SUMMARY
Fatty acid transport proteins (FATP) function in fatty acid trafficking pathways, several of which have been shown to participate in the transport of exogenous fatty acids into the cell. Members of this protein family also function as acyl CoA synthetases with specificity towards very long chain fatty acids or bile acids. These proteins have two identifying sequence motifs: The ATP/AMP motif, an approximately 100 amino acid segment required for ATP binding and common to members of the adenylate-forming super family of proteins, and the FATP/VLACS motif that consists of approximately 50 amino acid residues and is restricted to members of the FATP family. This latter motif has been implicated in fatty acid transport in the yeast FATP orthologue Fat1p. In the present studies using a yeast strain containing deletions in FAT1 (encoding Fat1p) and FAA1 (encoding the major acyl CoA synthetase (Acsl) Faa1p) as an experimental platform, the phenotypic and functional properties of specific murine FATP1-FATP4 and FATP6-FATP4 protein chimeras were evaluated in order to define elements within these proteins that further distinguish the fatty acid transport and activation functions. As expected from previous work FATP1 and FATP4 were functional in the fatty acid transport pathway, while and FATP6 was not. All three isoforms were able to activate the very long chain fatty acids arachidonate (C20:4) and lignocerate (C24:0), but with distinguishing activities between saturated and highly unsaturated ligands. A 73 amino acid segment common to FATP1 and FATP4 and between the ATP/AMP and FATP/VLACS motifs was identified by studying the chimeras, which is hypothesized to contribute to the transport function.
Keywords: FATP, transport, activation, protein chimera
INTRODUCTION
The fatty acid transport protein (FATP) family of proteins are emerging as central components in pathways involving the transport and trafficking of fatty acids and bile acids, including the activation of these compounds by esterification with coenzymeA. In mammalian systems, six distinct members of this family have been described. These proteins are also found in non-mammalian systems including Drosophila melanogaster, Caenorhabditis elegans, Saccharomyces cerevisiae and Mycobacteria tuberculosis attesting to their importance in overall fatty acid homeostasis [1–3]. Within mammalian systems, several members of this family (FATP1, FATP2, FATP4 and FATP6) have been shown to participate in the transport of exogenous fatty acids into the cell [2–11]. Additionally, some also condense either very long chain fatty acids (FATP1, FATP2, FATP3 and FATP4) or bile acids (FATP5) with CoA for downstream metabolism [8, 12–18]. The first FATP (now designated FATP1) was identified using expression cloning from a cDNA library prepared from murine 3T3-L1 adipocytes, directly demonstrating the physiological role of this protein in the net accumulation of fatty acids across a biological membrane [9]. The mammalian FATPs have broad tissue distribution, but there is a preference for specific isoforms in different tissues. FATP1 for example is highly expressed in skeletal muscle, but poorly expressed in intestine. FATP4, by comparison is highly expressed in intestine, skin and placenta, while FATP6 is highly expressed in heart [3, 7, 10].
The different FATP isoforms are well-conserved and have two domains that serve as distinguishing sequence elements or motifs: The ATP/AMP motif, common to all adenylate-forming enzymes, and the FATP/VLACS motif, which is restricted to this group of proteins. The ATP/AMP motif consists of an approximately 100 amino acid residue segment and contains a hydrophobic region that may associate with the membrane. Specific sequence elements within this motif are found within the larger family of adenylate-forming enzymes and mutational studies on members of the FATP and Acsl families of enzymes have confirmed their importance to function, including ATP binding [19–22]. The only studies reported to date defining the FATP/VLACS motif come from experiments on the yeast FATP orthologue, Fat1p, using directed mutagenesis [22]. These data are consistent with the proposal that this motif contributes to fatty acid transport. Several FATPs are localized, at least in part, to the plasma membrane further supporting their functional role in fatty acid transport [7, 9, 11, 23–25]. The transmembrane domains within the FATPs are amino-terminal proximal; one transmembrane domain has been identified in FATP1 [23] while two have been identified in Fat1p [26]. Both topological studies place the highly conserved ATP/AMP and FATP/VLACS motifs on the cytoplasmic face of the plasma membrane.
In our previous work, we used the yeast S. cerevisiae with deletions in FAT1 (encoding Fat1p, the yeast FATP orthologue) and FAA1 (encoding the major Acsl Faa1p) as a model system to express and characterize the individual murine FATP isoforms. These studies revealed the different FATPs had distinguishing biochemical properties when evaluated in yeast. Of particular note were the findings that FATP1, FATP2 and FATP4 supported fatty acid transport while FATP3, FATP5 and FATP6 did not [6]. We also evaluated the ability of these proteins to activate highly unsaturated very long chain (arachidonate, C20:4) and saturated very long chain (lignocerate, C24:0) fatty acids. FATP6 and FATP4 had 10- and 20-fold increases in C20:4-CoA synthetase activities respectively when compared to the null. Of the FATP isoforms, FATP4 was able to activate C24:0 at a rate nearly 10-fold higher than FATP6 and nearly 4-fold higher than FATP1. In this experimental system the Acsl Faa4p is intact and contributes the long chain acyl CoA synthetase activity. When the different FATP isoforms were expressed, the C18:1-CoA synthetase activity remained unchanged or was only slightly elevated (e.g., only up to 1.5-fold for FATP6) supporting the conclusion that long chain fatty acids are not the preferred substrates for the activation function of the FATPs. An important finding from these studies was a lack of correlation between fatty acid transport and the patterns of very long chain fatty acid activation. These results therefore were similar to those obtained employing directed mutagenesis of the FAT1 gene of yeast demonstrating the fatty acid transport and activation functions within this family of proteins are distinguishable. In the present work, we chose to generate protein chimeras between FATP1, FATP4, and FATP6 to further define elements within this family of proteins that contribute to fatty acid transport, fatty acid activation, or both. These studies demonstrate the transport and activation functions within these members of the FATP family can be distinguished. Further, the use of the different chimeras has identified a 73 amino acid region between the ATP/AMP and FATP/VLACS motifs common to FATP1 and FATP4 that is likely to be essential for fatty acid transport.
MATERIALS AND METHODS
Strains, media, and materials
The S. cerevisiae strain LS2086 with deletions within the FAT1 and FAA1genes (fat1Δ faa1Δ; MATa ura3-52 his3Δ200 ade2-101 lys2-801 leu2-3,112 faa1::HIS3 fat1Δ::G418) was used as the host for all of the studies detailed in this work [27]. Yeast expression plasmids encoding murine FATP1 (pDB282), FATP4 (pDB285) and FATP6 (pDB289) have been previously described [6]; the plasmids encoding the FATP chimeras used in this study are described below. Yeast cells were transformed using lithium acetate [28]. Yeast supplemented minimal media contained 0.67% yeast nitrogen base (YNB), 2% dextrose (YNBD), adenine (20 mg/L), uracil (20 mg/L), and amino acids as required. To induce protein expression, cells were grown in YNB containing 2% galactose and 2% raffinose (YNBGR). Growth in YNBGR media was monitored by optical density at 600nm; for all experiments cells were in mid-log phase at 30°C (1 × 107 cells/ml). For complementation studies, cells were grown at 30°C on YNBGR agar plates supplemented with 45μM cerulenin and 100μM oleate. Plasmids were maintained and propagated in the E. coli strain C600. Plasmids were purified using Qiagen kits and were sequenced to confirm the different chimeric constructs.
