Abstract
Nausea and vomiting are important as biological systems for drug side effects, disease co-morbidities, and defenses against food poisoning. Vomiting can serve the function of emptying a noxious chemical from the gut, and nausea appears to play a role in a conditioned response to avoid ingestion of offending substances. The sensory pathways for nausea and vomiting, such as gut and vestibular inputs, are generally defined but the problem of determining the brain’s final common pathway and central pattern generator for nausea and vomiting is largely unsolved. A neurophysiological analysis of brain pathways provides an opportunity to more closely determine the neurobiology of nausea and vomiting and its prodromal signs (e.g., cold sweating, salivation).
Keywords: nausea, vomiting, central pattern generator, vagus, brainstem, hindbrain, NTS, respiration, salivation
Nausea and vomiting are commonly studied at pharmacological, behavioral, and psychological levels of analysis. These approaches are represented by a large literature of human clinical research highlighting the efficacy of various anti-emetic agents. Extensive work has also been conducted to demonstrate that treatments for disease do not have negative effects, such as nausea and vomiting, that might limit their clinical application. The current scarcity of research on the neurobiological basis of nausea and vomiting is striking considering its clinical importance. For example, at the 2006 annual meeting of the Society for Neuroscience there were >14,000 presentation abstracts but only 19 contained the words vomiting, emesis, or nausea (www.sfn.org).
This review presents nausea and vomiting in the evolutionary context of food intake (i.e., what is the adaptive nature of these systems?), discusses the relevance of this topic to today’s world, and addresses the current understanding of the brain circuitry that generates nausea and vomiting.
Nausea and vomiting: Defenses against food poisoning
Animals possess an arsenal of special abilities for survival and many of these are used for the foraging and consumption of food. Food intake is a risky behavior leading to the exposure of internal organs to possible food-related ailments, including viral and bacterial infection, allergies, and food intolerance (Bischoff & Renzer, 2006). An important survival problem is to determine which foods are safe and animals possess a hierarchy of sensory systems that help in food identification. Many spoiled foods can be identified using olfactory cues and taste is an effective intake deterrent when food is sour or bitter.
Smell and taste, the gatekeepers of the alimentary tract, are not always effective in detecting the quality of food, and nausea and vomiting, as additional mechanisms for dealing with an unhealthy meal, play a large role in subsequent levels of defense. Emesis, along with diarrhea, helps rid the gastrointestinal tract of dangerous ingested toxins. The vomiting response is present in many species, appearing in most vertebrates (including representative members of fish, amphibia, reptiles, birds, and mammals, see Andrews, Axelsson, Franklin, & Holmgren, 2000; Andrews & Horn, 2006; Borison, Borison, & McCarthy, 1981) and at least one invertebrate, the gastropod pleurobanchaea (McClellan, 1983). However, the broad assessment of the emetic response across species is hampered by the problem of distinguishing emesis from processes of regurgitation and rumination; emesis is functionally different and likely represents a more forceful ejection of gastric contents.
Several commonly used laboratory animals appear to lack a vomiting response (e.g., rat, mouse, guinea pig, and rabbit). It is worth noting however that only a few strains of these species have been tested for emesis, using a limited set of stimuli, and it is unknown whether all members of these species lack the response. The possibility exists that rodents possess a degenerate “emetic” response rather than an absent one (Andrews & Horn, 2006). There is an isolated report of “retching” in mice (Furukawa & Yamada, 1980) and rats have a gag reflex, which has similar features to a single retch, triggered by mechanical stimulation of the pharynx (Andrew, 1956). There are structural differences in the rat and mouse esophagus and diaphragm that would make it difficult to generate the emetic response (Andrews, 1995). Perhaps the vomiting response became an unneeded level of protection in rodents because they possess other efficient ways to deal with potential toxicosis, including a finely tuned ability to develop conditioned flavor aversions (CFA) (Garcia & Koelling, 1967).
