Abstract
The mechanisms underlying the taxonomic assembly of montane biotas are still poorly understood. Most hypotheses have assumed that the diversification of montane biotas is loosely coupled to Earth history and have emphasized instead the importance of multiple long-distance dispersal events and biotic interactions, particularly competition, for structuring the taxonomic composition and distribution of montane biotic elements. Here we use phylogenetic and biogeographic analyses of species in the parrot genus Pionus to demonstrate that standing diversity within montane lineages is directly attributable to events of Earth history. Phylogenetic relationships confirm three independent biogeographic disjunctions between montane lineages, on one hand, and lowland dry-forest/wet-forest lineages on the other. Temporal estimates of lineage diversification are consistent with the interpretation that the three lineages were transported passively to high elevations by mountain building, and that subsequent diversification within the Andes was driven primarily by Pleistocene climatic oscillations and their large-scale effects on habitat change. These results support a mechanistic link between diversification and Earth history and have general implications for explaining high altitudinal disjuncts and the origin of montane biotas.
Keywords: montane biotas, historical biogeography, speciation, diversification, Andes, Psittacidae
1. Introduction
Formed behind an eastward-dipping ocean–continental subduction zone, the Andes stretch more than 7000 km from Colombia to Chile and have extensive land area above 4000 m, particularly from Peru through northern Chile and Argentina. This topographic complexity, and the fact that the Andes border the megadiverse lowland Amazonian biota, has contributed to their harbouring the world's most diverse montane avifauna in terms of numbers of species as well as endemics (Fjeldså & Krabbe 1990; Stotz et al. 1996).
The origin of the montane Andean flora and fauna, and specifically that of birds, is poorly understood. Two general hypotheses have been proposed. The most widely invoked is that montane elements have been largely derived from lowland relatives via long-distance dispersal (Descimon 1986; Monasterio & Vuilleumier 1986; van der Hammen & Cleef 1986) or by colonization as populations expanded their ranges upslope, became isolated due to climatically induced habitat change, and then became differentiated (Brumfield & Edwards 2007). An alternative hypothesis is that ancestral populations were distributed across both lowland and pre-Andean landscapes, with tectonic uplift passively transporting some to increasingly higher elevations where they differentiated within a variety of montane habitats (Lynch 1986; Reig 1986; van der Hammen & Cleef 1986; Hall 2005).
Neither hypothesis would be expected to be applicable across all groups, and it might not always be possible to distinguish between them in specific cases. What has generally been lacking in earlier studies, however, is a corroborated phylogenetic hypothesis, which, along with inferences about the spatial and temporal history of the group, could be used to discriminate between these two alternatives. Following vicariance biogeographic principles (Nelson & Platnick 1981), Lynch (1986) outlined such a test, applied it to several small clades of frogs in the genus Eleutherodactylus, and proposed that lowland/montane disjunctions were the result of vicariance. Later, Bates & Zink (1994) implied altitudinal vicariance for four species of tyrannid flycatchers (Leptopogon). Using a general mitochondrial clock calibration of 2% divergence per million years (Myr), they estimated a lowland/montane age of vicariance at 9–6 Myr (ago). Recently, Hall (2005) constructed a phylogeny for 11 species of butterflies (Ithomiola), and inferred a vicariance origin for the montane taxa, but did not provide a temporal framework.
Here we describe the phylogenetic and biogeographic history of species within a single monophyletic genus of parrots (Pionus) and infer three independent instances of lowland/high montane vicariance and subsequent diversification. We use phylogenetic and biogeographic patterns, as well as multiple estimates of divergence times based on molecular sequence data, to propose a causal link between diversification and Earth history, including mountain building and climatic oscillations, as understood from tectonic, palaeogeographic and palaeobotanical data. We discuss the implications these results have for investigating the origins of montane biotas and for understanding mechanisms of biological diversification.
2. Material and methods
(a) Taxonomic diversity and phylogenetic species
Discovery of all smallest diagnosable taxonomic units, or phylogenetic species, is critical for describing how a group's taxonomic diversity originated across space and time (Cracraft 1997). Our starting point was to test whether previously described subspecies of Pionus are diagnosably distinct (phylogenetic species); therefore, we scored 18 external morphological characters for all subspecies (Forshaw 1989; Juniper & Parr 1998) using a large series of museum study skins (498 individuals; see table S1 and ’Taxonomic diversity and distributions’, both in the electronic supplementary material).
(b) DNA sequence acquisition
We collected different sets of molecular data. For reconstructing species-level phylogenetic relationships, we obtained 2181 base pairs (bp) of sequence for the complete cytochrome b (cyt b, GenBank accession numbers EF517602–EF517636) and NADH dehydrogenase 2 (ND2, GenBank accession numbers EF517637–EF517671) mitochondrial genes from 35 individuals representing all 19 presumptive phylogenetic species within Pionus (table S2 in the electronic supplementary material). Monophyly of Pionus was confirmed by undertaking preliminary analyses using a diversity of South American parrots with presumed close and distant relationships (Tavares et al. 2004, 2006; Ribas et al. 2005; for further details see ‘methods’ in the electronic supplementary material). From these analyses, close relatives of Pionus, including Gypopsitta barrabandi, Amazona farinosa, Amazona xanthops and Graydidascalus brachyurus, were chosen as outgroups.
For the molecular clock analyses, sequences from the nuclear recombination activating genes, RAG-1 and RAG-2, were obtained for several genera of parrots (table S3 in the electronic supplementary material). Most RAG-1 sequences were obtained from GenBank from a recent study (Tavares et al. 2006) that focused on Neotropical genera. RAG-2 sequences were obtained in our laboratory for selected genera including all main lineages of parrots, all main Neotropical lineages, and five Pionus species (table S3).
(c) DNA extraction, sequencing and phylogenetic data analysis
DNA extraction, amplification, sequencing and alignment were performed as described (Ribas et al. 2005, 2006; for further details see ‘methods’ in the electronic supplementary material). All primers used are shown in table S4 (in the electronic supplementary material).