Yeast extract, yeast peptone, and yeast nitrogen base were obtained from Difco. Oleic acid was obtained from Sigma. 4-Difluoro-5-methyl-4-bora-3a,4a-diaza-s-indacene-3-dodecanoic acid (C1-BODIPY-C12) was purchased from Molecular Probes. Enzymes required for all DNA manipulations were from Promega, Invitrogen, or New England BioLabs. PCR primers for plasmid construction and sequencing were purchased from Integrated DNA Technologies. Arachidonic acid [5, 6, 8, 9, 11, 12, 14, 15-3H(N)] (92 Ci/mmol) was obtained from Perkin Elmer. Lignoceric acid [1-14C] (44 mCi/mmol) was obtained from Movarek Biochemicals, Inc.
Construction of expression plasmids encoding chimeras of mmFATP1, mmFATP4 and mmFATP6
The yeast expression vector pDB121 and the expression plasmids encoding murine FATP1, FATP4 and FATP6 tagged with a T7 epitope have previously been described [6, 22]. The expression plasmids encoding the chimeric constructs were generating using overlap extension PCR [29]. Three sites were chosen as juncture points in the construction of the FATP chimeras: I – at the first half of the ATP/AMP motif (between residues Y246 and I247 of FATP1); II – at the beginning of the FATP/VLACS motif (between residues Y503 and F504 of FATP1); and III – at the end of the FATP/VLACS motif (between residues M553 and A554 of FATP1). The five different FATP chimeras shown in Figure 1 were constructed. All PCR reactions were carried out according to cycling and reagent conditions specified by the manufacturer. The completed plasmid constructs were sequenced to verify the junctions within the chimeric constructs and to insure no mutations were generated in the PCR reactions.
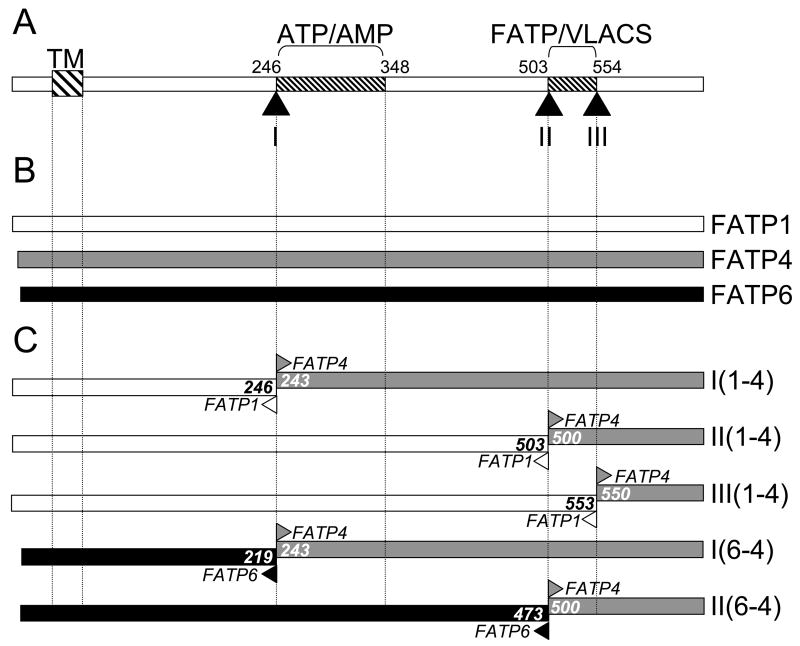
Figure 1. Schematic diagrams of FATP1, FATP4, FATP6 and the FATP1-FATP4 and FATP6-FATP4 chimeras.
A. Functional domains within FATP; the bold-hatched box (TM) corresponds to the region of the protein shown to contain a least one transmembrane spanning region, ATP/AMP corresponds to the highly conserved ATP/AMP motif that is involved in ATP binding and adenylate formation and FATP/VLACS corresponds to the conserved FATP/VLACS motif that contributes to fatty acid transport. The numbers correspond to residues in FATP1, the black triangles noted I, II, and III correspond to juncture points for the different chimeric constructs. B. Domain orientation of FATP1 (white), FATP4 (gray) and FATP6 (black). The Dashed lines extending from A show the positions of the conserved motifs and TM region. C. FATP1-FATP4 and FATP6-FATP4 chimeras. The junction points are noted by arrowheads pointing in opposite directions; white – FATP1; gray – FATP4; and black – FATP6; the numbers correspond to the last residue before the junction point and the first residue after the junction point. The nomenclature of the different constructs is given on the right.
Expression of FATPs and FATP chimeras
Cells (fat1Δ faa1Δ) transformed with pDB121 (vector) or plasmids encoding FATP1, FATP4, FATP6 or the different chimeric constructs were grown under inducing conditions in YNBGR (with appropriate supplements) to mid-log phase for all experiments. In all experiments, the vector control and FATP4 expression were included as controls. The expression of the target proteins was monitored using western blots probed with anti-T7 antibody as previously detailed [6]. Each lane on the SDS gel was adjusted to contain 20 μg total cellular protein. The images from the western blots were scanned using Epson Perfection 3170 Photo and the protein expression of the different FATPs and FATP chimeras was quantified using NIH Image (1.63). The expression of FATP4, included on each gel as a control (with the 1-4 or 6-4 chimeras) was assigned an arbitrary value of 1.
Determination of acyl-CoA synthetase activities
Acyl-CoA synthetase activity in cell extracts expressing FATP1, FATP4, FATP6 or the chimeric derivatives were measured using standard conditions as previously described using the very long chain fatty acids arachidonate (C20:4) or lignocerate (C24:0) as substrates [6]. The standard reaction mixtures contained 0.1–0.2 mg protein in the cell extract in 50mM Tris/HCl, pH 8.0, 10mM ATP, 10mM MgCl2, 0.3mM dithiothreitol (DTT), 0.001% Triton X-100, 50μM [14C] or [3H] fatty acid (prepared as free fatty acid 10X stocks in 10mg/ml α-cyclodextrin) and 200μM coenzyme A (CoA) in a final volume of 500μl. The reaction was initiated by the addition of CoA at 30°C, continued for 15min and terminated by the addition of 2.5ml of isopropanol/η-heptane/1M H2SO4 (40:10:1). The free fatty acid was removed by sequential organic extractions using η-heptane. Acyl-CoA formed during the reaction remained in the aqueous fraction and was quantified by scintillation counting. Protein concentrations were determined using the Bradford method [30]; all data were transformed into pmol acyl CoA formed/min/mg total cellular protein. The data presented are the means from at least three independent experiments. Data were analyzed by analysis of variance using JMP Software (SAS Institute Inc., Cary, NC).
