Abstract
We have previously shown that long-term treatment of airway smooth muscle (ASM) cells with a combination of TNF-α and IFN-γ impaired steroid anti-inflammatory action through the up-regulation of glucocorticoid receptor beta isoform (GRβ) (Mol Pharmacol 2006;69:588–596). We here found that steroid actions could also be suppressed by short-term exposure of ASM cells to TNF-α and IFN-γ (6 h) as shown by the abrogated glucocorticoid responsive element (GRE)-dependent gene transcription; surprisingly, neither GRα nuclear translocation nor GRβ expression was affected by cytokine mixture. The earlier induction of CD38, a molecule recently involved in asthma, seen with TNF-α and IFN-γ combination but not with cytokine alone, was also completely insensitive to steroid pretreatment. Chromatin-immunoprecipitation (IP) and siRNA strategies revealed not only increased binding of interferon regulatory factor 1 (IRF-1) transcription factor to CD38 promoter, but also its implication in regulating CD38 gene transcription. Interestingly, the capacity of fluticasone to completely inhibit TNF-α–induced IRF-1 expression, IRF-1 DNA binding, and transactivation activities was completely lost in cells exposed to TNF-α and IFN-γ in combination. This early steroid dysfunction seen with cytokine combination could be reproduced by enhancing IRF-1 cellular levels using constitutively active IRF-1, which dose-dependently inhibited GRE-dependent gene transcription. Consistently, reducing IRF-1 cellular levels using siRNA approach significantly restored steroid transactivation activities. Collectively, our findings demonstrate for the first time that IRF-1 is a novel alternative GRβ-independent mechanism mediating steroid dysfunction induced by pro-asthmatic cytokines, in part via the suppression of GRα activities.
Keywords: transcription factor, glucocorticoid, inflammation, asthma, mesenchymal cells
CLINICAL RELEVANCE
This research uncovered a novel molecule associated with pro-asthmatic signals involved in steroid resistance. Therefore, our findings will likely bring new insight into the development of novel therapeutic treatment of patients with steroid resistance.
Glucocorticoids (GCs) are the treatment of choice for chronic inflammatory diseases such as asthma (1). When administered in the airways, GCs may have several cellular targets that contribute to their therapeutic effectiveness in asthma management (2). Airway smooth muscle (ASM) has now been recognized as a novel player in the pathogenesis of asthma (3, 4) and might therefore be expected to be a prominent therapeutic target for inhaled steroids (5). In line with this, we and others showed that GCs were effective in abrogating the expression of a number of pro-inflammatory mediators including cytokines, chemokines, and adhesion molecules in ASM cells (5–10).
However, there are some circumstances in which ASM cell responsiveness to steroids could be dramatically reduced, especially when exposed to a mixture of inflammatory cytokines. For example, dexamethasone did not inhibit but on the contrary significantly enhanced fractalkine secretion induced by TNF-α/IFN-γ combination in ASM cells (9). We recently demonstrated that ASM cells treated for 24 hours with the same combination of TNF-α with IFNs, but not with IL-1β or IL-13, dramatically reduced steroid ability to inhibit CD38 expression (11). The failure of steroid to suppress CD38 expression could have clinical implications for multiple reasons. CD38 is an ectoenzyme that converts the cellular intermediary metabolite NAD+ to cyclic ADP ribose (cADPr), a Ca2+-mobilizing second messenger, and has been associated with a number of diseases (12). Because the CD38 pathway has been involved in ASM cell hyperresponsiveness induced by pro-asthmatic cytokines, including TNF-α (12), we and others proposed that abnormal CD38 activity and/or expression may represent a novel mechanism involved in the exaggerated airway narrowing observed in patients with asthma (13, 14). This hypothesis has been recently confirmed by Guedes and colleagues, who showed that CD38-deficient mice have reduced airway hyper-responsiveness to methacholine after IL-13 challenge (15). Thus, the lack of steroid ability to suppress induction of fractalkine or CD38 by cytokines in ASM cells is a strong experimental evidence to indicate that steroid resistance could also develop in structural airway cells. Together, these observations support the novel concept that the steroid resistance reported in a subset of individuals with asthma (2, 16–18) could result from the failure of steroids to suppress the expression of key pro-asthmatic molecules. Understanding the mechanisms mediating steroid resistance at the cellular/molecular level could have a major therapeutic relevance.
In this study, we have uncovered a novel in vitro model of inflammation-associated steroid resistance by reporting the failure of fluticasone to suppress CD38 expression in ASM cells exposed to TNF-α/IFN-γ for a short-term period (6 h). We took advantage of this model of early steroid resistance to demonstrate for the first time that activation of the transcription factor interferon regulatory factor 1 (IRF-1) not only regulates the transcriptional induction of CD38, but is also responsible for cytokine-induced steroid resistance in part via the suppression of GRα activities.
MATERIALS AND METHODS
ASM Cell Culture and Characterization
Human ASM cell culture was performed as described previously (19). Human trachea was obtained from lung transplant donors in accordance with procedures approved by the University of Pennsylvania Committee on Studies Involving Human Beings.
Flow Cytometry Analysis
Flow cytometry was performed as described previously (14). Antibody used for CD38 expression was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Fluorescein isothiocyanate–conjugated secondary antibody was bought from Jackson ImmunoResearch Laboratories (West Grove, PA). CD38 expression was expressed as the fold increases in mean fluorescence intensity over basal (untreated cells).
Reverse Transcription–Polymerase Chain Reaction Analysis
Total RNA was extracted from human ASM cells using RNeasy Mini Kit (Qiagen, Valencia, CA) as previously described (11). In preliminary experiments, we determined, for each primer pair, the melting temperature and number of amplification cycles necessary to yield the appropriate PCR product size. The PCR of CD38, GC receptor beta isoform (GRβ), IRF-1, and β-actin was performed using previously published primers (11, 14, 20). The semiquantitative PCR approach was performed in parallel by investigating the β-actin mRNA level. The intensity of the area density was analyzed using a Gel-Pro Analyzer (Media Cybernetics, Silver Spring, MD), and the final PCR product was expressed as a ratio of area density of CD38 and IRF-1 to β-actin (11).
SDS-Polyacrylamide Gel Electrophoresis and Western Blot Analysis
Immunoblot analyses for GRα, GRβ, and IRF-1 were performed as described previously (21). Anti-GRα– and anti-GRβ–specific antibodies were purchased from Affinity BioReagents (Golden, CO). Anti–IRF-1–specific antibody was bought from Santa Cruz Biotechnology. To ensure equal loading, the membranes were stripped and reprobed with anti–β-actin antibody (Santa Cruz Biotechnology).
Immunocytochemistry of GRα
Immunostaining for nuclear translocation experiments was performed as described previously (21) with the exception of the anti-GRα antibody (Affinity BioReagents). Isotype-matched antibodies (rabbit and mouse IgG from R&D Systems, Minneapolis, MN) were used as negative controls. After staining, the glass coverslips were mounted onto glass slides, examined under epifluorescence microscopy (Nikon, Tokyo, Japan), and photographed using Olympus 1X70 (Hitech Instruments, Inc., Edgemont, PA).
IRF-1–DNA Binding Activity
Nuclear extraction was performed as described previously (21). Ten micrograms of nuclear extracts were tested for IRF-1–DNA binding activity using TransAM IRF-1 kit according to the manufacturer's instructions (Active Motif, Carlsbad, CA). The results (optical densities measured at 450 nm) were expressed as a percentage of increase over untreated cells (11).
