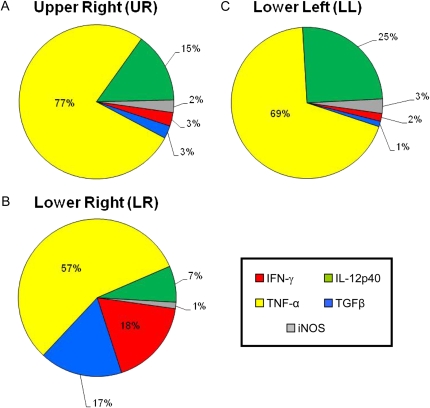
Figure 4.
Comprehensive charts mapping the relative contributions of cytokine mRNA expression to primary granulomas from the lung lobes of nonvaccinated guinea pigs. The sum of the relative expression levels of IL-12p40 mRNA (green), TNF-α mRNA (yellow), TGF-β mRNA (blue), IFN-γ mRNA (red), and iNOS mRNA (gray) in the primary granulomas microdissected from nonvaccinated guinea pigs from Figure 2 was used to calculate the relative proportion of transcripts represented by each cytokine.
Figure 5.
Cytokine mRNA expression of secondary granulomas from nonvaccinated guinea pigs aerosol-challenged with M. tuberculosis. Expression of IFN-γ, TGF-β, TNF-α, IL-12p40, and iNOS mRNA were measured from secondary granulomas microdissected from the upper left lung lobes of nonvaccinated guinea pigs 6 weeks after virulent challenge. Values for Ct were converted to “expression levels” to allow for fold comparisons between samples, expression level = 2(40-Ct). Data were normalized to HPRT rRNA followed by values derived from nongranulomatous tissues and expressed as means ± SEM (n = 8–10 capfuls of microdissected tissue from the upper left lung lobes). Inset: comprehensive chart mapping the relative contributions of cytokine mRNA expression in secondary granulomas from the upper left lung lobes of nonvaccinated guinea pigs. The sum of the relative expression levels of IL-12p40 mRNA (green), TNF-α mRNA (yellow), TGF-β mRNA (blue), IFN-γ mRNA (red), and iNOS mRNA (gray) in the secondary granulomas microdissected from nonvaccinated guinea pigs was used to calculate the relative proportion of transcripts represented by each cytokine.
Figure 6.
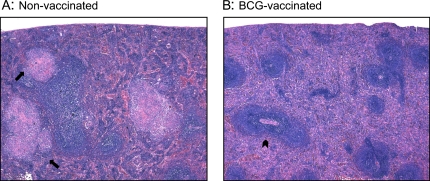
Spleens from (A) nonvaccinated and (B) BCG-vaccinated guinea pigs at 6 weeks after infection with low-dose M. tuberculosis were fixed in buffered formalin and stained with H&E. The arrows in A indicate the presence of splenic granulomas surrounding the lymphoid follicles of nonvaccinated guinea pigs. The arrowhead in B indicates the white pulp zone, located around a central arteriole, in the spleens of BCG-vaccinated guinea pigs. Total magnifications for both panels: ×500.
Statistical Analysis
ANOVA was used to determine statistical significances between mean differences of granulomas from nonvaccinated and BCG-vaccinated guinea pigs at a 95% confidence interval using Duncan post hoc analysis.
RESULTS
BCG-vaccinated and nonvaccinated guinea pigs were infected with virulent M.tb at a dose so low that not all lung lobes in each animal were infected by the airway. This infectious dose created an in vivo phenotype in which the individual lung lobes were populated with either large, primary (1°) or small, secondary (2°) lesions, or both (6). The 1° and 2° lesions were characterized according to their relative size and physical appearance as described previously (12). To determine whether the cytokine mRNA expression levels differed between 1° and 2° pulmonary tubercles, real-time PCR analysis of cells captured by laser microdissection from these granulomas was performed. Lung lobes were kept separate to determine whether the cytokine milieu was different among the two types of granulomas. The 6-week interval after infection was chosen to allow for complete hematogenous dissemination of bacilli and reseeding of the lung to occur.