Fatty acid transport monitored using the fluorescent fatty acid C1-BODIPY-C12
Following growth under inducing conditions as detailed above, cells were harvested and resuspended to 6 × 107 cells/ml. Accumulation of C1-BODIPY-C12, as an indicator or fatty acid transport, was monitored using confocal laser-scanning microscopy [6, 22, 25, 31]. Cells were incubated with 5μM C1-BODIPY-C12/5μM fatty acid free BSA for 3min, washed with PBS containing 15μM fatty acid-free bovine serum albumin 3 times, resuspended in PBS and placed on a poly-L-lysine-treated multiwell slide (ICN, Fisher Scientific, Pittsburgh, PA, USA) and visualized using confocal laser scanning microscopy. This time period results in labeling lipids with C1-BODIPY-C12, which necessarily required the production of C1-BODIPY-C12-CoA via vectorial acylation [32]. The instrument settings for brightness and contrast were optimized to ensure that the confocal laser-scanning microscope was set for full dynamic range relative to cells with no expression of any of the FATP isoforms (fat1Δ faa1Δ cells transformed with the vector pDB121) to those expressing FATP1 (fat1Δ faa1Δ cells transformed with pDB282 (encoding FATP1). The same settings were used for all subsequent image collections. An argon laser source was used for imaging with excitation at 488 nm and emission at 505 nm. For quantitative determinations strain LS2086 transformed with expression clones for FATP1, FATP4, FATP6 or one of the FATP chimeras were grown under inducing conditions as detailed above. Cells were harvested in mid-log phase and resuspended in PBS at a cell density of 6 × 107ml before dispensing to a 96-well assay plate as detailed by Li et al. [33]. To each well, a mixture of C1-BODIPY-C12 (final concentration 1.25μM), fatty acid free BSA (final concentration 2.5μM) and trypan blue (final concentration. 2.1mM) was added to give a final volume of 100μl. Trypan blue quenches the extracellular fluorescence; cell-associated fluorescence, which is reflective of transport, was measured after 3min using a Bio-Tek Synergy HT multi-detection microplate reader (Bio-Tek Instruments, Inc., Winooski, VT, USA) with filter sets of 485±20 nm excitation and 528±20 nm emission. This method allows fatty acid transport to be measured in real-time with no intervening washing steps. Data were converted to pmol C1-BODIPY-C12 transported/3min/1 × 108 cells using standard curves of varying C1-BODIPY-C12 concentrations versus fluorescent emission values. All data presented represent the mean from at least four independent experiments. Data were analyzed by analysis of variance using JMP Software (SAS Institute Inc., Cary, NC).
RESULTS
Construction of FATP chimeras
In order to assess functional elements within these proteins and to determine how they contribute to function, the FATP1-FATP4 and FATP6-FATP4 chimeras illustrated in Figure 1 were constructed using overlap extension PCR. FATP1 and FATP4 were chosen as they function in the fatty acid transport pathway; FATP6 was chosen as it does not function in fatty acid transport, but like FATP1 and FATP4 has very long chain activating activity. The sites of fusion were at the beginning of the highly conserved ATP/AMP motif (site I); at the beginning of the highly conserved FATP/VLACS motif (site II) or at the end of the FATP/VLACS motif (site III). In each of the chimeras, the conserved motifs remained intact (Figure 2), which allowed us to address the functional contributions of the less well-conserved region between the two motifs to transport and very long chain fatty acid activation. Previous work from our laboratory and from Shaffer’s laboratory has demonstrated the importance of both of these highly conserved motifs with regards to function [19–22]. Five different chimeras were constructed: I(1-4), FATP1-FATP4 with the juncture at site I; II(1-4), FATP1-FATP4 with the juncture at site II; III(1-4), FATP1-FATP4 with the juncture at site III; I(6-4), FATP6-FATP4 with the juncture at site I; and II(6-4), FATP6-FATP4 with the juncture at site II (Figure 1).
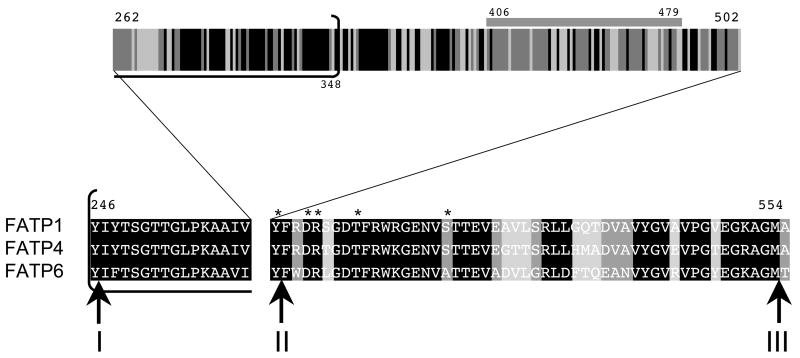
Figure 2. Sequence alignments and elements within the conserved ATP/AMP and FATP/VLACS motifs.
The sequences of FATP1, FATP4 and FATP6 are given with numbers corresponding to FATP1 beginning at amino acid 246. The ATP/AMP motif extends from 246–348 and is bracketed and underlined; the FATP/VLACS motif is from 503 – 554 (right sequence). Regions containing amino acid residues that are identical or highly conserved are black; regions with sequences that are shared by FATP1 and FATP4 are dark gray; regions with sequences that are not shared between FATP1, FATP4 and FATP6 are light gray. The juncture points for the different chimeric constructs are noted by the bold arrows and designated I, II, or III. The horizontal bar 406–479 represents the region that is highly conserved between FATP1 ad FATP4 and divergent with FATP6. The asterisks correspond to residues that when replaced by alanine in the yeast FATP orthologue, Fat1p, results in a mutant protein that did not function in fatty acid transport.