Tests were performed to assess incongruence of phylogenetic signal between the two mitochondrial genes and among all three codon positions (ILD test; Farris et al. 1995). We further determined whether there was evidence of saturation due to multiple substitutions and evaluated the uniformity of base composition (see ‘methods’ in the electronic supplementary material).
Phylogenetic hypotheses were constructed through heuristic maximum-parsimony (MP) and maximum-likelihood (ML) searches in PAUP* v. 4.0b10 (Swofford 2002), as well as with Bayesian analysis using MrBayes v. 3.1 (Huelsenbeck & Ronquist 2001). For MP analyses of the combined morphological and molecular data, the 18 morphological characters were added to a molecular matrix containing only one representative of each taxon. Support for nodes in MP and ML was assessed using the non-parametric bootstrap. Bayesian analyses were conducted with a mixed-model approach. Three independent analyses of 10 million generations each were performed (for further details on MP, ML and Bayesian analyses see ‘methods’ in the electronic supplementary material).
(d) Biogeographic analyses
Because montane versus lowland elevational distributions are discrete, they were scored as a binary character and each species was coded as being either lowland or montane (distributional data are provided in ‘taxonomic diversity and distributions’ in the electronic supplementary material). Historical biogeographic change in elevational distribution was assessed using ancestral character-state reconstruction with delayed optimization (Maddison & Maddison 1999).
(e) Molecular dating of divergence times
Estimating ages of divergence across lineages with precision is subject to many caveats (Arbogast et al. 2002; Lovette 2004), thus our goal was to seek an internally consistent estimate of divergence times. Because no fossil calibration exists for Pionus, three different strategies were implemented to test for consilience in age estimates.
Our first approach employed rates of molecular evolution in cyt b to bracket divergence time estimates in Pionus. The most commonly used rates for cyt b have been 1.6% (Fleischer et al. 1998) and 2% (Brown et al. 1979; Randi 1996) sequence divergence per million years (Myr). It is not known how applicable these rates are across birds as a whole, but the congruence of rates estimated from different studies lends some credibility to using them to bracket divergence times in groups for which no fossil record is available. As Pionus cyt b sequences evolve in a clock-like manner (p>0.1, likelihood ratio test), we adopted both 1.6 and 2% rates to convert ML distances to times of divergence within Pionus.
For the remaining two strategies, relaxed molecular clock methods were employed. Penalized likelihood (PL) allows for different rates of evolution across the tree, but applies a non-parametric roughness penalty (rate smoothing) that costs the model more if rates change too quickly between branches (Sanderson 2002). The parametric method proposed by Thorne et al. (1998) uses a probabilistic model based on priors to describe the change in evolutionary rates over time and uses the Markov chain Monte Carlo procedure to derive the posterior distribution of rates and times.
Previous phylogenetic studies have shown that the endemic New Zealand parrot genera, Nestor and Strigops, are basal to all other parrots (de Kloet & de Kloet 2005; Tavares et al. 2006). This provides a framework for calibrating this basal split using the date of geological separation of New Zealand from West Antarctica (e.g. Barker et al. 2004; Tavares et al. 2006), estimated to be 85–82 Myr ago (Yan & Kroenke 1993). Because saturation of nucleotide substitutions places limits on the use of mtDNA sequences for dating deep phylogenetic nodes (Jansa et al. 2006; Roelants et al. 2007), we employed nuclear sequences to estimate divergence dates across a generic tree for parrots using the New Zealand geological calibration. We then adopted these dates to calibrate the mtDNA tree for Pionus (see Wiens et al. 2007). Thus, we built a tree for RAG-2 sequences (1155 bp, GenBank accession numbers EF517676–EF517702) for 22 genera of parrots, including 5 Pionus species (table S3) and analysed their temporal diversification with both r8s (PL, Sanderson 2003) and Multidivtime (Thorne & Kishino 2002), fixing the age of the split between Nestor and all other genera at 85 Myr ago. Results from this analysis were then used to calibrate the mtDNA Pionus phylogeny (for details on the settings used for both r8s and Multidivtime analyses, see ‘methods’ in the electronic supplementary material).
Our final approach was designed to examine the precision of the ages determined by RAG-2. We obtained nuclear RAG-1 (2703 bp) sequences from GenBank (Tavares et al. 2006) for 30 parrot genera. As this pre-existing dataset was composed mostly of Neotropical genera, we added RAG-1 sequences from Nestor notabilis, Agapornis personata, Micropsitta bruijnii and Psittacus erithacus (table S3; GenBank accession numbers EF517672–EF517675), so that the same geological calibration could be applied. The final RAG-1 matrix had 34 terminals, including 14 genera in common with the RAG-2 dataset.
3. Results
(a) Basal taxa (phylogenetic species)
Morphological and genetic data support the recognition of 19 phylogenetic species, although additional sampling will be required to corroborate the status of several of these taxa (for details, see ’Taxonomic diversity and distributions’ in the electronic supplementary material).
(b) Phylogenetic analyses
No insertions or deletions were found, and start and stop codons were in the expected positions. Patterns of base composition were as expected for avian mtDNA. No base composition bias was detected (p=0.08 for cyt b and p=0.95 for ND2). The incongruence length difference test (ILD; Farris et al. 1995) rejected incongruence between the two genes (p>0.05) and among the three codon positions (cyt b p>0.15 and ND2 p>0.20). Plots of pairwise divergences (Ts and Tv versus p-distance) did not show evidence of saturation. The GTR +Γ model was implemented for cyt b (α=0.155) and combined (α=0.195) mitochondrial data, while TrN+Γ was applied to the ND2 (α=0.238) data.