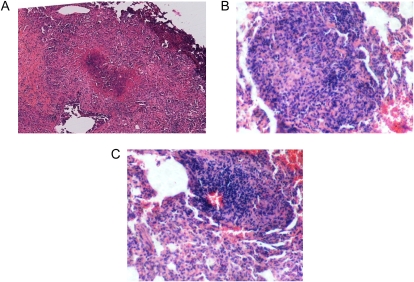
Primary granulomas from non-vaccinated guinea pigs stained with H&E were large, with an intensely necrotic center surrounded by loosely grouped scatterings of epithelioid macrophages (Figure 1A). Large, necrotic primary granulomas were not found in the UL lobes of the lung in the nonvaccinated guinea pigs. Conversely, 2° granulomas from nonvaccinated guinea pigs were smaller and remained highly cellular with clusters of fibronecrotic debris (Figure 1B). Secondary granulomas were found in all lung lobes of nonvaccinated animals. Primary lesions were found in the lung lobes of BCG-vaccinated guinea pigs, but no secondary lesions were found. These primary lesions were quite different histologically, as they were smaller than the primary lesions from nonvaccinated animals, and were non-necrotic (Figure 1C).
Figure 1.
Pulmonary granulomas fixed in buffered formalin and stained with hematoxylin and eosin (H&E) from a nonvaccinated (A and C) and bacillus Calmette-Guérin (BCG)-vaccinated (B) guinea pig. Primary lesions from nonvaccinated guinea pigs were characterized as large and necrotic (A), whereas secondary lesions from the same animals were small and non-necrotic (C). Primary granulomas from BCG-vaccinated guinea pigs were highly cellular, non-necrotic, and smaller than the primary granulomas found in the nonvaccinated group (B). Total magnification for A: ×100; for B and C: ×400.
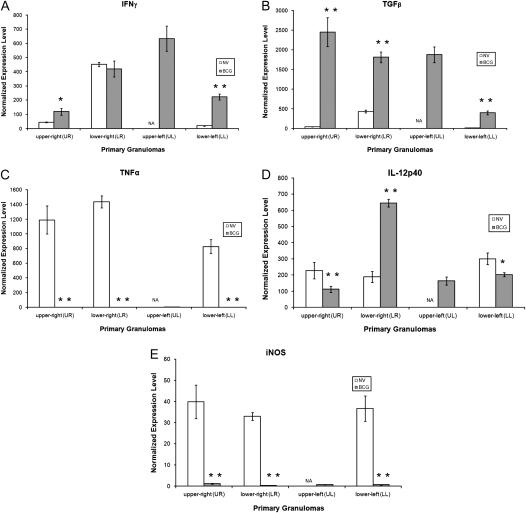
Figure 2 shows the in situ mRNA expression levels of IFN-γ, TNF-α, TGF-β, IL-12p40, and iNOS in primary lesions retrieved from the individual lung lobes of nonvaccinated and BCG-vaccinated guinea pigs infected with M.tb for 6 weeks. In general, levels of mRNA for IFN-γ and TGF-β in the primary granulomas from BCG-vaccinated guinea pigs were much higher in all lung lobes compared with the unimmunized guinea pigs (Figures 2A and 2B). For TNF-α and iNOS, mRNA expression levels were dramatically higher (20- to 1,000-fold) in the primary lesions of nonvaccinated animals than their BCG-vaccinated counterparts (Figures 2C and 2E). Primary lesions from BCG-vaccinated guinea pigs had much higher levels of IL-12p40 mRNA in the LR, but reduced levels were seen in both the UR and LL lobes of the lung (Figure 2D). Comprehensive charts (Figures 3 and 4) were constructed to illustrate the relative proportions of mRNA transcripts for each of the five cytokines measured. It is clear that 1° lesions from all lobes of the lungs from BCG-vaccinated guinea pigs are dominated by TGF-β, followed by IFN-γ and IL-12p40 cytokine mRNA (Figure 3). In primary lesions from nonvaccinated guinea pigs, TNF-α mRNA was the prevalent cytokine present (> 50%) in the mRNA pool, followed by IL-12p40 (7–25%) and TGFβ (17%) (Figure 4). No primary lesions were found in the UL lobes of nonvaccinated guinea pigs.