Nausea is an aversive experience that often accompanies emesis, and is a distinct perception, different from pain or stress. Although a rare condition, vomiting can occur without nausea (e.g., Visser, Hassink, Bonsel, Moen, & Kalkman, 2001). Nausea is not simply the result of a low level of stimulation to the emetic system, which if only increased in intensity would result in vomiting. Counter-intuitively, nausea is more difficult to treat than emesis using anti-vomiting medications. The severity of drug-induced emesis (e.g., from cancer chemotherapy) can be controlled with anti-emetic medications, such as 5-HT3 and NK1 receptor antagonists; but nausea is still a persistent problem (Sanger & Andrews, 2006). These facts suggest that nausea and vomiting are at least partially separate physiological processes. Arguably, nausea is the driving force behind the development of CFA – thus providing the potent unconditioned stimulus to support a learned response to avoid consumption of foods which make us sick (Scalera, 2002). Unfortunately, nausea is difficult to study in laboratory animals but animal behavior (e.g., salivation, conditioned aversion), under conditions that make humans nauseated, suggests the presence of a unique aversive state.
Pregnancy-induced nausea and vomiting has an adaptive advantage. Importantly, the first trimester is a period of rapid fetal growth, and includes critically the development of the CNS, which is highly susceptible to toxicosis. Pregnant women also appear to be picky eaters during this period and tend to avoid meat and fish products, which are more likely to contain pathogens that might harm the fetus (Flaxman & Sherman, 2000). In humans, the presence of pregnancy-induced nausea and vomiting in the first trimester is correlated with a healthy pregnancy (Weigel & Weigel, 1989). It is only in rare cases that pregnancy-induced nausea and vomiting extends beyond this time interval, compromising the health of mother and fetus; a condition called hyperemesis gravidarum (Verberg, Gillott, Al-Fardan, & Grudzinskas, 2005).
Why are nausea and vomiting important in today’s world?
In contrast to most other animals westernized humans are now surrounded by a plethora of food that is relatively safe, highly nutritious, and plentiful. But our physiological capabilities presumably were developed in an evolutionary window of time that was quite different from the one we now inhabit; a biology designed for racing across the savannah to spear the next meal is quite different from what is needed to make a trip to the supermarket. Despite our highly evolved world of refrigeration and food processing know-how, we still must deal with the real danger of food poisoning (2007). In the United States the CDC reports “76 million Americans get sick, more than 300,000 are hospitalized, and 5,000 people die from food-borne illnesses each year” (www.cdc.gov). Certainly, even in modern humans nausea and vomiting serve important roles in defense, although sometimes these defenses are insufficient.
Beyond the concern for tainted food, the systems for nausea and vomiting appear to have the inclination to become activated by a large number of modern conditions. Nausea and vomiting, as protective systems, cannot afford to make mistakes, and thus by necessity must have a low threshold for activation. Modern medicine is particularly effective at provoking nausea and vomiting, including many drug treatments and post surgery recovery. A significant impetus to develop anti-emetic drugs originated from a desire to inhibit nausea and vomiting produced by some anti-cancer agents with high emetic potential, such as cisplatin (Gralla et al., 1981). An assortment of other drugs also have side effects of nausea and vomiting in prescribed doses, and many drugs will produce these effects at high dosages. One important reason for investigating the systems for nausea and vomiting is the possibility to design “clean” drugs, which have little affect on nausea and vomiting but still retain efficacy for disease treatment.
We also have the unfortunate neurological connection between motion (or illusionary motion) and nausea and vomiting [“nausea,” refers to seasickness, derived from the Greek word “naus,” meaning ship]. Motion-induced emesis appears to have a very early evolutionary origin because it is present in most animal models of emesis. Motion-induced nausea and vomiting is thought to result from sensory conflict regarding body position in space (Yates, Miller, & Lucot, 1998), yet no satisfactory theory exists as to why animals have this mechanism in the first place (Yates et al., 1998). It seems unlikely that we evolved this input for nausea and vomiting to keep us away from boats, cars, and airplanes!