Expression and complementation of the fat1Δ faa1Δ strain
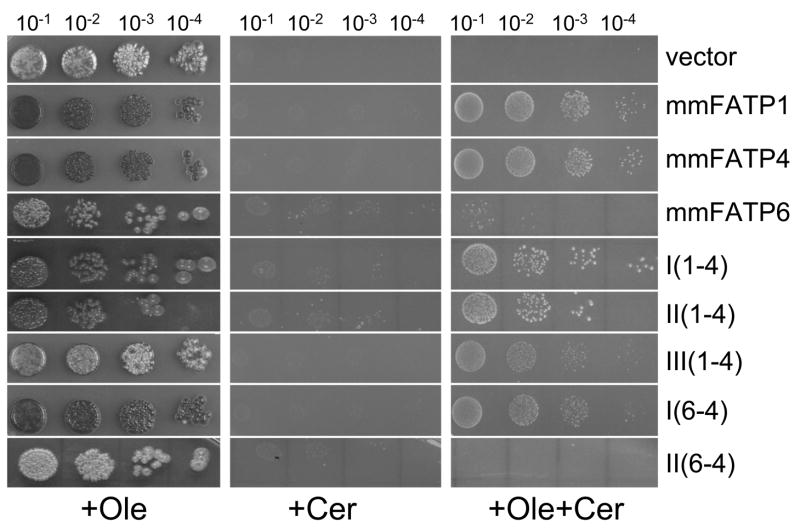
Each of the constructs encoding the FATP chimeras along with those for FATP1, FATP4 and FATP6 were transformed into the fat1Δ faa1Δ strain of yeast, expressed and analyzed for function in accordance with previous studies [6]. The expression of each of these proteins was under the control of the GAL10 promoter of yeast; inclusion of 2% galactose as detailed in Materials and Methods resulted in comparable levels of expression of the native and chimeric proteins (Figure 3). We initially evaluated each of these chimeras along with the native FATPs for complementation of the fat1Δ faa1Δ strain on plates containing [1] oleate, [2] cerulenin (to block endogenous fatty acid synthesis thereby generating a conditional auxotrophy for long chain fatty acids) and [3] oleate plus cerulenin (Figure 4). In line with our previous work, FATP1 and FATP4 fully complemented the fat1Δ faa1Δ strain on the oleate cerulenin plates while FATP6 did not. All three of the FATP1-FATP4 chimeras (I(1-4), II(1-4) and III(1-4)) were able to complement the fat1Δ faa1Δ strain indicating the ability to transport oleate into the cell remained intact. The FATP6-FATP4 constructs gave differing results. The I(6-4) chimera was able to complement the fatty acid transport defects as shown by growth on the oleate cerulenin plates while II(6-4) did not. The I(6-4) chimera contains the first 219 residues of FATP6 and the last 400 residues of FATP4; II(6-4) has the first 473 residues of FATP6 and the last 143 residues of FATP4. These data suggest the region between sites I and II, which includes the ATP/AMP motif also contains distinguishing sequence elements that contribute to the fatty acid transport property of FATP4. This region of the protein contains the complete ATP/AMP motif in addition to other regions having significant amino acid identity between FATP1, FATP4 and FATP6 (Figure 2). Of particular note from these studies was the identification of a 71–73 amino acid residue segment that was essentially identical in FATP1 and FATP4, but divergent in FATP6 (residues 406–479 in FATP1, noted by the horizontal gray bar in Figure 2).
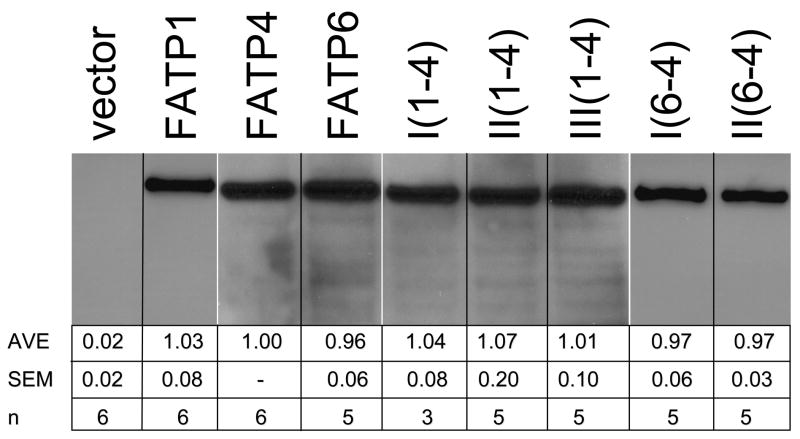
Figure 3. Western blot showing expression of FATP1, FATP4, FATP6 and the different FATP1-FATP4 and FATP6-FATP4 chimeras.
20 μg of total protein (from cells expressing FATP1, FATP4, FATP6 and the different chimeras) was used per lane on the SDS gel prior to the western blot. Using NIH Image, the blots were scanned and expression levels of each defined relative to FATP4, which was included on each gel. AVE – average expression values of FATP1, FATP6 or the different chimeras relative to FATP4 (set to 1); SEM – standard error of the mean with replicates (n) noted. The white vertical lines denote that different blots were used to generate this composite figure.
Figure 4. Complementation patterns of FATP1, FATP4, FATP6 and the different FATP when expressed in the faa1Δ fat1Δ strain of S. cerevisiae.
A. Growth patterns on minimal YNBGR plates supplemented with 100μM oleate (C18:1)(+Ole); B. Growth patterns on YNBGR plates containing 45μM cerulenin +Cer); C. Growth patterns on YNB-Gal plates supplemented with 100μM C18:1 and containing 45μM cerulenin (+Ole+Cer). Cells were grown to mid-logarithmic phase (A600 = 1.0) in YNBD, harvested, resuspended in the original volume of YNB and diluted 10−1, 10−2, 10−3 and 10−4 in YNB. Three microliters of the original volume and each dilution were spotted to the plates noted above, which were then incubated up to 120 hr at 30°C.