Figure 2.
Cytokine mRNA expression of primary granulomas from guinea pigs aerosol- challenged with Mycobacterium tuberculosis. Expression of (A) IFN-γ mRNA, (B) TGF-β mRNA, (C) TNF-α mRNA, (D) IL-12p40 mRNA, and (E) iNOS mRNA were measured from granulomas microdissected from the four lung lobes (upper right, lower right, upper left, lower left) of nonvaccinated (open bars) and BCG-vaccinated (shaded bars) guinea pigs 6 weeks after virulent challenge. Values for threshold cycle (Ct) were converted to “expression levels” to allow for fold comparisons between samples, expression level = 2(40-Ct). Data were normalized to HPRT rRNA followed by values derived from nongranulomatous tissues and expressed as means ± SEM (n = 8–10 capfuls of microdissected tissue from various regions of lung lobes). Large, necrotic, primary lesions were not found in the upper left lung lobe of nonvaccinated animals. Asterisks indicate significant (*P < 0.05) or highly significant (**P < 0.01) differences found between granulomas from nonvaccinated and BCG-vaccinated guinea pigs.
Figure 5 illustrates similar measurements taken from small, non-necrotic secondary lesions found only in the UL lung lobes of unimmunized animals. Relative mRNA expression levels of TGF-β were highest (∼ 20-fold) among the five cytokines measured, indicating a more anti-inflammatory phenotype than the primary lesions found within the same animal. The corresponding comprehensive chart reveals that the 2° lesions from nonvaccinated guinea pigs were overwhelmed with TGF-β mRNA (80%), and to a lesser extent, by IL-12p40 (11%) and IFN-γ (4%) mRNA. This cytokine profile is very different from that observed in primary lesions from the same (nonvaccinated) guinea pigs (Figure 4), but strikingly similar to that seen in the primary lesions found in BCG-vaccinated animals (Figure 3).
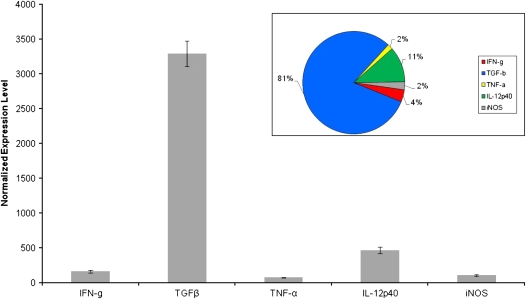
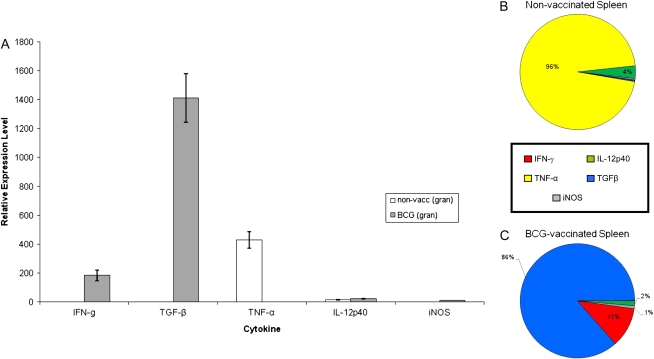
To determine the cytokine milieu of granulomas that developed in extrapulmonary organs after dissemination from the lung during the first 2 to 3 weeks of infection, granulomas were also microdissected from the spleen using LCM (Figure 6). The arrows in Figure 6A indicate the presence of splenic granulomas surrounding the lymphoid follicles of nonvaccinated guinea pigs. In contrast, fewer lesions were seen in the spleens of BCG-vaccinated animals (lesions per section: BCG = 2.3 ± 1.67, non-vacc = 10.54 ± 3.57; n = 20 sections ± SEM) and the white pulp zone, located around a central arteriole, was well-organized in the spleens of BCG-vaccinated guinea pigs (arrowhead, Figure 6B). Real-time PCR analysis of splenic granulomas from nonvaccinated guinea pigs revealed a phenotype much like that seen in the large, pulmonary granulomas from the same animals in which the cytokine mRNA pool was dominated by TNF-α and to a much lesser extent, IL-12p40 (Figure 7). Splenic lesions from previously BCG-immunized guinea pigs showed a dramatic increase (200- to 1,400-fold) in IFN-γ and TGF-β mRNA expression compared with the nonvaccinated group (Figure 7), which was similar to the profile seen in the pulmonary primary lesions found in the same animal (Figure 4). These results strongly suggest that the cytokine milieu of both pulmonary and extrapulmonary granulomas are almost identical and are driven by the pre-challenge immune status of the guinea pigs.