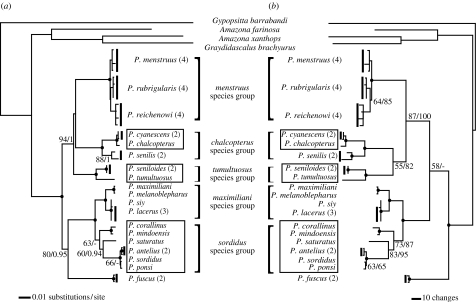
The trees found by ML and MP analyses are shown in figure 1. Bayesian analysis yielded the same topology as the ML tree. Five clades (species groups) were recovered, four of which (menstruus, chalcopterus, tumultuosus and maximiliani) have strong support. The sordidus species group was less well supported but was monophyletic in all analyses, especially when morphological characters were added to the molecular data (figure 1b). There was consistently high support for a relationship between the menstruus, chalcopterus and tumultuosus species groups, on one hand, and the maximiliani and sordidus groups on the other, but two phylogenetic uncertainties were also revealed. First, both ML and Bayesian analyses united the menstruus and chalcopterus groups with low support (figure 1a), whereas MP clustered the chalcopterus and tumultuosus groups. This latter clade had low MP support with molecular data alone but had moderate support when morphological characters were included (figure 1b). Second, Pionus fuscus is an old lineage, and its association with the maximiliani + sordidus clade appeared in ML and Bayesian analyses with high support. In contrast, the MP analysis placed P. fuscus basal to all other Pionus taxa, but this arrangement had very low support (figure 1b).
Figure 1.
Topologies generated by the combined analysis of cyt b and ND2 (2181 bp). Numbers in parenthesis indicate the number of specimens sampled. Boxes highlight the tree montane clades. (a) ML topology using the GTR+Γ model. Numbers along branches indicate ML bootstrap values greater than 50%/Bayesian posterior probabilities greater than 0.9. Solid circles indicate a bootstrap value greater than 95% and a posterior probability of 1.0. (b) MP topology. Numbers along branches are MP bootstrap values greater than 50% without/with the morphological characters included in the analysis. Solid circles indicate a bootstrap value greater than 95%.
(c) Biogeographic patterns within Pionus
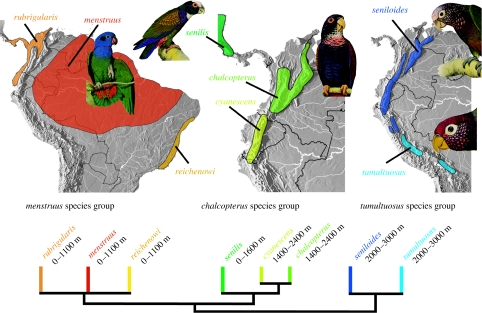
Phylogenetic results revealed that the three Andean clades are not jointly monophyletic but instead have independent relationships to lowland taxa (figure 1). Pionus chalcopterus and Pionus cyanescens are mid-montane sister taxa of the northern Andes (figure 2). Together they are related to P. senilis, a lowland species of Middle America and Mexico. On the ML tree, these three species are related to the lowland menstruus species group, which exhibits classic cis-, trans-Andean and Brazilian Serra do Mar tropical forest distributions. The sister group of these two species groups, as determined by ML, is the tumultuosus species group. The latter contains two high montane species, Pionus tumultuosus and Pionus seniloides. The MP solution (figure 1b) united the chalcopterus and tumultuosus species groups, and thus on the MP tree montane origins are optimized ambiguously: either there was a single origin for the chalcopterus and tumultuosus species groups, with a reversal back to lowlands in Pionus senilis, or these species groups became montane independently. Divergence time estimates, discussed below, suggest P. senilis separated from P. cyanescens/P. chalcopterus 2.2–1.2 Myr ago (table 1, node 5), after the emplacement of continuous Panamanian Isthmus lowlands (Duque-Caro 1990). This finding, and the fact that the current distribution of P. senilis is centred in the lowlands, strongly implies a primitively lowland distribution for this species. Both trees of figure 1 thus point to three independent montane origins.
Figure 2.
Distribution and altitudinal ranges of the menstruus, chalcopterus and tumultuosus species groups. Phylogenetic relationships are based on ML analysis (figure 1a). See electronic supplementary material for detailed discussion about distributions and altitudinal ranges. Plates courtesy of William T. Cooper (Parrots of the world, 1st edn, 1973).
Table 1.
Estimates of divergence time (Myr ago) within Pionus. Nodes are numbered as in figure 4. Fixed calibration times marked with asterisks are derived from a RAG-2 analysis as described in §2 (see also electronic supplementary material, table S5).
| node | cytochrome b | cytochrome b+ND2 | cytochrome b+ND2 | |||
|---|---|---|---|---|---|---|
| 1.6% | 2.0% | penalized likelihood (r8s) | Bayesian (Multidivtime) | |||
| mean | 95% interval | mean | 95% interval | |||
| 1 | — | — | 9.21* | — | 13.30* | — |
| 2 | 5.83 | 4.66 | 4.28* | — | 6.90* | — |
| 3 | 4.38 | 3.50 | 3.36 | 2.08–4.71 | 5.58 | 4.46–6.69 |
| 4 | 0.43 | 0.34 | 0.37 | 0.15–0.63 | 0.76 | 0.33–1.41 |
| 5 | 1.93 | 1.54 | 1.22 | 0.78–1.74 | 2.18 | 1.27–3.30 |
| 6 | 0.25 | 0.20 | 0.19 | 0.12–0.45 | 0.45 | 0.12–0.92 |
| 7 | 1.50 | 1.20 | 1.29 | 0.75–1.91 | 2.34 | 1.41–3.50 |
| 8 | 5.00 | 4.00 | 3.81 | 2.04–5.14 | 6.31 | 5.30–6.91 |
| 9 | 2.38 | 1.90 | 1.68 | 1.26–2.16 | 2.98 | 1.98–4.18 |
| 10 | 1.15 | 0.92 | 0.64 | 0.38–0.98 | 1.18 | 0.53–2.06 |
| 11 | 0.06 | 0.05 | 0.04 | 0.00–0.15 | 0.15 | 0.01–0.45 |
| 12 | 0.04 | 0.03 | 0.02 | 0.00–0.11 | 0.12 | 0.01–0.35 |
| 13 | 1.94 | 1.55 | 1.35 | 0.95–1.83 | 2.41 | 1.52–3.50 |
| 14 | 0.66 | 0.53 | 0.23 | 0.005–0.49 | 0.34 | 0.02–0.91 |
| 15 | 1.66 | 1.33 | 1.14 | 0.84–1.54 | 2.01 | 1.18–3.02 |
| 16 | 0.25 | 0.20 | 0.11 | 0.00–0.27 | 0.32 | 0.06–0.73 |
Nearly all species of Pionus, particularly the montane ones, have their distributions in one or more long-recognized areas of endemism (Cracraft 1985a). Shared patterns of endemism are most parsimoniously explained in terms of a common vicariance history (Nelson & Platnick 1981; Cracraft 1992, 1994), and indeed sister species within montane Pionus are allopatric, so that their distributions are consistent with vicariance. Species ranges within Pionus are limited by many of the same barriers that affect other montane groups, primarily strong topographical lows having disparate ecologies (e.g. hotter lowland forest or drier intermontane shrubland) when compared with adjacent montane forests.