Fatty acid transport properties of the FATP chimeras
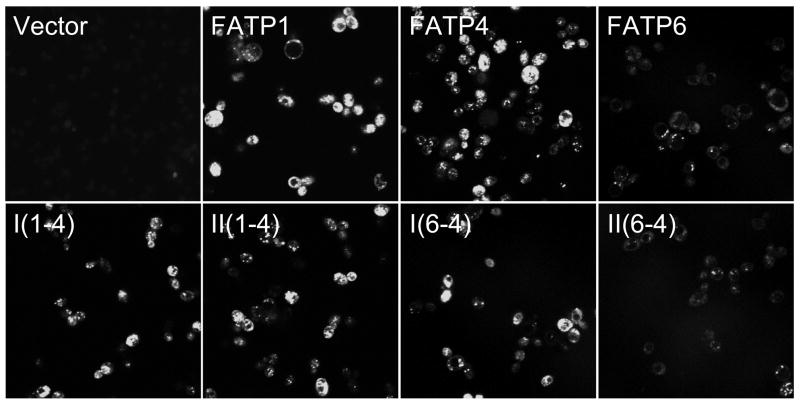
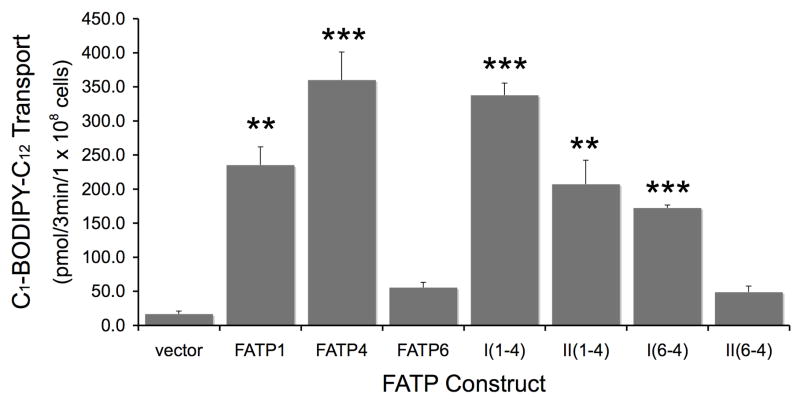
The complementation data provided some insights into regions likely to contribute the transport properties in each of the FATP chimeras. In order to evaluate this further, we expressed each chimera along with FATP1, FATP4 or FATP6 in the fat1Δ faa1Δ strain and defined the patterns of fatty acid transport using the fluorescent long chain fatty acid analogue C1-BODIPY-C12 using confocal microscopy as detailed in Materials and Methods (Figure 5). C1-BODIPY-C12 is rapidly incorporated into complex lipids following transport [32]. These experiments mirrored the complementation data: FATP1 and FATP4 were able to transport the C1-BODIPY-C12 while FATP6 did not; I(1-4), II(1-4) and I(6-4) were proficient at transport while II(6-4) was not. We next completed experiments to quantify the uptake of the fluorescent long chain fatty acid in real time using fluorometry to address whether there were more subtle differences in the transport levels that could not be detected using confocal microscopy (Figure 6). The results of these experiments showed that FATP4 was the most proficient at fatty acid transport; FATP6-mediated levels of transport were only slightly higher than the vector control but not of statistical significance. Only chimera I(1-4) gave levels that were equivalent to FATP4. Chimera II(1-4) was more equivalent to FATP1, while II(6-4) was similar to FATP6. The finding that I(6-4) was able to facilitate transport while II(6-4) was not, is consistent with the conclusion that the region of FATP4 between the ATP/AMP and FATP/VLACS motifs contributes substantially to the fatty acid transport function. Previous directed mutagenesis studies on the yeast FATP orthologue Fat1p have shown specific amino acid residues within the FATP/VLACS motif contribute to transport [22]. Of particular note in these previous studies were alanine substitutions in Fat1p corresponding to Y503, D506, R507, F512 and S520 in FATP1 that essentially eliminated transport (asterisks as noted in Figure 2) [22]. It is of interest to note that FATP6, which does not function in fatty acid transport in yeast, contains an alanyl residue corresponding to S520. The transport data generated using the chimeras is consistent with the conclusion that additional specific elements between these highly conserved motifs also contribute to fatty acid transport and are likely common between FATP1 and FATP4, but divergent with FATP6. As noted above and illustrated in Figure 2, this region contains a stretch of 71–73 amino acid residues (406–479 in mmFATP1) that is essentially identical between FATP1 and FATP4, but is divergent in FATP6. The finding that II(6-4) did not facilitate transport indicates the FATP/VLACS motif from FATP4 was insufficient, further supporting the conclusion that FATP6 must lack specific elements within this 71–73 amino acid region (Figure 7). We conclude from these data, the divergent 71 amino acid residue region unique to FATP6 results in an FATP isoform unable to facilitate transport.
Figure 5. Transport of the long-chain fatty acid analogue C1-BODIPY-C12 monitored using confocal microscopy in the faa1Δ fat1Δ strain of S. cerevisiae expressing A. FATP1, FATP4, FATP6 and the different FATP chimeras.
Shown are representative images from at least 5 independent experiments for each construct.
Figure 6. Quantitative fatty acid transport monitored using a live cell assay in the faa1Δ fat1Δ strain of S. cerevisiae expressing FATP1, FATP4, FATP6, and FATP chimeras and the fluorescent long chain fatty acid C1-BODIPY-C12.
The error bars indicate the standard error of the mean (n=4); **p≤0.01; ***p≤0.005 relative to vector control.
Figure 7. Alignment of the region between FATP1, FATP4 and FATP6 likely to contribute to the differential activities of these proteins.
This region corresponds to the area noted by the horizontal bar in Figure 2 and includes residues 406–479 of FATP1. The asterisks denote identical residues between FATP1, FATP4 and FATP6.
Acyl CoA synthetase activities of the FATP chimeras
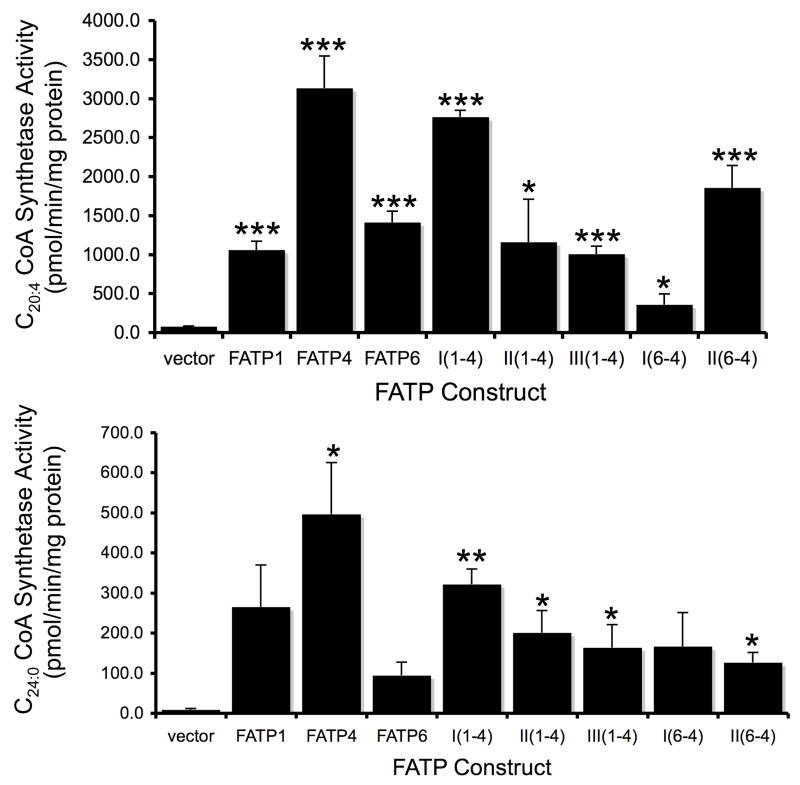
We next addressed whether there were changes in the acyl CoA synthetase activities of the different chimeras using highly unsaturated fatty very long chain (arachidonate, C20:4) and saturated very long chain (lignocerate, C24:0) fatty acids. Our previous work had demonstrated essentially no differences in the C18:1-CoA synthetase activities in fat1Δ faa1Δ cells expressing FATP1, FATP4 and FATP6 [6] and thus we did not evaluate long chain fatty acid substrates; in this experimental system the long chain acyl CoA synthetase activity comes from the endogenous Acsl, Faa4p. The most interesting data came from studies using C20:4 as the very long chain fatty acid substrate. In agreement with our previous work, all three FATP isoforms had C20:4-CoA synthetase activity with FATP4 being the highest and FATP1 and FATP6 having similar activities (Figure 8). The I(1-4) chimera had C20:4-CoA synthetase activity comparable to FATP4. As was the case in fatty acid transport, this indicates the region from the ATP/AMP motif to the carboxyl terminus of FATP4 contributes this activity. When the amino terminus and the ATP/AMP and FATP/VLACS motifs were derived from FATP1 (II(1-4) and III(1-4)), the activities were more like FATP1 indicating the functional importance of the region between the two highly conserved motifs as contributing to fatty acid activation. Thus, like transport, this region plays a crucial role in the activation of very long chain highly unsaturated fatty acids. The data from the FATP6-FATP4 chimeras were of particular interest. I(6-4) had low levels of C20:4-CoA synthetase activity while II(6-4) gave levels intermediate between FATP6 and FATP4. When the region of mmFATP4 including the FATP/VLACS motif to the carboxyl terminus of the protein was linked to the amino terminus of FATP6 (II(6-4)), there were significant levels of C20:4-CoA synthetase activity. By contrast I(6-4) had low levels of activity. This indicates FATP6 contains elements between the two highly conserved motifs that are involved in C20:4-CoA synthetase activity, which necessarily have to be distinct from those involved in transport. As noted above, I(6-4) was proficient in transport and II(6-4) was deficient in transport. These results indicate these activities are separable and point to elements between the ATP/AMP and FATP/VLACS motifs, including the 71–73 amino acid segment noted above, that are involved in conferring these distinguishing properties (Figure 7). The data using the very long chain fatty acid substrate C24:0 was not quite as informative (Figure 8). FATP6 did not activate C24:0 with the same efficiency as FATP1 and FATP4 and was in agreement with our previous studies. All five chimeras had levels that were equivalent to FATP1 (I(1-4)) or intermediate between FATP1 and FATP6 (II(1-4), III(1-4), I(6-4) and II(6-4)).