Figure 7.
Cytokine mRNA expression of splenic granulomas from guinea pigs aerosol-challenged with M. tuberculosis. Expression of IFN-γ, TGF-β, TNF-α, IL-12p40, and iNOS mRNA were measured from granulomas microdissected from the spleen of nonvaccinated (open bars) and BCG-vaccinated (shaded bars) guinea pigs 6 weeks after virulent challenge (A). Values for Ct were converted to “expression levels” to allow for fold comparisons between samples, expression level = 2(40-Ct). Data were normalized to HPRT rRNA followed by values derived from nongranulomatous tissues and expressed as means ± SEM (n = 8–10 capfuls of microdissected tissue from the spleen). Comprehensive charts mapping the relative contributions of cytokine mRNA expression to granulomas from the spleens of (B) nonvaccinated and (C) BCG-vaccinated guinea pigs. The sum of the relative expression levels of IL-12p40 mRNA (green), TNF-α mRNA (yellow), TGF-β mRNA (blue), IFN-γ mRNA (red), and iNOS mRNA (gray) in the splenic granulomas microdissected from nonvaccinated and BCG-vaccinated guinea pigs was used to calculate the relative proportion of transcripts represented by each cytokine.
DISCUSSION
A very low-dose aerosol infection allowed us to compare primary and secondary, bloodborne tubercles that develop after early infection in the lungs of guinea pigs (6). Primary lesions (from vaccinated and nonvaccinated guinea pigs) were clearly distinguished from secondary lesions (from nonvaccinated guinea pigs only) based on their size and cellular complexity (12). This low infectious dose allowed some lung lobes to escape without receiving an infectious particle by the airway. In these lobes, only secondary lesions were present. We chose the 6-week post-challenge interval to ensure that the secondary lesions had developed to sufficient size to allow analysis by LCM.
In situ microdissection of primary lesions by LCM clearly show that the cytokine mRNA profile in nonvaccinated guinea pigs was overwhelmed by the proinflammatory cytokine, TNF-α mRNA, and to a much lesser extent, IL-12p40 mRNA, 6 weeks after infection (Figure 4). In animals that had been immunized with BCG several weeks before prior to aerosol exposure, the cytokine milieu of primary lesions was dominated by the anti-inflammatory cytokine, TGF-β mRNA (Figure 3). These data confirm previous results from our laboratory, which demonstrated that the cytokine profile of granulomas from BCG-vaccinated guinea pigs shifted from a type 1 cytokine mRNA profile (IFN-γ, IL-12p40) at 3 weeks to a predominantly anti-inflammatory environment (TGF-β) at 6 weeks after infection, whereas the cytokine profile in unimmunized animals remained proinflammatory (TNF-α) (10).