Plasmid
Constitutively active IRF-1 was kindly provided by Dr. Yokosawa (Faculty of Pharmaceutical Sciences, Hokkaido University, Japan) (22).
Transfection of ASM Cells
ASM cells were transfected using Basic Nucleofector Kit for Primary Smooth Muscle Cells according to manufacturer's instructions using Amaxa Nucleofector II device (program U-25) (Amaxa Biosystems, Cologne, Germany) (11, 23). This device enabled us to reach transfection efficiencies of 70% using pmaxGFP (green fluorescent protein) vector and over 50% using constitutively active GRβ–GFP construct (11). For the different transfection experiments, we used 2 μg of constitutively active constructs, 2 μg of glucocorticoid responsive element (GRE)-dependent secreted alkaline phosphatase (SEAP) reporter plasmid (used to monitor steroid transactivation activity) (Stratagene, La Jolla, CA), 2 μg of IRF-1–dependent luciferase reporter plasmid (Panomics, Inc., Fremont, CA), and 1 μg of β-galactosidase vector (used to normalize transfection efficiency) (Promega, Madison, WI). For all constitutively active transfection experiments, controls included the parallel use of pcDNA3 empty vector (Stratagene).
IRF-1 Small Interfering RNA
ASM cells were transfected with 100 nM with the combination of three different Silencer Pre-designed small interfering RNA (siRNA) IRF-1 or nonsilencing siRNA control (Ambion, Austin, TX). siRNA IRF-1 was introduced into ASM cells using siRNA Test Kit for Cell Lines and Adherent Primary Cells according to manufacturer's instructions (Amaxa Biosystems).
Chromatin Immunoprecipitation
To study the ability of transcription factors or co-activators to bind specific DNA sites in gene promoters, chromatin immunoprecipitation (ChIP) assay was performed, using ChIP-IT kit (Active Motif) according to the manufacturer's instructions. One portion of the soluble chromatin was used as DNA input control (to verify equal loading), and the remains were immunoprecipitated with antibody against IRF-1 or isotype-matched antibodies (Santa Cruz Biotechnology). The purified eluted DNA from the immunoprecipitated complexes of antibody–protein–DNA was then analyzed by semiquantitative PCR (22 cycles). To examine the recruitment of IRF-1 to CD38 promoter, we used primer pairs spanning CD38 promoter regions (GenBank accession number: D84284) that contain IRF-1–binding sites: 5′-AAATGGTGCTGGGAAAACTG-3′ and 5′-CCCATGCCTATGTCCTGAAT-3′. The 175-bp resulting PCR products were resolved by 2% agarose–ethidium bromide gel electrophoresis, visualized by ultraviolet light, and analyzed with Gel-Pro Analyzer (Media Cybernetics, Silver Spring, MD) (11).
SEAP, Luciferase, and β-Galactosidase Assays
The activities of SEAP, luciferase, and β-galactosidase were evaluated using Great EscAPe SEAP detection kit (Clontech, Mountain View, CA), luciferase, and β-galactosidase detection kits (Promega), respectively, according to their manufacturer's instructions (11, 21).
Materials and Reagents
Tissue culture reagents and primers were obtained from Invitrogen (Carlsbad, CA). Human recombinant (r) TNF-α and rIFN-γ were provided by Roche Diagnostics (Indianapolis, IN). Fluticasone propionate (FP) was purchased from Sigma (St. Louis, MO).
Statistical Analysis
Data points from individual assays represent the mean values of triplicate measurements. Significant differences among groups were assessed with analysis of variance (Bonferroni-Dunn test) or by t test analysis, with values of P < 0.05 sufficient to reject the null hypothesis for all analyses. Each set of experiments was performed with a minimum of three different human ASM cell lines.
RESULTS
IRF-1 Induction by TNF-α and IFN-γ Combination in ASM Cells Is Insensitive to Steroid Treatment
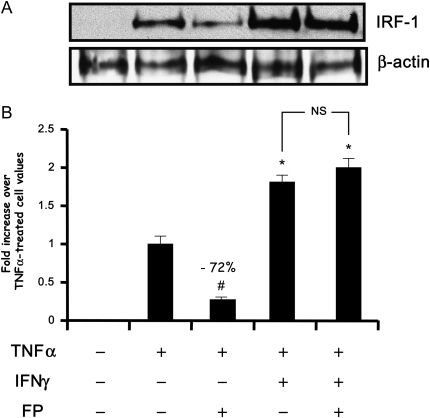
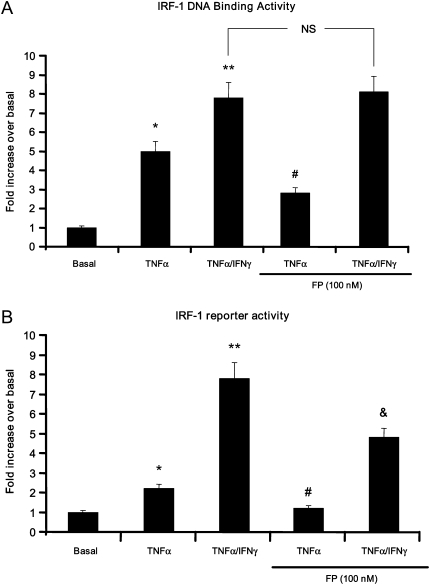
IRF-1 is an important transcriptional factor involved in the regulation of TNF-α– and IFN-inducible genes (24, 25). To date, little is known as to whether cytokines and steroids modulate IRF-1 expression and/or function in ASM cells. We first examined the effect of steroid on IRF-1 expression and activity in cells treated with cytokines. Immunoblot analysis showed that TNF-α and IFN-γ used either alone or in combination for 6 hours induced a significant increase of IRF-1 expression in ASM cells (Figure 1). IRF-1 induction was sensitive to fluticasone in TNF-α–treated cells but not in TNF-α/IFN-γ–treated cells (Figure 1). To further confirm whether IRF-1 up-regulation is functional, we next studied IRF-1 DNA-binding as well as transactivation activities. As shown in Figures 2A and 2B, IRF-1 DNA binding and reporter activities were significantly enhanced by TNF-α. Interestingly, the combination of TNF-α and IFN-γ further enhanced both IRF-1 activities, responses that were less affected by fluticasone treatment (Figure 2). Together, these data indicate that the TNF-α and IFN-γ combination induces a steroid-insensitive activation of IRF-1 in ASM cells.
Figure 1.
Fluticasone inhibits interferon regulatory factor 1 (IRF-1) expression induced by TNF-α alone but not by TNF-α/IFN-γ combination. (A) Human airway smooth muscle (ASM) cells were treated with TNF-α (10 ng/ml) either alone or in combination with IFN-γ (500 IU/ml) for 6 hours in the presence or absence of fluticasone (FP) (100 nM) added 2 hours before. Cells were then lysed and nuclear extracts were prepared and assayed for IRF-1 by immunoblot analysis. Results are representative of three separate blots. (B) Scanning densitometry of three representative immunoblots with each condition normalized over the area density of the corresponding β-actin content. The results are expressed as the fold increase over TNF-α–treated cell values. #P < 0.01 compared with TNF-α–treated cells; *P < 0.05 compared with TNF-α–treated cells; NS, not significant compared with TNF-α/IFN-γ–treated cells.
Figure 2.