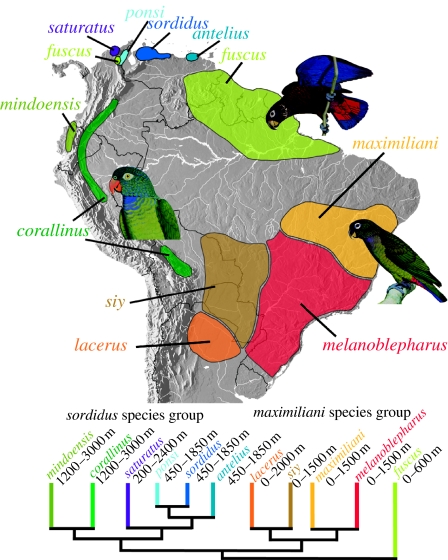
Examining distributions of Andean species groups phylogenetically reveals shared montane ecological histories within, but disparity among, these clades (figures 2 and 3). Of the three montane clades, the two species of the tumultuosus species group occur at the highest elevations in the high temperate zone and both range between 2000 and 3000 m (figure 2). Intermediate in altitude are the highland species within the chalcopterus species group (figure 2), with both species being found in mid-montane cloud forests. Finally, taxa of the sordidus species group have the lowest altitudinal distributions of the three montane clades and occupy low- to mid-montane forests and may range to lower elevations, especially in northern Venezuela (figure 3; for further details about distributions, see ‘taxonomic diversity and distributions’ in the electronic supplementary material).
Figure 3.
Distribution and altitudinal ranges of the sordidus and maximiliani species groups and P. fuscus. Phylogenetic relationships are based on ML analysis (figure 1a). See electronic supplementary material for detailed discussion about distributions and altitudinal ranges. Plates courtesy of William T. Cooper (Parrots of the world, 1st edn. 1973).
(d) Temporal pattern of diversification.
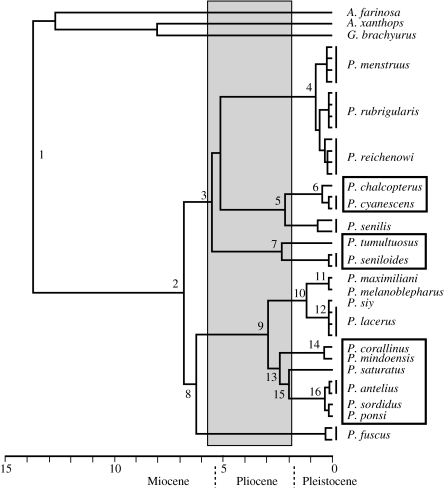
Based on age estimates using rates of 2.0 and 1.6% sequence divergence per Myr, the basal split within Pionus (figure 4, node 2) took place between ca 4.7 Myr ago and a maximum of ca 5.8 Myr ago (table 1). Importantly, the estimated dates bracket the origins of the three montane lineages as having taken place between ca 4.4 and 1.5 Myr ago (table 1, nodes 3, 5 and 9) and also suggest that the radiation of extant species was almost entirely within the Pleistocene.
Figure 4.
Bayesian Multidivtime chronogram based on the combined ND2 and cyt b datasets. Nodes 1 and 2 are fixed calibration points derived from a Bayesian Multidivtime analysis of RAG-2 data. Time scale is in millions of years. Numbered nodes correspond to those of table 1. Montane clades are shown in boxes. Shaded area shows estimated window of time during which montane/lowland vicariance occurred.
MP and ML analyses of the RAG-2 dataset resulted in a topology that agrees with previously inferred phylogenetic relationships among parrot genera (de Kloet & de Kloet 2005; Tavares et al. 2006), with the species of Pionus forming a well-supported clade, and the New Zealand genus Nestor resolved as the sister group of all other parrots (figure S1 in the electronic supplementary material). A PL analysis of the RAG-2 ML tree, using the external geological calibration, estimated the split between Pionus and its sister genera, Graydidascalus and Amazona, at ca 9.2 Myr ago (95% CI: 3.9–18.5 Myr ago), whereas the most basal split within Pionus took place ca 4.3 Myr ago (95% CI: 1.2–12.4 Myr ago; nodes 8 and 9; table S5 in the electronic supplementary material). The Bayesian analysis of the same RAG-2 data estimated these two splits at ca 13.3 Myr ago (95% CI: 5.2–26 Myr ago and 6.9 Myr ago (95% CI: 1.7–16 Myr ago), respectively (nodes 8 and 9, table S5).