Lastly, insight into the controls for nausea and vomiting has great utility for the study of feeding behavior. Traditionally, emesis research was conducted in areas of biology and physiology using cats, dogs, and ferrets. Laboratory research on feeding behavior grew out of experimental psychology, mostly using rats, and more recently mice. In animal psychology, perhaps owing to the lack of a vomiting response in rodents, researchers use CFA testing (and sometimes pica, e.g., clay ingestion; Mitchell et al., 1976) to assess possible aversive effects on feeding behavior. For example, it is still an important issue for researchers working on the satiation of food intake in non-human species (with implications for the control of obesity) to distinguish the actions of variables that reduce feeding from those that produce malaise. CFA or pica testing can provide only a partial answer to this dilemma since it is still not clear how these responses relate to aversive states such as nausea and vomiting.
Neurobiology of nausea and vomiting
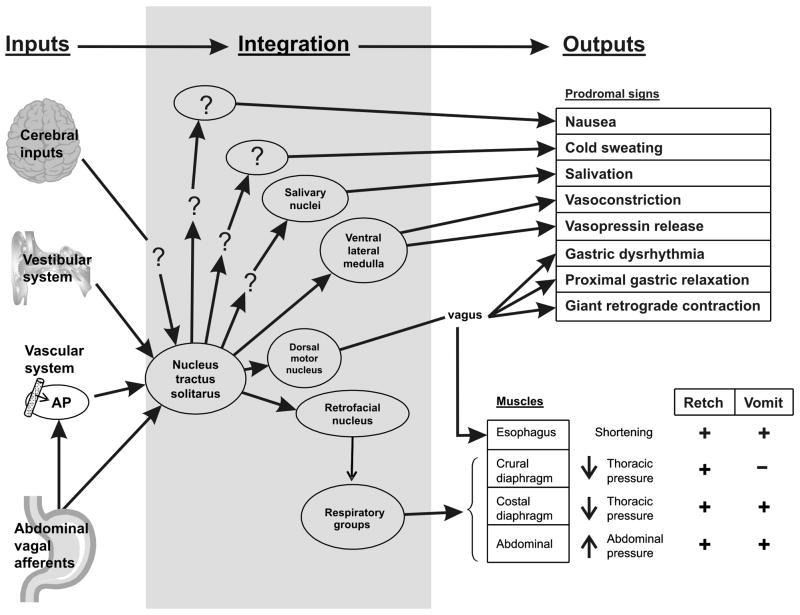
An important issue for understanding the neurobiology of nausea and vomiting is the large number of associated outputs (Fig. 1). There are many prodromal signs and some of these are not uniquely related to nausea and vomiting (e.g., salivation and sweating). Clearly the autonomic nervous system, with outputs of sweating, salivation, gastric function, and often vasoconstriction, is intimately connected to the neural pathways for nausea and vomiting (Fig. 1). The complexity of the emetic response in animal experiments is not often measured. A vomit (expulsion of gastric contents) is usually preceded by several retching responses, but retching and vomiting can occur separately (Andrews et al., 1990) and involve different sets of muscles (Fig. 1) (see Grelot & Miller, 1994). During a retch, thoracic pressure is decreased and abdominal pressure is increased, which may serve to position gastric contents and overcome esophageal resistance (Andrews et al., 1990). Conversely, a vomit occurs with increased thoracic and abdominal pressure. Retches and vomits are commonly lumped together in behavioral analyses and consequently the neural controls for these processes are not well delineated.
Fig. 1.
Model of neural pathways for nausea and vomiting. Inputs: Afferent input from the cerebral cortex, vestibular system, area postrema (AP), and gut vagal afferent fibers converge on the nucleus of the solitary tract (NTS) in the lower brainstem. Integration: The final common neural pathways and central pattern generator for nausea and vomiting and other prodromal signs are largely unknown. The NTS and region of the retrofacial nucleus are thought to play important integrative roles in nausea and vomiting. Outputs: Prodromal signs usually occur prior to retching and vomiting. Proximal gastric relaxation and a giant retrograde contraction of the intestine, mediated by the vagus, serve to position gastrointestinal contents for expulsion by vomiting. The sequence of muscles engaged in retching are different from those used in vomiting (expulsion). ?, indicates unknown elements in these pathways. Some neural regions are omitted for the sake of simplicity (e.g., hypothalamic pathway for vasopressin release).