Fluticasone inhibits IRF-1 DNA binding and transcriptional activities induced by TNF-α alone but not by TNF-α/IFN-γ combination. (A) Human ASM cells were treated with TNF-α (10 ng/ml) either alone or in combination with IFN-γ (500 IU/ml) for 6 hours in the presence or absence of fluticasone (FP) (100 nM) added 2 hours before. Cells were then lysed and nuclear extracts were prepared and tested for IRF-1–DNA binding activity using the TransAM/TM IRF-1 kit as described in Materials and Methods. *P < 0.05 compared with untreated cells; **P < 0.01 compared with untreated cells; #P < 0.05 compared with cells treated with TNF-α alone; NS, not significant compared with cells treated with TNF-α and IFN-γ in combination. (B) Cells were transfected with 2 μg of IRF-1 luciferase reporter vector. After 48 hours, growth-arrested ASM cells were treated as described above, then lysed, and the luciferase activity was measured as described in Materials and Methods. *P < 0.05 compared with untreated cells; **P < 0.01 compared with untreated cells; #P < 0.01 compared with cells treated with TNF-α alone; &P < 0.05 compared with cells treated with TNF-α and IFN-γ combination. Experiments were performed in triplicate using three different cell lines.
The TNF-α and IFN-γ Combination Induces an Early Suppression of Steroid Transactivation Activities Partially through IRF-1 Cellular Accumulation
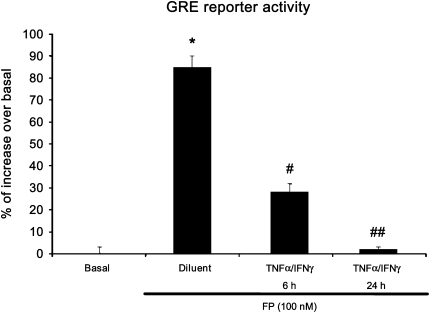
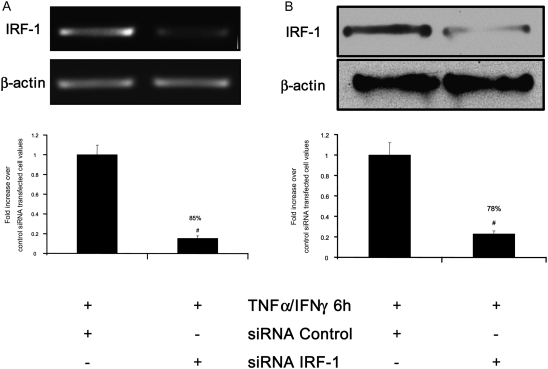
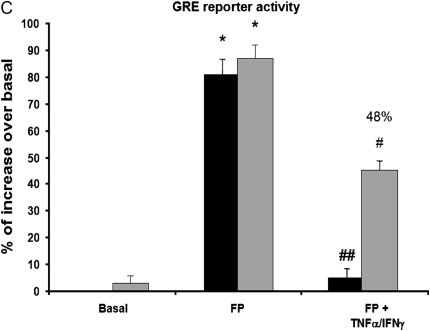
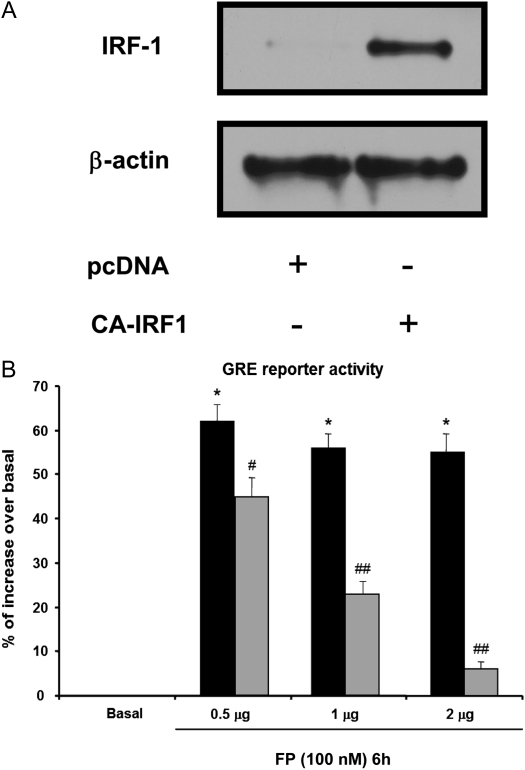
We have previously shown that cytokine combination abrogates steroid transactivation activity after 24 hours of stimulation (11). We here investigated whether similar phenomena may occur at earlier time points. In ASM cells transfected with a reporter construct containing SEAP reporter gene driven by GRE motifs, we found that TNF-α and IFN-γ combination significantly inhibits fluticasone-induced SEAP activity as early as 6 hours (68%), an inhibition that was even more pronounced at 24 hours (98% of inhibition) (Figure 3). These data suggest that impairment of steroid transactivation activity can develop in ASM cells exposed to cytokines for a shorter time period. We next investigate whether steroid-insensitive activation of IRF-1 observed at 6 hours (Figures 1 and 2) was involved in the inhibition of steroid transactivation activity seen at this early time point. To this end, IRF-1 was silenced by transfecting ASM cells with 100 nM of the combination of three different Silencer Pre-designed siRNA IRF-1 or nonsilencing, siRNA control for 48 hours before cytokines were added for another 6 hours. As shown in Figures 4A and 4B, specific siRNA IRF-1 (but not siRNA control) dramatically reduced TNF-α/IFN-γ–induced IRF-1 mRNA (Figure 4A) and protein (Figure 4B) contents. Reporter promoter assays showed that fluticasone-induced GRE reporter activity, which was completely suppressed by TNF-α and IFN-γ in siRNA control-transfected cells (Figure 4C, solid bars), was restored by more than 48% in IRF-1–deficient cells (transfected with siRNA IRF-1) (Figure 4C, shaded bars). The capacity of IRF-1 to modulate steroid transactivation activity was shown by the observation that overexpressing constitutively active IRF-1 (Figure 5A), but not the empty vector pcDNA3, inhibited in a dose-dependent manner fluticasone-induced GRE reporter activity (complete inhibition was achieved at 2 μg of constitutively active IRF-1 [Figure 5B], from 53.5 ± 5.4% to 6.72 ± 2.1% [percentage of increase over basal in cells transfected with pcDNA3 or constitutively active IRF-1, respectively]). This finding indicates that IRF-1 is an important factor mediating TNF-α/IFN-γ–induced steroid dysfunction in ASM cells.
Figure 3.
The TNF-α and IFN-γ combination induces an early inhibition of steroid-associated glucocorticoid receptor (GR)-dependent transcriptional activity. ASM cells were transfected with 2 μg of secreted alkaline phosphatase (SEAP) reporter construct containing glucocorticoid responsive element (GRE) motifs. After 48 hours, cells were treated with TNF-α (10 ng/ml) and IFN-γ (500 IU/ml) in combination for 6 hours and 24 hours in the presence or absence of fluticasone (FP) (100 nM) added 2 hours before. Cells were then lysed, and the SEAP activity was performed as described in Materials and Methods. *P < 0.05 compared with untreated cells; #P < 0.05 compared with cells treated with fluticasone alone; ##P < 0.01 compared with cells treated with fluticasone alone. Experiments were performed in triplicate using three different cell lines.
Figure 4.