Figure 8. Acyl CoA synthetase activities using the very long chain fatty acids arachidonate (C20:4) and lignocerate (C24:0) as substrates in cell extracts prepared from the fat1Δ faa1Δ strain of S. cerevisiae expressing FATP1, FATP4, FATP6, and FATP chimeras.
The error bars indicate the standard error of the mean (n=3); *p≤0.05; **p≤0.01; ***p≤0.005 relative to vector control.
DISCUSSION
The identification of the different FATP isoforms has come from experiments directed at identifying proteins involved in fatty acid transport and enzymes involved in activating very long chain fatty acids. The FATP proteins have been designated as members of the solute carrier 27 protein family (Slc27); a designation that does not take into account their functional properties as activating enzymes. It is clear that only select members of this family are involved in the transport of exogenous fatty acids. There is evidence both from the yeast model system and experiments using 3T3-L1 adipocytes that several FATP isoforms work in concert with specific long chain acyl CoA synthetases. A general understanding that is emerging from a number of studies is that members of the FATP protein family function in different fatty acid trafficking pathways, including the transport of exogenous fatty acids, and thus provide important roles in maintaining overall fatty acid homeostasis [1–3, 5, 6, 10, 14, 34–41]. In terms of functional properties of these proteins, we prefer to broaden the descriptive roles of this group of proteins to include fatty acid trafficking, which includes the function of these proteins in the transport of exogenous long chain fatty acids and in intracellular fatty acid metabolism, both of which require fatty acid activation.
In the present work, we constructed protein chimeras between FATP1, FATP4 and FATP6 in an effort to define elements within these three proteins that confer their unique biochemical properties. FATP1 and FATP4 are fully functional in the fatty acid transport pathway when expressed in yeast containing deletions in FAT1 (encoding the FATP orthologue Fat1p) and FAA1 (encoding the major Acsl Faa1p). In this strain, the native long chain acyl CoA synthetase activity comes from the Acsl Faa4p; this activity accounts for 10–20% of wild type activity [6, 25]. FATP6, by contrast, was not functional in the fatty acid transport pathway but like FATP1 and FATP4 was able to activate very long chain fatty acids (C20:4 and C24:0) (not a property of yeast Faa4p). Despite the two highly conserved motifs (ATP/AMP and FATP/VLASC) within these family members, the functions of FATP1 and FATP4 (transport and activation) differ from FATP6 (activation) suggesting the non-conserved regions contribute to these differences. The five chimeras generated (I(4-1), II(4-1), III (4-1), I (6-4) and II(6-4)) were constructed in a manner to maintain the ATP/AMP and FATP/VLACS motifs from one protein while mixing the non-conserved regions from another. The amino terminus of several FATP isoforms has been shown to contain membrane-spanning regions; as it seems likely all of the different isoforms share this property, we chose to generate chimeras that maintained the amino terminus of FATP1 or FATP6. Two key findings emerged from these studies. The first was that the ability to complement the fat1Δ faa1Δ strain of yeast on oleate-cerulenin plates was correlated with the ability to transport the fluorescent long chain fatty acid C1-BODIPY-C12. The second were the functional properties of the different chimeras that led to the identification of a 71–73 amino acid segment between the ATP/AMP and FATP/VLACS motifs that is highly conserved between FATP1 and FATP4, but divergent in FATP6. We hypothesize this region is important in conferring the unique properties of these three proteins, particularly with regards to fatty acid transport (Figure 7). Within this region, FATP1 and FATP4 share identity in 53/73 (73%) residues; when taking into account conservation of amino acid residues with similar chemical properties, this approaches 90%. FATP6 is considerably more divergent within this region (12/71 (16%) identity), but given the placement of five glycine and two proline residues that are identical to those in FATP1 and FATP4, it seems likely this region will adopt a similar conformation in all three proteins. Several additional differences between FATP1 and FATP4 relative to FATP6 were notable. The first are two missing residues in FATP6 that correspond to Y466 and V467 in FATP1 and Y462 and L463 of FATP4. The second is the change of a number of charged residues to uncharged or aliphatic residues and, third, are residues where there is a charge reversal. Each of these differences suggest a region of the protein that may adopt a similar tertiary structure, but contain specific residues that seem likely to function as determinates in the different functional properties of FATP6, when compared to FATP1 and FATP4.
The present work demonstrates the FATP family of proteins contributes two activities: the transport of exogenous long chain fatty acids and activation of very long chain fatty acids. Data from this work and our previous studies [6] demonstrate FATP1, FATP2 and FATP4 have both transport and very long chain activation activities. A 73 amino acid segment in FATP1 and FATP4 was identified by studying these protein chimeras that appears to be crucial for the transport activity. We suspect that in the context of transport in the yeast experimental system, each of these proteins functionally interact with the endogenous Acsl Faa4p. The very long chain activation activity is likely to be distinct from that required for transport via vectorial acylation and thus provides an important role in other aspects of fatty acid homeostasis. FATP6 does not function in transport in the yeast experimental system, but has considerable very long chain activating activity. Current studies using the yeast experimental system are directed to further address the functional importance of the highly conserved 73 amino acid segment common between FATP1 and FATP4 and how this region of these proteins specifically contribute to long chain fatty acid transport.