Unlike a simple reflex, the occurrence of which can be predicted from the intensity of stimulation, the threshold for the emetic response is more variable. The response is modifiable by experience and can be conditioned (Stockhorst, Steingrueber, Enck, & Klosterhalfen, 2006). Even in well-controlled animal studies, the timing of the emetic response is quite variable. Following emetogenic treatments, the latency to the first emetic episode and inter-response interval are difficult to predict with precision, with responses sometimes separated by minutes to hours. Cyclic vomiting syndrome (CVS) in humans is a particularly mysterious problem because the separation of emetic episodes can be 2 to 4 weeks (Li & Misiewicz, 2003). It is unknown what determines these variable temporal patterns; certainly the type (e.g., chemotherapy versus a motion stimulus) and amount of stimulation play a role but it might also be related to the propensity of other neural systems to adjust the tone of emetic circuitry. For example, cardiovascular inputs from carotid baro- and chemoreceptors modulate the emetic response (Uchino, Kuwahara, Ebukuro, & Tsubone, 2006). Early work also suggested the existence of brainstem circuits containing opioid receptors that modulate emetic pathways (Rudd & Naylor, 1995). More recent studies indicate modulation by the cannabinoid system (Parker, Limebeer, & Kwaitkowska, 2005).
Nausea is more difficult to analyze using experimental animals. Although CFA testing has been used as a marker of nausea, mostly in the rat and mouse, it is difficult to know whether this index truly reflects nausea, especially since some drugs with reinforcing properties also produce conditioned flavor avoidance (Parker, 1995). Furthermore, the neural pathways mediating CFA are inherently difficult to assess because of the long delay between input and response and the complexity of a system that also depends on learning and memory. Even though rodents lack a vomiting response they display pica when injected with toxins or subjected to strong motion, and pica can be inhibited by anti-emetic drugs (review, Andrews & Horn, 2006). Research suggests a relationship between pica, CFA, and emesis but the neurobiological substrates remain to be determined (Smith, Friedman, & Andrews, 2001; Rabin & Hunt, 1992).
Emetic-like responses using in vivo animal preparations provide the opportunity for a detailed analysis of neural circuitry. In vivo preparations showing retching or vomiting responses have been developed for the cat, dog, ferret, and house musk shrew (e.g., Smith, Paton, & Andrews, 2002; Fukuda et al., 2003; Umezaki, Zheng, Shiba, & Miller, 1997; Van, Oland, Mackie, Davison, & Sharkey, 2003). Since prodromal outputs, including nausea, are connected to the emetic circuitry this level of analysis should yield insights into brain pathways that also mediate prodromal responses (Fig. 1). For example, it seems reasonable that the emetic central pattern generator or final common pathway should connect to forebrain areas involved in nausea (the amygdala is a possible candidate: Horn, Ciucci, & Chaudhury, 2007) and these putative pathways could be assessed with electrophysiological methods during the induction of emetic-like responses.
There is a critical need to delineate the emetic circuitry better. The final common neural pathway for emesis has not been defined and the location of a “vomiting center” or central pattern generator for emesis is controversial (e.g., Miller & Wilson, 1983; Miller, Nonaka, & Jakus, 1994). Anti-emetic drugs, such as NK1 receptor antagonists, that block many types of emesis (induced by drugs, motion, vagal stimulation, etc.) strongly indicate the presence of a final common pathway for emesis. Cerebral, vestibular, area postrema, and gut afferent inputs for nausea and vomiting converge on the nucleus of the solitary tract (NTS) in the caudal hindbrain (Fig. 1). Based on sensory inputs the NTS seems to be the logical candidate as a final common pathway for emesis. Toxic agents in the blood might act on the area postrema, which has a weak blood brain barrier, to produce nausea and vomiting but there are serious problems in establishing this mechanism because manipulations of the area postrema can also potentially affect NTS and vagal function. Results from lesion, electrical stimulation, and neurophysiological experiments indicate that the NTS provides input to the emetic central pattern generator located in the area of the retrofacial nucleus of the reticular formation, which provides control over the repiratory groups that mediate muscular movements for retching and vomiting (Fukuda et al., 2003; Miller et al., 1994) (Fig. 1).