IRF-1 silencing restores the steroid responsiveness in cells exposed to cytokine combination. (A and B) ASM cells were transfected with 100 nM of nonsilencing small interfering RNA (siRNA) control or with 100 nM of three different Silencer Pre-designed siRNA IRF-1 oligonucleotides for 48 hours before TNF-α (10 ng/ml) and IFN-γ (500 IU/ml) were added for 6 hours. (A) Total mRNA (2 μg) was subjected to RT-PCR with β-actin and IRF-1 primers. PCR products were separated on a 1% agarose gel and stained with ethidium bromide (top). (Bottom) Scanning densitometric of two gels with each value normalized over the mean density of the corresponding β-actin PCR products; #P < 0.01 compared with control siRNA transfected cell. (B) Total cell lysates were prepared and assayed for β-actin and IRF-1 by immunoblot analysis (top). (Bottom) Scanning densitometric of two blots with each value normalized over the mean density of the corresponding β-actin bands; #P < 0.01 compared with control siRNA transfected cell. (C) ASM cells were co-transfected with siRNA control (solid bars) or IRF-1 (shaded bars) and 2 μg of SEAP reporter construct containing GRE motifs and treated with TNF-α (10 ng/ml) in combination with IFN-γ (500 IU/ml) for 6 hours in the presence or absence of fluticasone (FP) (100 nM) added 2 hours before. Cells were then lysed, and the SEAP activity was performed as described in Materials and Methods. *P < 0.05 compared with untreated cells; #P < 0.05 compared with cells treated with fluticasone alone; ##P < 0.01 compared with cells treated with fluticasone alone. Experiments were performed in triplicate using three different cell lines.
Figure 5.
Overexpressing IRF-1 suppresses fluticasone-induced GR-dependent transactivation activity. (A) ASM cells were transfected with 2 μg of constitutively active (CA) IRF-1 construct for 48 hours. Total cell lysates were then prepared and assayed for β-actin and IRF-1 by immunoblot analysis. (B) ASM cells were co-transfected with 0.5 to 2 μg of pcDNA3 empty vector (solid bars) or CA IRF-1 construct (shaded bars) and 2 μg of SEAP reporter plasmid driven by GRE motifs. Cells were then treated with fluticasone (FP) (100 nM) for 6 hours. After that, cells were lysed, and the SEAP activity was performed as described in Materials and Methods. The results are expressed as a percentage of control values from untreated cells. Data are representative of three separate experiments. *P < 0.05 compared with untreated cells; #P < 0.05 compared with pcDNA3-transfected cells treated with fluticasone; ##P < 0.01 compared with pcDNA3-transfected cells treated with fluticasone.
Steroid Treatment Failed to Repress the Expression of IRF-1–Dependent Gene CD38 in TNF-α and IFN-γ–Treated ASM Cells
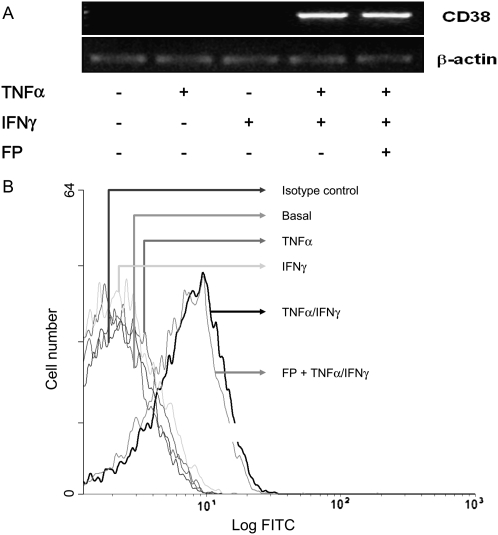
We have previously shown that steroid treatment failed to repress CD38 expression in cytokine combination-treated ASM cells at 24 hours of stimulation (11). We here investigated whether similar phenomena may occur at earlier time points (6 h). As shown in Figures 6A and 6B, while TNF-α or IFN-γ had no effect when used alone, TNF-α and IFN-γ in combination induced an earlier and significant increase of CD38 protein and mRNA contents that was completely insensitive to fluticasone.
Figure 6.
The early induction CD38 gene and protein induced by cytokine combination is insensitive to steroid action. ASM cells were treated with TNF-α (10 ng/ml) either alone or in combination with IFN-γ (500 IU/ml) for 6 hours in the presence or absence of fluticasone (FP) (100 nM) added 2 hours before. (A) Total mRNA was isolated from each condition and subjected to reverse transcriptase PCR with the primers for β-actin and CD38. PCR products were separated on a 1% agarose gel and stained with ethidium bromide. Data are representative of mRNA obtained from three different experiments. (B) CD38 protein expression was assessed by flow cytometry as described in Materials and Methods. Flow cytometry histograms are representative of three independent experiments.
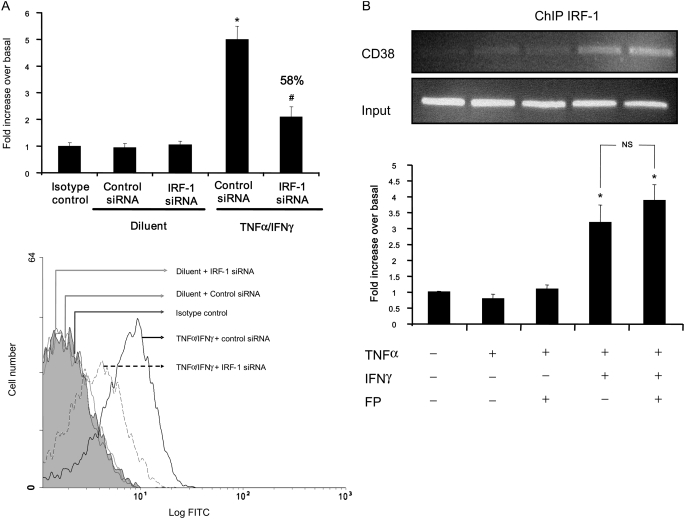
Using Motif Genome Software (http://motif.genome.jp/), we found several IRF-1–binding sites in the promoter of CD38 gene (GenBank accession number: D84284). Whether IRF-1 plays any role in regulating CD38 expression has not been demonstrated in any cell types. Flow cytometry analyses using anti-CD38 antibody showed that siRNA IRF-1 dramatically reduces the ability of TNF-α and IFN-γ to induce CD38 protein expression (∼ 70%, Figure 7A). Of note, siRNA control had no effect. To further confirm the direct role of IRF-1 in CD38 transcriptional induction, ChIP assay was used to assess the in vivo binding of IRF-1 to CD38 promoter. Interestingly, IRF-1 IPs revealed a marked enrichment of CD38 promoter DNA in cells treated with TNF-α and IFN-γ in combination for 6 hours (175-bp fragment containing IRF-1–binding sites, Figure 7B, lane 4) compared with untreated cells (lane 1), indicating IRF-1 binding to the CD38 promoter. Interestingly, fluticasone failed to repress the IRF-1 binding to CD38 promoter in TNF-α/IFN-γ–treated ASM cells (Figure 7B, lane 5 versus lane 4).
Figure 7.