Splits inside Pionus were estimated using the mtDNA dataset and applying the RAG-2 dates as calibration points. Excluding the most basal (fixed-age) node, divergence dates within Pionus ranged from 3.81 to 0.02 Myr ago using PL and from 6.31 to 0.12 Myr ago in the Bayesian analysis. The dates and confidence intervals are given in table 1, and the chronogram derived from the Bayesian analysis is shown in figure 4.
PL and Bayesian analyses of the RAG-1 dataset using the same settings and calibration point as that for RAG-2 resulted in the dates and confidence intervals shown in table S5. Despite disparate taxon sampling between the RAG-1 and RAG-2 datasets, estimated ages for the nodes common to both analyses were similar (figures S1 and S2, and table S5), strengthening our calibration of the mtDNA Pionus tree.
We found slight biases related to the different datasets and method of analysis. RAG-1 age estimates are slightly older than RAG-2 using the same geological calibration point and same methods, and Bayesian estimates are older than those for PL, given the same calibration and data. As a result of this, the mtDNA age estimates within the genus are slightly younger using PL (table 1). Owing to the lack of multiple calibration points, confidence and credibility intervals are large and overlap substantially.
Despite the variance just noted, multiple approaches to dating diversification within Pionus yielded a consistent interpretation: (i) major lineages within the genus arose in the Late Miocene or Pliocene and (ii) most of the species arose in the Pleistocene (figure 4).
4. Discussion
(a) Vicariance origin of montane Pionus and Earth history
Taken as a whole, several lines of evidence are consistent with a vicariance hypothesis for the origin of the three montane clades within Pionus. First, distributions of these clades are strongly allopatric relative to their lowland sister clades, and in each case the putative barrier is intervening low montane forest or lowland moist or dry habitats created as the Andes uplifted. These highland/lowland disjunctions are therefore better explained by vicariance rather than three independent long-distance dispersal events. Second, within each montane clade, species are distributed allopatrically in well-known areas of endemism that are shared by many other montane clades of birds. Such congruent patterns of endemism imply a vicariant history in generating species diversity within those clades (Cracraft 1994). Finally, the reconstructed temporal history of diversification (figure 4) is correlative with the uplift history of the Andes, thus establishing a plausible causal linkage to the process of vicariance.
The Central Andean Altiplano and Eastern Cordillera of Peru and northern Bolivia had attained approximately 40–50% of its current elevation by 10 Myr ago, and 2300–3500 m additional uplift has taken place since then (Gregory-Wodzicki 2000). Palaeofloras (Gregory-Wodzicki et al. 1998; Gregory-Wodzicki 2000, 2002; Graham et al. 2001) and geological analyses (Barke & Lamb 2006; Ghosh et al. 2006) for the Eastern Cordillera of the Central Andes indicate Miocene–Pliocene (ca 6–5 Myr ago) palaeoaltitudes of approximately 1000–2000 m, which is much lower than present-day elevations (3500 m or greater). At that time, our data suggest that several lineages of Pionus were diversifying in this region (figure 4). A 1000–2000 m palaeoaltitude is at the lower part of the current altitudinal range of one of these lineages, the P. tumultuosus species group, which is also the oldest montane clade. The habitat at 1000–1500 m would have been near the cloud forest/tropical forest interface (Graham et al. 2001). We estimate that between 5.58 and 2.34 Myr ago (nodes 3 and 7, table 1 and figure 4) the ancestor of P. tumultuosus/P. seniloides would have been uplifted approximately 650–1000 m (at 0.2–0.3 mm/yr assuming continuous uplift; Gregory-Wodzicki 2000) and an additional 450–700 m since then, thereby transporting the lineage and its habitat from a subtropical/tropical zone to a temperate one.
In the northern Andes of Ecuador and Colombia, in contrast, the chalcopterus species group arose later in time (2.18 Myr ago; node 5, figure 4) and is distributed at lower elevations (figure 2), which may reflect differences in tectonic history. The Ecuadorian Cordillera began developing in the Middle Miocene, with uplift of approximately 6100 m taking place over Plio–Pleistocene times (Steinmann et al. 1999; Coltorti & Ollier 2000). The Eastern Cordillera of Colombia was a continuous range by 11.8 Myr ago (Hoorn et al. 1995; Flynn et al. 1997; Guerrero 1997), but at an elevation of approximately 700 m it had lowland, or perhaps lower montane, forest depending on location (Gregory-Wodzicki 2000). The Eastern Cordillera of Colombia and the Merida Andes of Venezuela experienced rapid Plio–Pleistocene uplift (Gregory-Wodzicki 2000; Chacín et al. 2005), and montane forest environments were established via 1500–1800 m of uplift within the past 4 Myr (Gregory-Wodzicki 2000). This time frame of uplift is consistent with the origins of the P. chalcopterus/cyanescens clade (node 5, figure 4) and the P. sordidus species group (node 9, figure 4). Within the later, the first split (node 13, figure 4) isolated northern Colombian/Venezuelan taxa (saturatus, antelius, sordidus and ponsi) from the Central and Northern Andean taxa (corallinus and mindoensis) that occur today at higher elevations. This split is dated at ca 2.4 Myr ago, a time when the Northern Andes were undergoing their most recent significant burst of uplift. The northern Colombian/Venezuelan clade was isolated in the Mérida, Perijá and Santa Marta ranges and diversified during a period of uplift (Cediel et al. 2003), with the Santa Marta endemic (Pionus saturatus) being the first to be isolated at ca 2 Myr ago (node 15, figure 4). Finally, our temporal estimates (figure 4; table 1) suggest that P. senilis separated from chalcopterus/cyanescens ca 2.2 Myr ago, well after the Panamanian Isthmus was formed (Duque-Caro 1990). What isolated senilis and why it is not found in southern Panama are unknown.