Acknowledgments
This work has been supported by a grant from the National Institutes of Health (GM056840).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Black PN, DiRusso CC. Transmembrane movement of exogenous long-chain fatty acids: proteins, enzymes, and vectorial esterification. Microbiol Mol Biol Rev. 2003;67:454–472. doi: 10.1128/MMBR.67.3.454-472.2003. table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Doege H, Stahl A. Protein-mediated fatty acid uptake: novel insights from in vivo models. Physiology (Bethesda) 2006;21:259–268. doi: 10.1152/physiol.00014.2006. [DOI] [PubMed] [Google Scholar]
- 3.Hirsch D, Stahl A, Lodish HF. A family of fatty acid transporters conserved from mycobacterium to man. Proc Natl Acad Sci U S A. 1998;95:8625–8629. doi: 10.1073/pnas.95.15.8625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Black PN, DiRusso CC. Vectorial acylation: Linking fatty acid transport and activation to metabolic trafficking. In: Goode J, editor. Fatty acids and lipotoxicity in obesity and diabetes (Novartis Foundation Symposium) Vol. 286. Wiley; London: 2007. pp. 127–141. [DOI] [PubMed] [Google Scholar]
- 5.DiRusso CC, Connell EJ, Faergeman NJ, Knudsen J, Hansen JK, Black PN. Murine FATP alleviates growth and biochemical deficiencies of yeast fat1Δ strains. Eur J Biochem. 2000;267:4422–4433. doi: 10.1046/j.1432-1327.2000.01489.x. [DOI] [PubMed] [Google Scholar]
- 6.DiRusso CC, Li H, Darwis D, Watkins PA, Berger J, Black PN. Comparative biochemical studies of the murine fatty acid transport proteins (FATP) expressed in yeast. J Biol Chem. 2005;280:16829–16837. doi: 10.1074/jbc.M409598200. [DOI] [PubMed] [Google Scholar]
- 7.Gimeno RE, Ortegon AM, Patel S, Punreddy S, Ge P, Sun Y, Lodish HF, Stahl A. Characterization of a heart-specific fatty acid transport protein. J Biol Chem. 2003;278:16039–16044. doi: 10.1074/jbc.M211412200. [DOI] [PubMed] [Google Scholar]
- 8.Milger K, Herrmann T, Becker C, Gotthardt D, Zickwolf J, Ehehalt R, Watkins PA, Stremmel W, Fullekrug J. Cellular uptake of fatty acids driven by the ER-localized acyl-CoA synthetase FATP4. J Cell Sci. 2006;119:4678–4688. doi: 10.1242/jcs.03280. [DOI] [PubMed] [Google Scholar]
- 9.Schaffer JE, Lodish HF. Expression cloning and characterization of a novel adipocyte long chain fatty acid transport protein. Cell. 1994;79:427–436. doi: 10.1016/0092-8674(94)90252-6. [DOI] [PubMed] [Google Scholar]
- 10.Stahl A. A current review of fatty acid transport proteins (SLC27) Pflugers Arch. 2004;447:722–727. doi: 10.1007/s00424-003-1106-z. [DOI] [PubMed] [Google Scholar]
- 11.Stahl A, Hirsch DJ, Gimeno RE, Punreddy S, Ge P, Watson N, Patel S, Kotler M, Raimondi A, Tartaglia LA, Lodish HF. Identification of the major intestinal fatty acid transport protein. Mol Cell. 1999;4:299–308. doi: 10.1016/s1097-2765(00)80332-9. [DOI] [PubMed] [Google Scholar]
- 12.Berger J, Truppe C, Neumann H, Forss-Petter S. A novel relative of the very-long-chain acyl-CoA synthetase and fatty acid transporter protein genes with a distinct expression pattern. Biochem Biophys Res Commun. 1998;247:255–260. doi: 10.1006/bbrc.1998.8770. [DOI] [PubMed] [Google Scholar]
- 13.Coe NR, Smith AJ, Frohnert BI, Watkins PA, Bernlohr DA. The fatty acid transport protein (FATP1) is a very long chain acyl-CoA synthetase. J Biol Chem. 1999;274:36300–36304. doi: 10.1074/jbc.274.51.36300. [DOI] [PubMed] [Google Scholar]
- 14.Doege H, Baillie RA, Ortegon AM, Tsang B, Wu Q, Punreddy S, Hirsch D, Watson N, Gimeno RE, Stahl A. Targeted deletion of FATP5 reveals multiple functions in liver metabolism: alterations in hepatic lipid homeostasis. Gastroenterology. 2006;130:1245–1258. doi: 10.1053/j.gastro.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 15.Hall AM, Smith AJ, Bernlohr DA. Characterization of the Acyl-CoA synthetase activity of purified murine fatty acid transport protein 1. J Biol Chem. 2003;278:43008–43013. doi: 10.1074/jbc.M306575200. [DOI] [PubMed] [Google Scholar]
- 16.Hall AM, Wiczer BM, Herrmann T, Stremmel W, Bernlohr DA. Enzymatic properties of purified murine fatty acid transport protein 4 and analysis of acyl-CoA synthetase activities in tissues from FATP4 null mice. J Biol Chem. 2005;280:11948–11954. doi: 10.1074/jbc.M412629200. [DOI] [PubMed] [Google Scholar]
- 17.Jia Z, Moulson CL, Pei Z, Miner JH, Watkins PA. FATP4 is the principal very long-chain fatty acyl-CoA synthetase in skin fibroblasts. J Biol Chem. 2007 doi: 10.1074/jbc.M700568200. [DOI] [PubMed] [Google Scholar]
- 18.Watkins PA, Pevsner J, Steinberg SJ. Human very long-chain acyl-CoA synthetase and two human homologs: initial characterization and relationship to fatty acid transport protein. Prostaglandins Leukot Essent Fatty Acids. 1999;60:323–328. doi: 10.1016/s0952-3278(99)80007-6. [DOI] [PubMed] [Google Scholar]
- 19.Stuhlsatz-Krouper SM, Bennett NE, Schaffer JE. Substitution of alanine for serine 250 in the murine fatty acid transport protein inhibits long chain fatty acid transport. J Biol Chem. 1998;273:28642–28650. doi: 10.1074/jbc.273.44.28642. [DOI] [PubMed] [Google Scholar]
- 20.Stuhlsatz-Krouper SM, Bennett NE, Schaffer JE. Molecular aspects of fatty acid transport: mutations in the IYTSGTTGXPK motif impair fatty acid transport protein function. Prostaglandins Leukot Essent Fatty Acids. 1999;60:285–289. doi: 10.1016/s0952-3278(99)80001-5. [DOI] [PubMed] [Google Scholar]