IRF-1 binds to and is essential for cytokine-induced expression of CD38 gene. (A) ASM cells were transfected with 100 nM of nonsilencing siRNA control or with 100 nM of the combination of three different Silencer Pre-designed siRNA IRF-1 oligonucleotides. After 48 hours, cells were treated with TNF-α (10 ng/ml) and IFN-γ (500 UI/ml) for 6 hours. CD38 protein expression was assessed by flow cytometry as described in Materials and Methods, and the results are expressed as the -fold increase in mean fluorescence intensity over basal (untreated cells) ± SEM of three separate experiments. *P < 0.05 compared with untreated cells; #P < 0.05 compared with control siRNA-transfected cells treated with TNF-α and IFN-γ (top). (Bottom) Representative flow cytometry histograms of siRNA IRF-1 effect on CD38 expression. (B) Confluent cultures of ASM cells were treated with TNF-α either alone or in combination with IFN-γ for 6 hours in the presence or absence of fluticasone (FP, 100 nM) added 2 hours before. Chromatin fragments were immunoprecipitated with antibody against IRF-1 as described in Materials and Methods. CD38 promoter region containing IRF-1 binding sites was amplified by PCR (22 cycles). The input represents PCR products from chromatin pellets prior to immunoprecipitation (top). (Bottom) Scanning densitometric of the three ChIP assays with each value normalized over the mean density of the corresponding input. *P < 0.05 compared with untreated cells; NS, not significant compared with cells treated with TNF-α and IFN-γ in combination.
Collectively, these data demonstrate that CD38 is an IRF-1–dependent gene in TNF-α/IFN-γ–treated ASM cells. These data also suggest that steroid resistant responses (CD38 expression and IRF-1 activation) could develop in cells exposed to cytokine combination.
Early Steroid Resistance Induced by Cytokine Combination Is Not Associated with Changes in GRα Nuclear Translocation and Involves GRβ-Independent Mechanisms
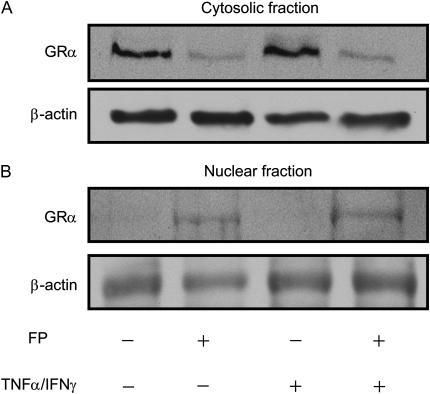
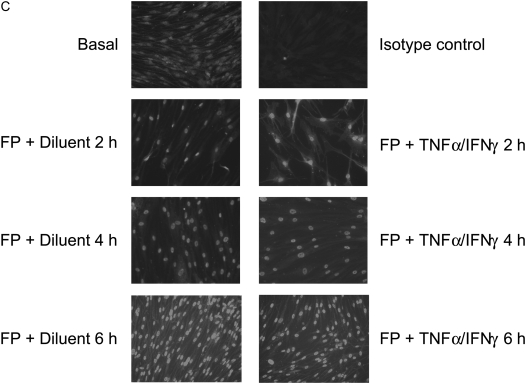
We next examined whether steroid ability to induce GRα nuclear translocation is altered after short-term incubation with cytokines. Both immunoblot (Figures 8A and 8B) and immunostaining analyses (Figure 8C) showed that fluticasone treatment induced GRα nuclear translocation, an effect that was not altered when the TNF-α and IFN-γ combination was added for 2, 4, and 6 hours.
Figure 8.
TNF-α and IFN-γ combination does not interfere with GRα subcellular trafficking. (A and B) ASM cells were treated with fluticasone (FP) for 2 hours before TNF-α (10 ng/ml) and IFN-γ (500 IU/ml) were added for another 6 hours. Then cytosolic (A) and nuclear (B) extracts were prepared and assayed for GRα by immunoblot analysis. Results are representative of three separate blots. (C) ASM cells were treated with FP for 2 hours before TNF-α (10 ng/ml) and IFN-γ (500 IU/ml) or diluent were added for another 2, 4, and 6 hours. ASM cells were then fixed, permeabilized, and assayed for GRα by immunocytochemistry using rabbit anti-GRα followed by a secondary phycoerythrin anti-IgG antibody. These results are representative of three separate experiments.
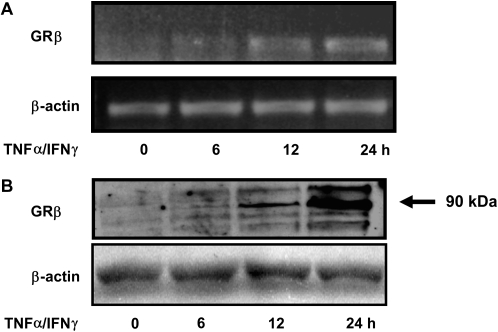
We previously showed that the TNF-α and IFN-γ combination alters steroid responses in ASM cells after 24 hours of stimulation by a mechanism involving the up-regulation of GRβ isoform (11). Interestingly, time course study using RT-PCR and immunoblot analyses failed to detect expression of GRβ after short-term treatment while still being induced at later time points (12, 24 h) (Figures 9A and 9B), suggesting that GRβ-independent mechanisms likely contribute to the development of steroid resistance induced by TNF-α/IFN-γ–treated cells after short incubation time.
Figure 9.
The TNF-α and IFN-γ combination induces a delayed expression of GRβ. (A and B) ASM cells were stimulated with TNF-α (10 ng/ml) and IFN-γ (500 IU/ml) in combination for 6, 12, and 24 hours. (A) Total mRNA (1 μg) was subjected to RT-PCR with β-actin and GRβ primers. PCR products were separated on a 1% agarose gel and stained with ethidium bromide. Results are representative of three separate RT-PCR experiments. (B) Total cell lysates were prepared and assayed for GRβ by immunoblot analysis. Results are representative of three separate blots.
DISCUSSION
In this study, we have demonstrated that the transcription factor IRF-1 is critical for the regulation of CD38 expression in response to cytokines. This finding is supported by the following observations: (1) silencing IRF-1 dramatically reduced cytokine-induced CD38 expression, and (2) cytokine treatment significantly increased the in vivo binding of IRF-1 to CD38 promoter. We also found that induction of CD38 seen at early time point after cytokine stimulation (6 h) is resistant to the suppressive action of steroids. We also provided evidence that short-term treatment with cytokines inhibits, in a GRβ-independent manner, steroid abilities to induce transactivation partially through the cellular accumulation of IRF-1. To our knowledge, this is the first report showing that cytokine-induced IRF-1 up-regulation impairs steroid functions in any cell types.
Up-regulation of CD38 is now seen as a novel factor associated with a number of disease states, including asthma (26–28). Thus, a better characterization of the mediators as well as the molecular mechanisms regulating CD38 expression could have therapeutic applications. Earlier reports using either pharmacologic inhibitors or IκB mutants demonstrated a major role played by NF-κB pathway in the transcriptional regulation of CD38 expression in different cell types such as osteoclasts and ASM (29, 30). The early induction of CD38 in ASM cells by cytokine combination observed here does not seem to involve an NF-κB–dependent pathway, since we found that NF-κB activation is dramatically inhibited in TNF-α/IFN-γ–treated cells at these time points (31). Instead, we investigated a possible role of the transcription factor IRF-1 based on multiple indirect lines of evidence: first, IFNs and retinoic acids, the classical inducers of IRF members (24, 25, 32), up-regulate CD38 expression in different cell types, including ASM cells (14, 33, 34); second, three IRF-1–binding sites are present in the 5′ flanking region of the human CD38 gene (35), although their functional role has not yet been demonstrated in any cell types. We previously reported that TNF-α induced an early and sustained activation of IRF-1 in human ASM cells (21). Here, the use of luciferase reporter construct allowed us to demonstrate that IRF-1 activation induced by TNF-α is rapid and transcriptionally active in regulating gene expression (Figures 1 and 2). More importantly, using chromatin IP and silencing strategies (Figure 7), we provide the first evidence that, upon cytokine stimulation, IRF-1 not only binds to CD38 promoter (TNF-α/IFN-γ) but also participates in the transcriptional induction of CD38 gene. We therefore identify IRF-1 as a novel transcription factor mediating cytokine-induced CD38 expression.