The complexity of the neural systems for nausea and vomiting guarantees that its secrets will not be revealed easily, particularly because these systems are contained within the highly overlapping neuronal network of the caudal hindbrain. It will be important to distinguish brainstem systems for respiration, cardiovascular control, and swallowing from those involved in nausea and vomiting. Studies in invertebrate systems reveal overlapping neural architecture that simply switches between “behavioral states” (e.g., rejection and ingestion responses in the marine snail Aplysia, Jing et al., 2007) and this also seems operative in mammals (e.g., the role of the respiratory network in the emetic response: Fukuda et al., 2003). The sensory pathways for nausea and vomiting are generally well understood (e.g., vagal and vestibular inputs) but the pivotal problem of defining the convergent neural circuitry that generates nausea and vomiting is still largely unsolved. An answer to this puzzle would likely represent a rich source of information for designing effective treatments to control nausea and vomiting and yield significant insight into understanding gut-brain communication.
Acknowledgments
Based on a presentation to the Columbia University Seminar on Appetitive Behavior, April 5, 2007, Harry R. Kissileff, Chairman, supported in part by GlaxoSmithKline and The New York Obesity Research Center, St. Luke’s/Roosevelt Hospital. The work of Charles Horn is supported by NIH funding (DK065971). The author thanks Drs. Mark Friedman, Michael Tordoff, and Bart DeJonghe for helpful comments on this manuscript.
Footnotes
Important Note: This is not a short communication (SC) any longer and Dr. David Booth, Executive Editor, has approved the longer version.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Food for thought. Nature. 2007;445:683–684. doi: 10.1038/445683b. [DOI] [PubMed] [Google Scholar]
- Andrew BL. The nervous control of the cervical oesophagus of the rat during swallowing. Journal of Physiology. 1956;134:729–740. doi: 10.1113/jphysiol.1956.sp005679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews PL, Axelsson M, Franklin C, Holmgren S. The emetic reflex in a reptile (Crocodylus porosus) Journal of Experimental Biology. 2000;203(Pt 10):1625–1632. doi: 10.1242/jeb.203.10.1625. [DOI] [PubMed] [Google Scholar]
- Andrews PL, Horn CC. Signals for nausea and emesis: Implications for models of upper gastrointestinal diseases. Autonomic Neuroscience: Basic and Clinical. 2006;125:100–115. doi: 10.1016/j.autneu.2006.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews PLR. Why do some animals lack a vomiting reflex? Physiological Zoology. 1995;68:61. [Google Scholar]
- Andrews PLR, Bhandari P, Garland S, Bingham S, Davis CJ, Hawthorn J, et al. Does retching have a function?: An experimental study in the ferret. Pharmacodynamics and Therapeutics. 1990;9:135–152. [Google Scholar]
- Bischoff SC, Renzer C. Nausea and nutrition. Autonomic Neuroscience: Basic and Clinical. 2006;129:22–27. doi: 10.1016/j.autneu.2006.07.011. [DOI] [PubMed] [Google Scholar]
- Borison HL, Borison R, McCarthy LE. Phylogenic and neurologic aspects of the vomiting process. Journal of Clinical Pharmacology. 1981;21:23S–29S. doi: 10.1002/j.1552-4604.1981.tb02569.x. [DOI] [PubMed] [Google Scholar]