- 21.Weimar JD, DiRusso CC, Delio R, Black PN. Functional role of fatty acyl-coenzyme A synthetase in the transmembrane movement and activation of exogenous long-chain fatty acids. Amino acid residues within the ATP/AMP signature motif of Escherichia coli FadD are required for enzyme activity and fatty acid transport. J Biol Chem. 2002;277:29369–29376. doi: 10.1074/jbc.M107022200. [DOI] [PubMed] [Google Scholar]
- 22.Zou Z, DiRusso CC, Ctrnacta V, Black PN. Fatty acid transport in Saccharomyces cerevisiae. Directed mutagenesis of FAT1 distinguishes the biochemical activities associated with Fat1p. J Biol Chem. 2002;277:31062–31071. doi: 10.1074/jbc.M205034200. [DOI] [PubMed] [Google Scholar]
- 23.Lewis SE, Listenberger LL, Ory DS, Schaffer JE. Membrane topology of the murine fatty acid transport protein 1. J Biol Chem. 2001;276:37042–37050. doi: 10.1074/jbc.M105556200. [DOI] [PubMed] [Google Scholar]
- 24.Richards MR, Listenberger LL, Kelly AA, Lewis SE, Ory DS, Schaffer JE. Oligomerization of the murine fatty acid transport protein 1. J Biol Chem. 2003;278:10477–10483. doi: 10.1074/jbc.M212469200. [DOI] [PubMed] [Google Scholar]
- 25.Faergeman NJ, DiRusso CC, Elberger A, Knudsen J, Black PN. Disruption of the Saccharomyces cerevisiae homologue to the murine fatty acid transport protein impairs uptake and growth on long-chain fatty acids. J Biol Chem. 1997;272:8531–8538. doi: 10.1074/jbc.272.13.8531. [DOI] [PubMed] [Google Scholar]
- 26.Obermeyer T, Fraisl P, DiRusso CC, Black PN. Topology of the yeast fatty acid transport protein Fat1p: Mechanistic implications for functional domains on the cytosolic surface of the plasma membrane. J Lipid Res. 2007:2354–2364. doi: 10.1194/jlr.M700300-JLR200. [DOI] [PubMed] [Google Scholar]
- 27.Zou Z, Tong F, Faergeman NJ, Borsting C, Black PN, DiRusso CC. Vectorial acylation in Saccharomyces cerevisiae. Fat1p and fatty acyl-CoA synthetase are interacting components of a fatty acid import complex. J Biol Chem. 2003;278:16414–16422. doi: 10.1074/jbc.M210557200. [DOI] [PubMed] [Google Scholar]
- 28.Gietz RD, Schiestl RH, Willems AR, Woods RA. Studies on the transformation of intact yeast cells by the LiAc/SS-DNA/PEG procedure. Yeast. 1995;11:355–360. doi: 10.1002/yea.320110408. [DOI] [PubMed] [Google Scholar]
- 29.Urban A, Neukirchen S, Jaeger KE. A rapid and efficient method for site-directed mutagenesis using one-step overlap extension PCR. Nucleic Acids Res. 1997;25:2227–2228. doi: 10.1093/nar/25.11.2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical biochemistry. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 31.Faergeman NJ, Black PN, Zhao XD, Knudsen J, DiRusso CC. The Acyl-CoA synthetases encoded within FAA1 and FAA4 in Saccharomyces cerevisiae function as components of the fatty acid transport system linking import, activation, and intracellular Utilization. J Biol Chem. 2001;276:37051–37059. doi: 10.1074/jbc.M100884200. [DOI] [PubMed] [Google Scholar]
- 32.Li H, Black PN, Chokshi A, Sandoval-Alvarez A, Vatsyayan R, Sealls W, DiRusso CC. High throughput screening for fatty acid uptake inhibitors in humanized yeast identifies atypical antipsychotics that cause dyslipidemias. J Lipid Res. 2008 doi: 10.1194/jlr.D700015-JLR200. [DOI] [PubMed] [Google Scholar]
- 33.Li H, Black PN, DiRusso CC. A live-cell high-throughput screening assay for identification of fatty acid uptake inhibitors. Analytical biochemistry. 2005;336:11–19. doi: 10.1016/j.ab.2004.09.025. [DOI] [PubMed] [Google Scholar]
- 34.Chiu HC, Kovacs A, Blanton RM, Han X, Courtois M, Weinheimer CJ, Yamada KA, Brunet S, Xu H, Nerbonne JM, Welch MJ, Fettig NM, Sharp TL, Sambandam N, Olson KM, Ory DS, Schaffer JE. Transgenic Expression of FATP1 in the Heart Causes Lipotoxic Cardiomyopathy. Circ Res. 2004 doi: 10.1161/01.RES.0000154079.20681.B9. [DOI] [PubMed] [Google Scholar]
- 35.Fisher RM, Gertow K. Fatty acid transport proteins and insulin resistance. Curr Opin Lipidol. 2005;16:173–178. doi: 10.1097/01.mol.0000162322.39548.b1. [DOI] [PubMed] [Google Scholar]
- 36.Herrmann T, Buchkremer F, Gosch I, Hall AM, Bernlohr DA, Stremmel W. Mouse fatty acid transport protein 4 (FATP4): characterization of the gene and functional assessment as a very long chain acyl-CoA synthetase. Gene. 2001;270:31–40. doi: 10.1016/s0378-1119(01)00489-9. [DOI] [PubMed] [Google Scholar]
- 37.Herrmann T, Grone HJ, Langbein L, Kaiser I, Gosch I, Bennemann U, Metzger D, Chambon P, Stewart AF, Stremmel W. Disturbed epidermal structure in mice with temporally controlled fatp4 deficiency. J Invest Dermatol. 2005;125:1228–1235. doi: 10.1111/j.0022-202X.2005.23972.x. [DOI] [PubMed] [Google Scholar]
- 38.Martin G, Poirier H, Hennuyer N, Crombie D, Fruchart JC, Heyman RA, Besnard P, Auwerx J. Induction of the fatty acid transport protein 1 and acyl-CoA synthase genes by dimer-selective rexinoids suggests that the peroxisome proliferator-activated receptor-retinoid X receptor heterodimer is their molecular target. J Biol Chem. 2000;275:12612–12618. doi: 10.1074/jbc.275.17.12612. [DOI] [PubMed] [Google Scholar]
- 39.Memon RA, Fuller J, Moser AH, Smith PJ, Grunfeld C, Feingold KR. Regulation of putative fatty acid transporters and Acyl-CoA synthetase in liver and adipose tissue in ob/ob mice. Diabetes. 1999;48:121–127. doi: 10.2337/diabetes.48.1.121. [DOI] [PubMed] [Google Scholar]
- 40.Richards MR, Harp JD, Ory DS, Schaffer JE. Fatty acid transport protein 1 and long-chain acyl coenzyme A synthetase 1 interact in adipocytes. J Lipid Res. 2006;47:665–672. doi: 10.1194/jlr.M500514-JLR200. [DOI] [PubMed] [Google Scholar]
- 41.Schaffer JE. A novel adipocyte long chain fatty acid transport protein. Eur J Med Res. 1996;1:176–180. [PubMed] [Google Scholar]