A number of studies mostly performed in immune cells reported that inflammatory cytokines can alter steroid cellular responses (36–39). We recently provided the first demonstration that a similar phenomenon occurred in airway resident cells. Indeed, while it is well established that CD38 expression is sensitive to steroids in ASM cells treated with TNF-α alone for 24 hours (11, 30), such expression becomes completely insensitive to the suppressive action of steroids in ASM cells treated with a combination of TNF-α and IFNs for 24 hours (11). This cellular steroid resistance seen at late time points (24 h) occurred via the induction of GRβ, which suppressed the transcriptional activity of GRα. An increased expression of GRβ isoform has also been associated with cytokine-induced steroid resistance in HelaS3 cells (40). The fact that cytokines suppress GC transcriptional actions as early as 6 hours (Figure 3), times at which GRβ is expressed at low levels (Figure 9), strongly suggests the involvement of GRβ-independent mechanisms. A defect in GRα nuclear translocation as seen in peripheral blood mononuclear cells (PBMCs) derived from steroid-resistant individuals with asthma (41) has been proposed as a possible mechanism responsible for steroid resistance in asthma. In contrast, we failed to observe any change in fluticasone-induced GRα nuclear translocation in cells treated with TNF-α and IFN-γ combination for 2, 4, and 6 hours (Figure 8), suggesting that this early steroid resistance induced by cytokines likely occurs at the nuclear level. An enhanced level of transcription factors has been shown to dramatically affect steroid responsiveness (42, 43). High levels of AP-1 were previously reported in human PBMCs of steroid-resistant individuals with asthma (42). Similarly, steroid resistance can be recreated in COS-1 cells by overexpressing NF-κB subunit p65, which inhibits the ability of steroid to induce transactivation (44, 45). Interestingly, the suppression of steroid signaling detected at early time points shown here was paralleled with a dramatic enhancement of IRF-1 levels (Figure 1) and activities (Figure 2), raising the question of whether IRF-1 could play a critical role in mediating steroid resistance induced by short-term treatment with cytokines.
IRF-1 is an early response gene involved in a diverse set of transcriptional regulatory processes (21, 25). Interestingly, a strong association was found between IRF-1 polymorphism and childhood atopic asthma (46, 47). Further, mortality associated with injection of a lethal dose of lipopolysaccharide was significantly reduced in IRF-1 knockout mice (48). In line with this, using a similar model, Zhao and colleagues showed that lipopolysaccharide-induced pro-inflammatory gene expression in the mouse lung was abolished in IRF-1(−/−) mice (49). Together, these observations indicate the essential role of IRF-1 in modulating airway inflammation. We here found that IRF-1 is both a target and regulator of GC function in human ASM cells. We showed that IRF-1 expression, IRF-1 DNA-binding activities, and IRF-1 transactivation activities were steroid-sensitive in cells exposed to TNF-α alone. We propose that interfering with IRF-1 activation is likely one of the multiple mechanisms underlying the anti-inflammatory action of steroid against CD38 expression. This is a unique finding, since the sensitivity of IRF-1 to steroid seems to be highly cell specific. In T cells and monocytes, IRF-1 induction by IFN-γ or IL-12 was completely inhibited by dexamethasone at both mRNA and protein levels (50–52). Conversely, in COS-1 cells, GRα overexpression significantly enhances IRF-1 reporter activities as well as IRF-1 protein–DNA binding activities in a dexamethasone-dependent manner (53). We here found that in cells exposed to the TNF-α/IFN-γ combination, steroid failed to suppress IRF-1 activation. It is possible that the dramatic activation of IRF-1 seen in TNF-α/IFN-γ–treated cells (shown by increased protein level and transcriptional activity, Figures 1 and 2) represents one mechanism explaining how cytokine combination reduces steroid responsiveness. This hypothesis is supported by the facts that (1) overexpressing IRF-1 levels is enough to suppress GRα transactivation activities, and (2) reducing IRF-1 levels in TNF-α/IFN-γ–treated cells partially restores steroid responsiveness. Interestingly, while GRα overexpression in COS-7 cells interfered with IRF-1–dependent gene expression in a dexamethasone-dependent fashion, no studies have shown whether IRF-1 in turn modulates GC functions. Our study is the first to suggest that IRF-1 is another transcription factor regulating GRα function in any given cell type and represents a novel mechanism underlying cytokine-induced impaired steroid function. Because protein–protein interaction between IRF-1 and GRα has been demonstrated in dexamethasone-treated COS-1 cells (53), it is possible that this represents a possible mechanism by which IRF-1 suppresses steroid function. However, further studies are needed to determine the precise transcriptional mechanisms by which IRF-1 interferes with steroid signaling in ASM cells.
To our knowledge, our report is the first to show that IRF-1 is involved in CD38 transcriptional induction, further confirming the pathogenic role of IRF-1 in modulating airway inflammation. Our report also provides novel evidence that enhanced levels of IRF-1 in cells exposed to multiple cytokines reduce steroid responsiveness. The fact that different studies showed that the expression of IRF-1 was largely increased after viral infections (25, 54), combined with the suppressive effect of IRF-1 on GC signaling (present study), may explain the reduced steroid responsiveness seen in patients with asthma experiencing viral infections (16–18). Our study opens a new area of investigation, namely, to determine the molecular mechanisms by which IRF-1 promotes steroid resistance in airway structural cells.
Acknowledgments
The authors thank Mary McNichol for her assistance in the preparation of the manuscript.
This work was supported by National Institutes of Health grants HL064063 (to Y.A.) and 1 K99 HL089409-01 (to O.T.), the University of Pennsylvania Research Foundation (to Y.A.), American Lung Association grants CI-21948-N (to Y.A.) and RG-49342-N (to O.T.), and by the Parker B. Francis Family Foundation (to O.T.).