- Flaxman SM, Sherman PW. Morning sickness: a mechanism for protecting mother and embryo. Quarterly Review of Biology. 2000;75:113–148. doi: 10.1086/393377. [DOI] [PubMed] [Google Scholar]
- Fukuda H, Koga T, Furukawa N, Nakamura E, Hatano M, Yanagihara M. The site of the antiemetic action of NK1 receptor antagonists. In: Donnerer J, editor. Antiemetic therapy. New York, NY: Karger; 2003. pp. 33–77. [Google Scholar]
- Furukawa T, Yamada K. The alpha-naphthoxyacetic acid-elicited retching involves dopaminergic inhibition in mice. Pharmacology, Biochemistry, and Behavior. 1980;12:735–738. doi: 10.1016/0091-3057(80)90158-6. [DOI] [PubMed] [Google Scholar]
- Garcia J, Koelling RA. A comparison of aversions induced by x-rays, toxins, and drugs in the rat. Radiation Research Supplement. 1967;7:439–450. [PubMed] [Google Scholar]
- Gralla RJ, Itri LM, Pisko SE, Squillante AE, Kelsen DP, Braun DW, Jr, et al. Antiemetic efficacy of high-dose metoclopramide: randomized trials with placebo and prochlorperazine in patients with chemotherapy-induced nausea and vomiting. New England Journal of Medicine. 1981;305:905–909. doi: 10.1056/NEJM198110153051601. [DOI] [PubMed] [Google Scholar]
- Grelot L, Miller AD. Vomiting - Its Ins and Outs. News in Physiological Sciences. 1994;9:142–147. [Google Scholar]
- Horn CC, Ciucci M, Chaudhury A. Brain Fos expression during 48 h after cisplatin treatment: neural pathways for acute and delayed visceral sickness. Autonomic Neuroscience: Basic and Clinical. 2007;132:44–51. doi: 10.1016/j.autneu.2006.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing J, Vilim FS, Horn CC, Alexeeva V, Hatcher NG, Sasaki K, et al. From hunger to satiety: reconfiguration of a feeding network by Aplysia neuropeptide Y. Journal of Neuroscience. 2007;27:3490–3502. doi: 10.1523/JNEUROSCI.0334-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li BU, Misiewicz L. Cyclic vomiting syndrome: a brain-gut disorder. Gastroenterology Clinics of North America. 2003;32:997–1019. doi: 10.1016/s0889-8553(03)00045-1. [DOI] [PubMed] [Google Scholar]
- McClellan AD. Higher order neurons in buccal ganglia of Pleurobranchaea elicit vomiting motor activity. Journal of Neurophysiology. 1983;50:658–670. doi: 10.1152/jn.1983.50.3.658. [DOI] [PubMed] [Google Scholar]
- Miller AD, Nonaka S, Jakus J. Brain areas essential or non-essential for emesis. Brain Research. 1994;647:255–264. doi: 10.1016/0006-8993(94)91325-0. [DOI] [PubMed] [Google Scholar]
- Miller AD, Wilson VJ. ‘Vomiting center’ reanalyzed: an electrical stimulation study. Brain Research. 1983;270:154–158. doi: 10.1016/0006-8993(83)90805-3. [DOI] [PubMed] [Google Scholar]
- Mitchell D, Wells C, Hoch N, Lind K, Woods SC, Mitchell LK. Poison induced pica in rats. Physiology and Behavior. 1976;17:691–697. doi: 10.1016/0031-9384(76)90171-2. [DOI] [PubMed] [Google Scholar]
- Parker LA. Rewarding drugs produce taste avoidance, but not taste aversion. Neuroscience and Biobehavioral Reviews. 1995;19:143–57. doi: 10.1016/0149-7634(94)00028-y. [DOI] [PubMed] [Google Scholar]
- Parker LA, Limebeer CL, Kwaitkowska M. Cannabinoids: effects on vomiting and nausea in animal models. In: Mechoulam R, editor. Cannabinoids as Therapeutics. Switzerland: Birkhauser Verlag/Switzerland; 2005. pp. 183–200. [Google Scholar]
- Rabin BM, Hunt WA. Relationship between vomiting and taste aversion learning in the ferret: studies with ionizing radiation, lithium chloride, and amphetamine. Behavioral and Neural Biology. 1992;58:83–93. doi: 10.1016/0163-1047(92)90291-b. [DOI] [PubMed] [Google Scholar]