(b) Speciation and Pleistocene climate change
Except for P. tumultuosus, P. seniloides and P. saturatus, all montane species apparently originated within the last 0.5 Myr (table 1). Distributional patterns and relationships of the latter are consistent with vicariance driven by cyclic climatic changes that shifted montane environments to lower elevations during cooler/drier (glacial) times and back to higher elevations during warmer/wetter (interglacial) periods (Haffer 1974). The elevation of the upper forest line has been estimated to have oscillated between 2000 and 3400 m in the Colombian Andes (Hooghiemstra & van der Hammen 2004; Weng et al. 2007). During interglacial conditions montane populations of Pionus would have been isolated by intermontane valleys, tropical forest habitats or both. This mechanism has also been suggested to have promoted diversification in several other groups of Andean birds (Roy et al. 1997; García-Moreno et al. 1999a,b; Chesser 2000).
(c) Vicariance, origin and evolution of montane avifaunas
Although some phylogenetic studies of Andean birds have suggested that montane clades originated prior to the Pleistocene (Bates & Zink 1994; Bleiweiss 1998; Garcia-Moreno et al. 1998, 2001; Burns & Naoki 2004; Chesser 2004; Pérez-Emán 2005), the origins of those clades were not explicitly attributed to vicariance via Andean uplift (Bates & Zink (1994) and Bleiweiss (1998) implied a role for Andean uplift in establishing montane groups). Many authors, in contrast, have proposed that subsequent diversification within these groups was strongly influenced by tectonic activity and climate change (Garcia-Moreno et al. 1998, 2001; Pérez-Emán 2005; Cadena et al. in press; Chaves et al. 2007; among others). Thus, Andean orogenesis has been invoked more frequently as being important for generating diversity within the Andes rather than as a mechanism underlying the origin of the montane avifauna.
We propose that highland–lowland vicariance has been underappreciated for several reasons: (i) the relevant phylogenetic–biogeographic patterns have been overlooked, (ii) avian divergences are generally thought to be too young relative to the time scale of mountain building, (iii) it has long been assumed that ecological (biotic) factors structure altitudinal distributions, (iv) a vicariance split between disjunct montane and tropical lowland clades has been seen as improbable, and (v) some biogeographic methods, especially those that estimate ancestral nodal distributions across a tree using either a parsimony or model-based framework, can themselves create a bias for accepting a dispersalist interpretation. In contrast to Bates & Zink (1994) and this paper, other researchers have identified biogeographic disjunctions between highland and lowland sister groups but did not link their divergence to vicariance via mountain building, for example in Henicorhina wood-wrens (Dingle et al. 2006) and Thamnophilus antshrikes (Brumfield & Edwards 2007). The latter authors propose a ‘colonization’ model for some highland taxa, which they proposed post-dated Andean uplift, in contrast to the history suggested here for Pionus. Moreover, they applied their model to taxa that have large overlaps in altitudinal distribution, which further suggests it is not applicable to biogeographic patterns such as discussed here.
These observations lead to the conclusion that more attention should be paid to deciphering the phylogenetic and biogeographic histories of montane biotas. In addition to the Andes, mountain ranges in Eurasia, New Guinea and New Zealand underwent rapid elevational change in the Late Neogene; thus, it can be predicted that patterns of lowland/montane vicariance are likely to be more widespread than previously realized. This prediction is testable by biogeographic analysis of montane clades and their lowland sister groups. Many biogeographic studies of montane taxa do not include sampling of lowland relatives, thus lowland/highland vicariance has been probably overlooked. The approach discussed here can also be used to test the hypothesis that mountain biotas are a source for tropical lowland diversity (Fjeldså 1994). Support for this hypothesis has lacked rigorous phylogenetic and historical biogeographic testing (but see Weir 2006), and our results provide a falsifying instance.
Finally, the observation that allopatric or parapatric distributions of congeners along an altitudinal gradient can be explained as a function of biotic interactions (Terborgh 1971; Diamond 1973) needs critical re-examination. Without discounting possible secondary effects of biotic interactions on distributions, our results and those of others (Arctander & Fjeldså 1994; Bates & Zink 1994; Garcia-Moreno et al. 1998, 1999a,b; Cadena 2007) demonstrate that altitudinally parapatric congeners are often not sister taxa, thus providing falsifying instances of the hypothesis of parapatric speciation across altitudinal zones. Increasingly, these studies suggest that allopatric speciation driven by Earth history can explain north–south and altitudinal distributional patterns and is likely to be the primary mechanism underlying the taxonomic assembly of montane communities.
(d) Drivers of diversification
Historical biogeographic analysis interpreted within a framework of Earth history (Nelson & Platnick 1981; Cracraft 1985b, 1992, 1994) is fundamental for developing a mechanistic understanding of biological diversification. Because speciation in plants and animals appears to be largely allopatric (Coyne & Orr 2004), historical biogeographic methods can be used to test the hypothesis (Cracraft 1992, 1994) that allopatry is predominately driven by vicariance due to geological/environmental change rather than by long-distance dispersal. This approach is essential for testing alternative hypotheses about the rate control of speciation, which, along with extinction, is the first-order determinant of patterns of diversity across space and time. The results of this study provide evidence that spatio-temporal diversification in Pionus is causally linked to Andean tectonic and palaeoclimate change through vicariance. Because many other species in the Andes share similar spatial patterns, it is probable that these physical controls on diversification extend to them as well.