Previous analysis of the individual lung lobes at 6 weeks after infection suggested that some heterogeneity existed in the cytokine profiles between different lobes of the lung in nonvaccinated guinea pigs, specifically the UL lobes compared with the others (10). In the current study, we were able to distinguish between the cytokine profiles of 1° and 2° lesions based on their size and location in the lungs in nonvaccinated animals. In those animals, primary and secondary lesions were found in all lobes of the lung except for the UL lobe, in which only small, 2° lesions were found. These data imply that the UL lobe of the guinea pig lung did not become implanted with the inhaled bacilli, but rather only with the disseminated bacilli, that returned to the lung via the bloodstream during the “silent bacillemia” (1, 6, 12). In contrast, only typical 1° tubercles were observed in the lung lobes of BCG-vaccinated guinea pigs and no small, 2° lesions were seen. This supports previous studies suggesting that BCG vaccination prevents or delays hematogenous reseeding of the lung after low-dose pulmonary infection of guinea pigs (16). The precise mechanism by which this occurs remains to be elucidated.
Analysis of secondary, bloodborne lesions from the lungs of nonvaccinated guinea pigs revealed a cytokine mRNA profile (predominantly TGF-β mRNA; Figure 5) that was very different from the primary tubercles (predominantly TNF-α mRNA) from the same animals, but strikingly similar to the primary lesions from the BCG-vaccinated guinea pigs (predominantly TGF-β mRNA; Figure 3). This supports the hypothesis, based upon previous data, that 2° lesions in the lungs of nonvaccinated guinea pigs behave like 1° lesions in the lungs of vaccinated guinea pigs (6, 12). At a time when the growth of bacilli in primary lesions has been controlled in the nonvaccinated animals, the bacilli in the secondary lesions from the same guinea pigs continue to increase exponentially but are controlled at a much lower (1–2 log10 CFU lower) level (6). Thus, it appears that the primary, pulmonary infection “vaccinates” the previously uninvolved portions of the lung in nonvaccinated guinea pigs, allowing those lung lobes to respond much more successfully to bacilli arriving in the blood stream. This may be due to the development of mycobacteria-specific T cells, which home quickly to portions of the lung infected by the bloodstream 3 to 4 weeks after infection. The strength of the local T cell–mediated immune response and the lack of TNF-α–mediated necrosis in secondary lesions may facilitate the response to chemotherapy at these lung sites (8).
It has been suggested that 2° lung lesions might behave like primary lesions from the spleen, since the granulomas in the spleen develop at about the same time after infection as the secondary granulomas in the lungs in guinea pigs (7). The ability of antimycobacterial drugs to significantly reduce bacillary loads in guinea pig tubercles was remarkably similar in 1° lesion-free (i.e., 2° lesion-containing) lung lobes and the spleen (17). The similarity in response to chemotherapy implied that these two types of granulomas might share other similarities (e.g., cytokine profile). However, the present study shows that the cytokine mRNA profile in the lesions found in the spleens of nonvaccinated and vaccinated guinea pigs (Figure 7) closely resembled the profiles of primary lesions retrieved from the lungs of the same animals. We expected the spleen granulomas in the nonvaccinated guinea pigs would be like 2° lung granulomas because they develop at about the same time (i.e., during the onset of acquired resistance and a strong T cell response). This discrepancy may be explained, in part, by the duration of infection studied. Smith and coworkers reported that the tubercle bacilli recovered from primary lung lesions and spleens respond similarly to chemotherapy up to 40 to 50 days after infection, which coincides with our study interval (17). Furthermore, maximum bacterial loads in the spleens of nonvaccinated guinea pigs are as high as those seen in the lung lobes in the same animals in spite of the strong T cell responses (7). Thus, the cytokine profiles in the splenic granulomas may be driven to the over expression of TNF-α by high bacillary loads, in contrast to the low bacillary loads observed in secondary lung lesions (6). Alternatively, splenic granulomas in nonvaccinated animals may not need to modulate TNF-α levels by up-regulating TGF-β like the 2° lung lesions found in the same animals (Figure 5). In other words, TGF-β may help protect air exchange by down-modulating TNF-α–induced inflammation in the lung. However, this protective function may not be necessary in the spleen, revealing a fundamental difference in the immunomodulatory mechanisms in the lung versus the spleen during mycobacterial infection. In fact, since the principal role of the spleen is to sample bloodborne antigens for activation and expansion of T and B cell populations, TGF-β production may be controlled in the spleen to prevent inadvertent suppression of immune activation.