Originally Published in Press as DOI: 10.1165/rcmb.2007-0226OC on October 18, 2007
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Lemanske RF Jr, Busse WW. 6. Asthma. J Allergy Clin Immunol 2003;111:S502–S519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barnes PJ. Corticosteroid resistance in airway disease. Proc Am Thorac Soc 2004;1:264–268. [DOI] [PubMed] [Google Scholar]
- 3.Gounni AS. The high-affinity ige receptor (fcepsilonri): a critical regulator of airway smooth muscle cells? Am J Physiol Lung Cell Mol Physiol 2006;291:L312–L321. [DOI] [PubMed] [Google Scholar]
- 4.Halayko AJ, Amrani Y. Mechanisms of inflammation-mediated airway smooth muscle plasticity and airways remodeling in asthma. Respir Physiolo Neurobiol 2003;137:209–222. [DOI] [PubMed] [Google Scholar]
- 5.Hirst SJ, Lee TH. Airway smooth muscle as a target of glucocorticoid action in the treatment of asthma. Am J Respir Crit Care Med 1998;158:S201–S206. [DOI] [PubMed] [Google Scholar]
- 6.Ammit AJ, Lazaar AL, Irani C, O'Neill GM, Gordon ND, Amrani Y, Penn RB, Panettieri RA Jr. Tumor necrosis factor-alpha-induced secretion of rantes and interleukin-6 from human airway smooth muscle cells: modulation by glucocorticoids and beta-agonists. Am J Respir Cell Mol Biol 2002;26:465–474. [DOI] [PubMed] [Google Scholar]
- 7.Amrani Y, Lazaar AL, Panettieri RA Jr. Up-regulation of icam-1 by cytokines in human tracheal smooth muscle cells involves an nf-kappa b-dependent signaling pathway that is only partially sensitive to dexamethasone. J Immunol 1999;163:2128–2134. [PubMed] [Google Scholar]
- 8.Pang L, Knox AJ. Regulation of tnf-alpha-induced eotaxin release from cultured human airway smooth muscle cells by beta2-agonists and corticosteroids. FASEB J 2001;15:261–269. [DOI] [PubMed] [Google Scholar]
- 9.Sukkar MB, Issa R, Xie S, Oltmanns U, Newton R, Chung KF. Fractalkine/cx3cl1 production by human airway smooth muscle cells: induction by ifn-gamma and tnf-alpha and regulation by tgf-beta and corticosteroids. Am J Physiol Lung Cell Mol Physiol 2004;287:L1230–L1240. [DOI] [PubMed] [Google Scholar]
- 10.Vlahos R, Stewart AG. Interleukin-1alpha and tumour necrosis factor-alpha modulate airway smooth muscle DNA synthesis by induction of cyclo-oxygenase-2: inhibition by dexamethasone and fluticasone propionate. Br J Pharmacol 1999;126:1315–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tliba O, Cidlowski JA, Amrani Y. Cd38 expression is insensitive to steroid action in cells treated with tumor necrosis factor-alpha and interferon-gamma by a mechanism involving the up-regulation of the glucocorticoid receptor beta isoform. Mol Pharmacol 2006;69:588–596. [DOI] [PubMed] [Google Scholar]
- 12.Deshpande DA, Walseth TF, Panettieri RA, Kannan MS. Cd38/cyclic adp-ribose-mediated ca2+ signaling contributes to airway smooth muscle hyper-responsiveness. FASEB J 2003;17:452–454. [DOI] [PubMed] [Google Scholar]
- 13.Amrani Y, Tliba O, Deshpande DA, Walseth TF, Kannan MS, Panettieri RA Jr. Bronchial hyperresponsiveness: insights into new signaling molecules. Curr Opin Pharmacol 2004;4:230–234. [DOI] [PubMed] [Google Scholar]
- 14.Tliba O, Panettieri RA Jr, Tliba S, Walseth TF, Amrani Y. Tumor necrosis factor-alpha differentially regulates the expression of proinflammatory genes in human airway smooth muscle cells by activation of interferon-beta-dependent cd38 pathway. Mol Pharmacol 2004;66:322–329. [DOI] [PubMed] [Google Scholar]
- 15.Guedes AG, Paulin J, Rivero-Nava L, Kita H, Lund FE, Kannan MS. Cd38-deficient mice have reduced airway hyperresponsiveness following IL-13 challenge. Am J Physiol Lung Cell Mol Physiol 2006;291:L1286–L1293. [DOI] [PubMed] [Google Scholar]
- 16.Macek V, Sorli J, Kopriva S, Marin J. Persistent adenoviral infection and chronic airway obstruction in children. Am J Respir Crit Care Med 1994;150:7–10. [DOI] [PubMed] [Google Scholar]
- 17.Vianna EO, Westcott J, Martin RJ. The effects of upper respiratory infection on t-cell proliferation and steroid sensitivity of asthmatics. J Allergy Clin Immunol 1998;102:592–597. [DOI] [PubMed] [Google Scholar]
- 18.Yamada K, Elliott WM, Hayashi S, Brattsand R, Roberts C, Vitalis TZ, Hogg JC. Latent adenoviral infection modifies the steroid response in allergic lung inflammation. J Allergy Clin Immunol 2000;106:844–851. [DOI] [PubMed] [Google Scholar]
- 19.Panettieri RA, Murray RK, DePalo LR, Yadvish PA, Kotlikoff MI. A human airway smooth muscle cell line that retains physiological responsiveness. Am J Physiol 1989;256:C329–C335. [DOI] [PubMed] [Google Scholar]
- 20.Luo XM, Ross AC. Physiological and receptor-selective retinoids modulate interferon gamma signaling by increasing the expression, nuclear localization, and functional activity of interferon regulatory factor-1. J Biol Chem 2005;280:36228–36236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tliba O, Tliba S, Da Huang C, Hoffman RK, DeLong P, Panettieri RA Jr, Amrani Y. Tumor necrosis factor alpha modulates airway smooth muscle function via the autocrine action of interferon beta. J Biol Chem 2003;278:50615–50623. [DOI] [PubMed] [Google Scholar]
- 22.Nakagawa K, Yokosawa H. Degradation of transcription factor irf-1 by the ubiquitin-proteasome pathway: the c-terminal region governs the protein stability. Eur J Biochem 2000;267:1680–1686. [DOI] [PubMed] [Google Scholar]
- 23.Haleem-Smith H, Derfoul A, Okafor C, Tuli R, Olsen D, Hall DJ, Tuan RS. Optimization of high-efficiency transfection of adult human mesenchymal stem cells in vitro. Mol Biotechnol 2005;30:9–20. [DOI] [PubMed] [Google Scholar]
- 24.Dagia NM, Harii N, Meli AE, Sun X, Lewis CJ, Kohn LD, Goetz DJ. Phenyl methimazole inhibits tnf-alpha-induced vcam-1 expression in an ifn regulatory factor-1-dependent manner and reduces monocytic cell adhesion to endothelial cells. J Immunol 2004;173:2041–2049. [DOI] [PubMed] [Google Scholar]
- 25.Kroger A, Koster M, Schroeder K, Hauser H, Mueller PP. Activities of irf-1. J Interferon Cytokine Res 2002;22:5–14. [DOI] [PubMed] [Google Scholar]
- 26.Barata H, Thompson M, Zielinska W, Han YS, Mantilla CB, Prakash YS, Feitoza S, Sieck G, Chini EN. The role of cyclic-adp-ribose-signaling pathway in oxytocin-induced ca2+ transients in human myometrium cells. Endocrinology 2004;145:881–889. [DOI] [PubMed] [Google Scholar]
- 27.Deshpande DA, White TA, Guedes AG, Milla C, Walseth TF, Lund FE, Kannan MS. Altered airway responsiveness in cd38-deficient mice. Am J Respir Cell Mol Biol 2005;32:149–156. [DOI] [PubMed] [Google Scholar]
- 28.Partida-Sanchez S, Rivero-Nava L, Shi G, Lund FE. Cd38: an ecto-enzyme at the crossroads of innate and adaptive immune responses. Adv Exp Med Biol 2007;590:171–183. [DOI] [PubMed] [Google Scholar]