Acknowledgments
We thank David Willard and Shannon Hackett (the Field Museum of Natural History), James Dean (the National Museum of Natural History), Mark Robbins (the Kansas University Natural History Museum), Leo Joseph (the Academy of Natural Sciences of Philadelphia), Renato G. Lima, Marcos Raposo, Paulo Martuscelli, Carlos Yamashita and Rodrigo Teixeira for collecting and/or providing samples. John Flynn, Scott Schaefer, Jose Tello and Mark Robbins are greatly acknowledged for their valuable comments on the manuscript. William T. Cooper kindly provided permission to use images from ‘Parrots of the World’. This work was supported by Fundação de Amparoà Pesquisa do Estado de São Paulo, Coordenação de Aperfeiçoamento de Pessoal de Nível Superior and Conselho Nacional de Desenvolvimento Científico e Tecnológico. This paper is a contribution from the Monell Molecular Laboratory and the Cullman Research Facility in the Department of Ornithology, American Museum of Natural History and has received generous support from the Lewis B. and Dorothy Cullman Program for Molecular Systematics Studies, a joint initiative of the New York Botanical Garden and the American Museum of Natural History, the Sackler Institute of Comparative Genomics, and the L. J. and L. C. Sanford funds.
Supplementary Material
Description of diagnosable morphological characters and details on the altitudinal distribution for all basal taxa within Pionus
Details on laboratory protocols and methods of analysis used
Morphological character matrix, taxa sampled, primers used, and divergence dates obtained in the nuclear gene (RAG1 and RAG2) analyses
Chronograms obtained from the Bayesian analysis (Multidivtime) of the RAG1 and RAG2 datasets
References
- Arbogast B.S, Edwards S.V, Wakeley J, Beerli P, Slowinski J.B. Estimating divergence times from molecular data on phylogenetic and population genetic timescales. Annu. Rev. Ecol. Syst. 2002;33:707–740. doi:10.1146/annurev.ecolsys.33.010802.150500 [Google Scholar]
- Arctander P, Fjeldså J. Andean tapaculos of the genus Scytalopus (Aves, Rhinocryptidae): a study of speciation using DNA sequence data. In: Loeschke V, Tomiuk J, Jain S.K, editors. Conservation genetics. Birkhause Verlag; Basel, Switzerland: 1994. pp. 205–226. [DOI] [PubMed] [Google Scholar]
- Barke R, Lamb S. Late Cenozoic uplift of the Eastern Cordillera, Bolivian Andes. Earth Planet. Sci. Lett. 2006;249:350–367. doi:10.1016/j.epsl.2006.07.012 [Google Scholar]
- Barker F.K, Cibois A, Schikler P, Feinstein J, Cracraft J. Phylogeny and diversification of the largest avian radiation. Proc. Natl Acad. Sci. USA. 2004;101:11 040–11 045. doi: 10.1073/pnas.0401892101. doi:10.1073/pnas.0401892101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates J.M, Zink R.M. Evolution into the Andes: molecular evidence for species relationships in the genus Leptopogon. Auk. 1994;111:507–515. [Google Scholar]
- Bleiweiss R. Tempo and mode of hummingbird evolution. Biol. J. Linn. Soc. 1998;65:63–76. doi:10.1006/bijl.1998.0241 [Google Scholar]
- Brown W.M, George M, Jr., Wilson A.C. Rapid evolution of animal mitochondrial DNA. Proc. Natl Acad. Sci. USA. 1979;76:1967–1971. doi: 10.1073/pnas.76.4.1967. doi:10.1073/pnas.76.4.1967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brumfield R.T, Edwards S. Evolution into and out of the Andes: a Bayesian analysis of historical diversification in Thamnophilus antshrikes. Evolution. 2007;61:346–367. doi: 10.1111/j.1558-5646.2007.00039.x. doi:10.1111/j.1558-5646.2007.00039.x [DOI] [PubMed] [Google Scholar]
- Burns K.J, Naoki K. Molecular phylogenetics and biogeography of Neotropical tanagers in the genus Tangara. Mol. Phylogenet. Evol. 2004;32:838–854. doi: 10.1016/j.ympev.2004.02.013. doi:10.1016/j.ympev.2004.02.013 [DOI] [PubMed] [Google Scholar]
- Cadena C.D. Testing the role of interspecific competition in the evolutionary origin of elevational zonation: an example with Buarremon brush-finches (Aves, Emberizidae) in the Neotropical mountains. Evolution. 2007;61:1120–1136. doi: 10.1111/j.1558-5646.2007.00095.x. doi:10.1111/j.1558-5646.2007.00095.x [DOI] [PubMed] [Google Scholar]
- Cadena, C. D., Klicka, J. & Ricklefs, R. E. In press. Evolutionary differentiation in the Neotropical montane region: molecular phylogenetics and phylogeography of Buarremon brush-finches (Aves, Emberizidae). Mol. Phylogenet. Evol [DOI] [PubMed]
- Cediel, F., Shaw, R. P., Cáceres, C., 2003 Tectonic assembly of the Northern Andean Block. In The circum-Gulf of Mexico and the Caribbean: hydrocarbon habitats, basin formation, and plate tectonics (eds C. Bartolini, R. T. Buffler & J. Blickwede), pp. 815–848.