iNOS plays an essential role in the killing of M.tb by mononuclear phagocytes in mice (18). However, its role remains to be proven in the immunopathogenesis of TB in guinea pigs. Previously, we and others have been unable to detect iNOS using the nitrate assay in supernatants of IFN-γ–activated or mycobacteria-infected guinea pig macrophages (19–21). In the current study, we were able to detect iNOS mRNA production in situ in pulmonary granulomas of nonvaccinated guinea pigs (Figure 2) using previously published real-time PCR primer sequences (15). We extracted RNA from the kidneys of unimmunized, uninfected guinea pigs injected intraperitoneally with LPS to demonstrate that our assay was capable of detecting iNOS mRNA (15). However, the relative iNOS mRNA expression levels were minimal when compared with the four other cytokine mRNA species measured (IFN-γ, TGF-β, TNF-α, and IL-12p40; Figure 4), and essentially no iNOS mRNA was detected in the granulomas of BCG-vaccinated guinea pigs, successfully controlling bacillary loads. Therefore, iNOS expression was not correlated with bacterial control in BCG-vaccinated animals. Therefore, the contribution and importance of this antimicrobial cytokine to granuloma formation and bacillary control in guinea pigs remains speculative and must be analyzed more extensively.
These data, for the first time, confirm that two fundamentally different types of lesions exist in the nonvaccinated guinea pig lung during the pathogenesis of early pulmonary TB and that cytokine mRNA profiles of the secondary, bloodborne granulomas from those animals resemble primary lesions in BCG-vaccinated guinea pigs. Further studies will be needed to determine the relationship between the cytokine profiles and bacillary loads within these two types of lesions.
This work was supported in part, by the National Institutes of Health Grant RO1 AI-15495 to D.N.M. and P30ES0910607 to the Center of Environmental and Rural Health at Texas A&M University.
Originally Published in Press as DOI: 10.1165/rcmb.2007-0326OC on November 21, 2007
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Stead WW, Bates JH. Evidence of a “silent” bacillemia in primary tuberculosis. Ann Intern Med 1971;74:559–561. [DOI] [PubMed] [Google Scholar]
- 2.Sweany HC, Cook CE, Kegerreis R. A study of the position of primary cavities in pulmonary tuberculosis. Am Rev Tuberc 1931;24:558–589. [Google Scholar]
- 3.Medlar EM. The pathogenesis of minimal pulmonary tuberculosis: a study of 1225 necropsises in cases of sudden and unexpected death. Am Rev Tuberc 1948;58:583–611. [DOI] [PubMed] [Google Scholar]
- 4.Balasubramanian V, Wiegeshaus EH, Smith DW. Mycobacterial infection in guinea pigs. Immunobiology 1994. a;191:395–401. [DOI] [PubMed] [Google Scholar]
- 5.Balasubramanian V, Wiegeshaus EH, Taylor BT, Smith DW. Pathogenesis of tuberculosis: pathway to apical localization. Tuber Lung Dis 1994. b;75:168–178. [DOI] [PubMed] [Google Scholar]
- 6.Ho RS, Fok JS, Harding GE, Smith DW. Host-parasite relationships in experimental airborne tuberculosis: VII. Fate of Mycobacterium tuberculosis in primary lung lesions and in primary lesion-free lung tissue infected as a result of bacillemia. J Infect Dis 1978;138:237–241. [DOI] [PubMed] [Google Scholar]
- 7.Smith DW, McMurray DN, Wiegeshaus EH, Grover AA, Harding GE. Host-parasite relationships in experimental airborne tuberculosis. IV. Early events in the course of infection in vaccinated and nonvaccinated guinea pigs. Am Rev Respir Dis 1970;102:937–949. [DOI] [PubMed] [Google Scholar]