- 29.Iqbal J, Kumar K, Sun L, Zaidi M. Selective upregulation of the adp-ribosyl cyclases cd38 and cd157 by tnf but not by rank-l reveals differences in downstream signaling. Am J Physiol 2006;291:F557–F566. [DOI] [PubMed] [Google Scholar]
- 30.Kang BN, Tirumurugaan KG, Deshpande DA, Amrani Y, Panettieri RA, Walseth TF, Kannan MS. Transcriptional regulation of cd38 expression by tumor necrosis factor-alpha in human airway smooth muscle cells: role of nf-kappab and sensitivity to glucocorticoids. FASEB J 2006;20:1000–1002. [DOI] [PubMed] [Google Scholar]
- 31.Keslacy S, Tliba O, Baidouri H, Amrani Y. Inhibition of tumor necrosis factor-alpha-inducible inflammatory genes by interferon-gamma is associated with altered nuclear factor-kappaB transactivation and enhanced histone deacetylase activity. Mol Pharmacol 2007;71:609–618. [DOI] [PubMed] [Google Scholar]
- 32.Wang J, Peng Y, Sun YW, He H, Zhu S, An X, Li M, Lin MC, Zou B, Xia HH, et al. All-trans retinoic acid induces xaf1 expression through an interferon regulatory factor-1 element in colon cancer. Gastroenterology 2006;130:747–758. [DOI] [PubMed] [Google Scholar]
- 33.Bauvois B, Durant L, Laboureau J, Barthelemy E, Rouillard D, Boulla G, Deterre P. Upregulation of cd38 gene expression in leukemic b cells by interferon types i and ii. J Interferon Cytokine Res 1999;19:1059–1066. [DOI] [PubMed] [Google Scholar]
- 34.Drach J, McQueen T, Engel H, Andreeff M, Robertson KA, Collins SJ, Malavasi F, Mehta K. Retinoic acid-induced expression of cd38 antigen in myeloid cells is mediated through retinoic acid receptor-alpha. Cancer Res 1994;54:1746–1752. [PubMed] [Google Scholar]
- 35.Ferrero E, Malavasi F. Human cd38, a leukocyte receptor and ectoenzyme, is a member of a novel eukaryotic gene family of nicotinamide adenine dinucleotide+-converting enzymes: extensive structural homology with the genes for murine bone marrow stromal cell antigen 1 and aplysian adp-ribosyl cyclase. J Immunol 1997;159:3858–3865. [PubMed] [Google Scholar]
- 36.Goleva E, Kisich KO, Leung DY. A role for stat5 in the pathogenesis of IL-2-induced glucocorticoid resistance. J Immunol 2002;169:5934–5940. [DOI] [PubMed] [Google Scholar]
- 37.Pipitone N, Sinha M, Theodoridis E, Goulding N, Hall M, Lanchbury J, Corrigall V, Panayi G, Pitzalis C. The glucocorticoid inhibition of lfa-1 and cd2 expression by human mononuclear cells is reversed by IL-2, IL-7 and IL-15. Eur J Immunol 2001;31:2135–2142. [DOI] [PubMed] [Google Scholar]
- 38.Spahn JD, Szefler SJ, Surs W, Doherty DE, Nimmagadda SR, Leung DY. A novel action of IL-13: Induction of diminished monocyte glucocorticoid receptor-binding affinity. J Immunol 1996;157:2654–2659. [PubMed] [Google Scholar]
- 39.Strickland I, Kisich K, Hauk PJ, Vottero A, Chrousos GP, Klemm DJ, Leung DY. High constitutive glucocorticoid receptor beta in human neutrophils enables them to reduce their spontaneous rate of cell death in response to corticosteroids. J Exp Med 2001;193:585–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Webster JC, Oakley RH, Jewell CM, Cidlowski JA. Proinflammatory cytokines regulate human glucocorticoid receptor gene expression and lead to the accumulation of the dominant negative beta isoform: a mechanism for the generation of glucocorticoid resistance. Proc Natl Acad Sci USA 2001;98:6865–6870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Matthews JG, Ito K, Barnes PJ, Adcock IM. Defective glucocorticoid receptor nuclear translocation and altered histone acetylation patterns in glucocorticoid-resistant patients. J Allergy Clin Immunol 2004;113:1100–1108. [DOI] [PubMed] [Google Scholar]
- 42.Adcock IM, Lane SJ, Brown CR, Lee TH, Barnes PJ. Abnormal glucocorticoid receptor-activator protein 1 interaction in steroid-resistant asthma. J Exp Med 1995;182:1951–1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Leung DY, Bloom JW. Update on glucocorticoid action and resistance. J Allergy Clin Immunol 2003;111:3–22. (quiz 23). [DOI] [PubMed] [Google Scholar]
- 44.Caldenhoven E, Liden J, Wissink S, Van de Stolpe A, Raaijmakers J, Koenderman L, Okret S, Gustafsson JA, Van der Saag PT. Negative cross-talk between rela and the glucocorticoid receptor: A possible mechanism for the antiinflammatory action of glucocorticoids. Mol Endocrinol 1995;9:401–412. [DOI] [PubMed] [Google Scholar]
- 45.McKay LI, Cidlowski JA. Cross-talk between nuclear factor-kappa b and the steroid hormone receptors: mechanisms of mutual antagonism. Mol Endocrinol 1998;12:45–56. [DOI] [PubMed] [Google Scholar]
- 46.Nakao F, Ihara K, Kusuhara K, Sasaki Y, Kinukawa N, Takabayashi A, Nishima S, Hara T. Association of ifn-gamma and ifn regulatory factor 1 polymorphisms with childhood atopic asthma. J Allergy Clin Immunol 2001;107:499–504. [DOI] [PubMed] [Google Scholar]
- 47.Wang TN, Chu YT, Chen WY, Feng WW, Shih NH, Hsiang CH, Ko YC. Association of interferon-gamma and interferon regulatory factor 1 polymorphisms with asthma in a family-based association study in Taiwan. Clin Exp Allergy 2006;36:1147–1152. [DOI] [PubMed] [Google Scholar]
- 48.Senaldi G, Shaklee CL, Guo J, Martin L, Boone T, Mak TW, Ulich TR. Protection against the mortality associated with disease models mediated by tnf and ifn-gamma in mice lacking ifn regulatory factor-1. J Immunol 1999;163:6820–6826. [PubMed] [Google Scholar]
- 49.Zhao Z, Qian Y, Wald D, Xia YF, Geng JG, Li X. Ifn regulatory factor-1 is required for the up-regulation of the cd40-nf-kappa b activator 1 axis during airway inflammation. J Immunol 2003;170:5674–5680. [DOI] [PubMed] [Google Scholar]
- 50.Franchimont D, Galon J, Gadina M, Visconti R, Zhou Y, Aringer M, Frucht DM, Chrousos GP, O'Shea JJ. Inhibition of th1 immune response by glucocorticoids: dexamethasone selectively inhibits IL-12-induced stat4 phosphorylation in t lymphocytes. J Immunol 2000;164:1768–1774. [DOI] [PubMed] [Google Scholar]
- 51.Hu X, Li WP, Meng C, Ivashkiv LB. Inhibition of ifn-gamma signaling by glucocorticoids. J Immunol 2003;170:4833–4839. [DOI] [PubMed] [Google Scholar]
- 52.Nunez BS, Geng CD, Pedersen KB, Millro-Macklin CD, Vedeckis WV. Interaction between the interferon signaling pathway and the human glucocorticoid receptor gene 1a promoter. Endocrinology 2005;146:1449–1457. [DOI] [PubMed] [Google Scholar]
- 53.Jiang X, Norman M, Roth L, Li X. Protein-DNA array-based identification of transcription factor activities regulated by interaction with the glucocorticoid receptor. J Biol Chem 2004;279:38480–38485. [DOI] [PubMed] [Google Scholar]
- 54.Mamane Y, Heylbroeck C, Genin P, Algarte M, Servant MJ, LePage C, DeLuca C, Kwon H, Lin R, Hiscott J. Interferon regulatory factors: the next generation. Gene 1999;237:1–14. [DOI] [PubMed] [Google Scholar]