- Rudd JA, Naylor RJ. Opioid receptor involvement in emesis and antiemesis. In: Reynolds DJM, Andrews PLR, Davis CJ, editors. Serotonin and the scientific basis of anti-emetic therapy. Oxford, UK: Oxford Clinical Communications; 1995. pp. 208–221. [Google Scholar]
- Sanger GJ, Andrews PL. Treatment of nausea and vomiting: gaps in our knowledge. Autonomic Neuroscience: Basic and Clinical. 2006;129:3–16. doi: 10.1016/j.autneu.2006.07.009. [DOI] [PubMed] [Google Scholar]
- Scalera G. Effects of conditioned food aversions on nutritional behavior in humans. Nutritional Neuroscience. 2002;5:159–188. doi: 10.1080/10284150290013059. [DOI] [PubMed] [Google Scholar]
- Smith JE, Friedman MI, Andrews PL. Conditioned food aversion in Suncus murinus (house musk shrew) - a new model for the study of nausea in a species with an emetic reflex. Physiology and Behavior. 2001;73:593–598. doi: 10.1016/s0031-9384(01)00538-8. [DOI] [PubMed] [Google Scholar]
- Smith JE, Paton JF, Andrews PL. An arterially perfused decerebrate preparation of Suncus murinus (house musk shrew) for the study of emesis and swallowing. Experimental Physiology. 2002;87:563–574. doi: 10.1113/eph8702424. [DOI] [PubMed] [Google Scholar]
- Stockhorst U, Steingrueber HJ, Enck P, Klosterhalfen S. Pavlovian conditioning of nausea and vomiting. Autonomic Neuroscience: Basic and Clinical. 2006;129:50–57. doi: 10.1016/j.autneu.2006.07.012. [DOI] [PubMed] [Google Scholar]
- Uchino M, Kuwahara M, Ebukuro S, Tsubone H. Modulation of emetic response by carotid baro- and chemoreceptor activations. Autonomic Neuroscience: Basic and Clinical. 2006;128:25–36. doi: 10.1016/j.autneu.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Umezaki T, Zheng Y, Shiba K, Miller AD. Role of nucleus retroambigualis in respiratory reflexes evoked by superior laryngeal and vestibular nerve afferents and in emesis. Brain Research. 1997;769:347–356. doi: 10.1016/s0006-8993(97)00756-7. [DOI] [PubMed] [Google Scholar]
- Van S, Oland LD, Mackie K, Davison JS, Sharkey KA. Delta9-tetrahydrocannabinol selectively acts on CB1 receptors in specific regions of dorsal vagal complex to inhibit emesis in ferrets. American Journal of Physiology: Gastrointestinal and Liver Physiology. 2003;285:G566–G576. doi: 10.1152/ajpgi.00113.2003. [DOI] [PubMed] [Google Scholar]
- Verberg MF, Gillott DJ, Al-Fardan N, Grudzinskas JG. Hyperemesis gravidarum, a literature review. Human Reproduction Update. 2005;11:527–539. doi: 10.1093/humupd/dmi021. [DOI] [PubMed] [Google Scholar]
- Visser K, Hassink EA, Bonsel GJ, Moen J, Kalkman CJ. Randomized controlled trial of total intravenous anesthesia with propofol versus inhalation anesthesia with isoflurane-nitrous oxide: postoperative nausea with vomiting and economic analysis. Anesthesiology. 2001;95:616–626. doi: 10.1097/00000542-200109000-00012. [DOI] [PubMed] [Google Scholar]
- Weigel MM, Weigel RM. Nausea and vomiting of early pregnancy and pregnancy outcome. An epidemiological study. British Journal of Obstetrics and Gynaecology. 1989;96:1304–1311. doi: 10.1111/j.1471-0528.1989.tb03228.x. [DOI] [PubMed] [Google Scholar]
- Yates BJ, Miller AD, Lucot JB. Physiological basis and pharmacology of motion sickness: an update. Brain Research Bulletin. 1998;47:395–406. doi: 10.1016/s0361-9230(98)00092-6. [DOI] [PubMed] [Google Scholar]