- 8.Lenaerts AJ, Hoff D, Aly S, Ehlers S, Andries K, Cantarero L, Orine IM, Basaraba RJ. Location of persisting mycobacteria in a Guinea pig model of tuberculosis revealed by r207910. Antimicrob Agents Chemother 2007;51:3338–3345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith DW, Fok JS, Ho RS, Harding GE, Wiegeshaus E, Arora PK. Influence of BCG vaccination on the pathogenesis of experimental airborne tuberculosis. J Hyg Epidemiol Microbiol Immunol 1975;19:407–417. [PubMed] [Google Scholar]
- 10.Ly LH, Russell MI, McMurray DN. Microdissection of the cytokine milieu of pulmonary granulomas from tuberculous guinea pigs. Cell Microbiol 2007;9:1127–1136. [DOI] [PubMed] [Google Scholar]
- 11.Wiegeshaus EH, McMurray DN, Grover AA, Harding GE, Smith DW. Host-parasite relationships in experimental airborne tuberculosis: III. Relevance of microbial enumeration to acquired resistance in guinea pigs. Am Rev Respir Dis 1970;102:422–429. [DOI] [PubMed] [Google Scholar]
- 12.McMurray DN. Hematogenous reseeding of the lung in low-dose, aerosol-infected guinea pigs: unique features of the host-pathogen interface in secondary tubercles. Tuberculosis (Edinb) 2003;83:131–134. [DOI] [PubMed] [Google Scholar]
- 13.Cho H, Lasco TM, Allen SS, Yoshimura T, McMurray DN. Recombinant guinea pig tumor necrosis factor alpha stimulates the expression of interleukin-12 and the inhibition of Mycobacterium tuberculosis growth in macrophages. Infect Immun 2005;73:1367–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Allen SS, McMurray DN. Coordinate cytokine gene expression in vivo following induction of tuberculous pleurisy in guinea pigs. Infect Immun 2003;71:4271–4277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shirato M, Sakamoto T, Uchida Y, Nomura A, Ishii Y, Iijima H, Goto Y, Hasegawa S. Molecular cloning and characterization of Ca2+-dependent inducible nitric oxide synthase from guinea-pig lung. Biochem J 1998;333:795–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harding GE, Smith DW. Host-parasite relationships in experimental airborne tuberculosis: VI. Influence of vaccination with Bacille Calmette-Guerin on the onset and/or extent of hematogenous dissemination of virulent Mycobacterium tuberculosis to the lungs. J Infect Dis 1977;136:439–443. [DOI] [PubMed] [Google Scholar]
- 17.Smith DW, Balasubramanian V, Wiegeshaus E. A guinea pig model of experimental airborne tuberculosis for evaluation of the response to chemotherapy: the effect on bacilli in the initial phase of treatment. Tubercle 1991;72:223–231. [DOI] [PubMed] [Google Scholar]
- 18.Chan ED, Chan J, Schluger NW. What is the role of nitric oxide in murine and human host defense against tuberculosis? Current knowledge. Am J Respir Cell Mol Biol 2001;25:606–612. [DOI] [PubMed] [Google Scholar]
- 19.Sugawara I, Yamada H, Mizuno S. BCG vaccination enhances resistance to M. tuberculosis infection in guinea pigs fed a low casein diet. Tohoku J Exp Med 2007;211:259–268. [DOI] [PubMed] [Google Scholar]
- 20.Jeevan A, McFarland CT, Yoshimura T, Skwor T, Cho H, Lasco T, McMurray DN. Production and characterization of guinea pig recombinant gamma interferon and its effect on macrophage activation. Infect Immun 2006;74:213–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamada H, Udagawa T, Mizuno S, Hiramatsu K, Sugawara I. Newly designed primer sets available for evaluating various cytokines and iNOS mRNA expression in guinea pig lung tissues by RT-PCR. Exp Anim 2005;54:163–172. [DOI] [PubMed] [Google Scholar]