- Chacín L, Jácome M.I, Izarra C. Flexural and gravity modeling of the Mérida Andes and Barinas-Apuré Basin, Western Venezuela. Tectonophysics. 2005;405:155–167. doi:10.1016/j.tecto.2005.06.004 [Google Scholar]
- Chaves J.A, Pollinger J.P, Smith T.B, LeBuhn G. The role of geography and ecology in shaping the phylogeography of the speckled hummingbird (Adelomyia melanogenys) in Ecuador. Mol. Phylogenet. Evol. 2007;43:795–807. doi: 10.1016/j.ympev.2006.11.006. doi:10.1016/j.ympev.2006.11.006 [DOI] [PubMed] [Google Scholar]
- Chesser R.T. Evolution in the High Andes: the phylogenetics of Muscisaxicola ground-tyrants. Mol. Phylogenet. Evol. 2000;15:369–380. doi: 10.1006/mpev.1999.0774. doi:10.1006/mpev.1999.0774 [DOI] [PubMed] [Google Scholar]
- Chesser R.T. Systematics, evolution, and biogeography of the South American ovenbird genus Cinclodes. Auk. 2004;121:752–766. doi:10.1642/0004-8038(2004)121[0752:SEABOT]2.0.CO;2 [Google Scholar]
- Coltorti M, Ollier C.D. Geomorphic and tectonic evolution of the Ecuadorian Andes. Geomorphology. 2000;32:1–19. doi:10.1016/S0169-555X(99)00036-7 [Google Scholar]
- Coyne J.A, Orr H.A. Sinauer; Sunderland, MA: 2004. Speciation. [Google Scholar]
- Cracraft J. Historical biogeography and patterns of differentiation within the South American avifauna: areas of endemism. Ornithol. Monogr. 1985a;36:49–84. [Google Scholar]
- Cracraft J. Biological diversification and its causes. Annu. Missouri Bot. Gard. 1985b;72:794–822. doi:10.2307/2399222 [Google Scholar]
- Cracraft J. Explaining patterns of biological diversity: integrating causation at different spatial and temporal scales. In: Eldredge N, editor. Systematics, ecology, and the biodiversity crisis. Columbia University Press; New York, NY: 1992. pp. 59–76. [Google Scholar]
- Cracraft J. Species diversity, biogeography, and the evolution of biotas. Am. Zool. 1994;34:33–47. [Google Scholar]
- Cracraft J. Species concepts in systematics and conservation biology—an ornithological viewpoint. Syst. Assoc. Spec. Ser. 1997;54:325–339. [Google Scholar]
- de Kloet R.S, de Kloet S.R. The evolution of the spindlin gene in birds: sequence analysis of an intron of the spindlin W and Z gene reveals four major divisions of the Psittaciformes. Mol. Phylogenet. Evol. 2005;36:706–721. doi: 10.1016/j.ympev.2005.03.013. doi:10.1016/j.ympev.2005.03.013 [DOI] [PubMed] [Google Scholar]
- Descimon H. Origins of Lepidopteran faunas in the high tropical Andes. In: Vuilleumier F, Monasterio M, editors. High altitude tropical biogeography. Oxford University Press; New York, NY: 1986. pp. 500–532. [Google Scholar]
- Diamond J. Distributional ecology of New Guinea Birds. Science. 1973;179:759–769. doi: 10.1126/science.179.4075.759. doi:10.1126/science.179.4075.759 [DOI] [PubMed] [Google Scholar]
- Dingle C, Lovette I.J, Canaday C, Smith T. Elevational zonation and the phylogenetic relationships of the Henicorhina wood-wrens. Auk. 2006;123:119–134. doi:10.1642/0004-8038(2006)123[0119:EZATPR]2.0.CO;2 [Google Scholar]
- Duque-Caro H. Neogene stratigraphy, paleoceanography and paleobiogeography in northwest South America and the evolution of the Panama Seaway. Palaeogeogr. Palaeocl. 1990;77:203–234. doi:10.1016/0031-0182(90)90178-A [Google Scholar]
- Farris J.S, Kallersjo M, Kluge A.G, Bult C. Testing significance of incongruence. Cladistics. 1995;10:315–319. doi:10.1111/j.1096-0031.1994.tb00181.x [Google Scholar]
- Fjeldså J. Geographical patterns for relict and young species of birds in Africa and South America and implications for conservation priorities. Biodivers. Conserv. 1994;3:207–226. doi:10.1007/BF00055939 [Google Scholar]
- Fjeldså J, Krabbe N. Apollo Books; Copenhagen, Denmark: 1990. Birds of the High Andes. [Google Scholar]
- Fleischer R.C, Mcintosh C.E, Tarr C.L. Evolution on a volcanic conveyor belt: using phylogeographic reconstructions and K-Ar-based ages of the Hawaiian Islands to estimate molecular evolutionary rates. Mol. Ecol. 1998;7:533–545. doi: 10.1046/j.1365-294x.1998.00364.x. doi:10.1046/j.1365-294x.1998.00364.x [DOI] [PubMed] [Google Scholar]
- Flynn J.J, Guerrero J, Swisher C.C., III . Geochronology of the Honda Group. In: Kay R.F, Madden R.H, Cifelli R.L, Flynn J.J, editors. Verterbrate paleontology in the neotropics: the Miocene fauna of La Venta, Colombia. Smithsonian Institution Press; Washington, DC: 1997. pp. 44–59. [Google Scholar]
- Forshaw J. 3rd ed. Landsdowne Editions; Melbourne, Australia: 1989. Parrots of the World. [Google Scholar]
- Garcia-Moreno J, Arctander P, Fjeldså J. Pre-Pleistocene differentiation among chat-tyrants. Condor. 1998;100:629–640. doi:10.2307/1369744 [Google Scholar]
- Garcia-Moreno J, Arctander P, Fjeldså J. Strong diversification at the treeline among Metallura hummingbirds. Auk. 1999a;116:702–711. [Google Scholar]
- Garcia-Moreno J, Arctander P, Fjeldså J. A case of rapid diversification in the Neotropics: phylogenetic relationships among Cranioleuca spinetails (Aves, Furnariidae) Mol. Phylogenet. Evol. 1999b;12:273–281. doi: 10.1006/mpev.1999.0617. doi:10.1006/mpev.1999.0617 [DOI] [PubMed] [Google Scholar]
- García-Moreno J, Ohlson J, Fjeldså J. MtDNA sequences support monophyly of Hemispingus tanagers. Mol. Phylogenet. Evol. 2001;21:424–435. doi: 10.1006/mpev.2001.1027. doi:10.1006/mpev.2001.1027 [DOI] [PubMed] [Google Scholar]
- Ghosh P, Garzione C.N, Eiler J.M. Rapid uplift of the altiplano revealed through 13C–18O bonds in paleosol carbonates. Science. 2006;311:511–515. doi: 10.1126/science.1119365. doi:10.1126/science.1119365 [DOI] [PubMed] [Google Scholar]
- Graham A, Gregory-Wodzicki K, Wright K.L. Studies in Neotropical paleobotany, XV. A Mio–Pliocene palynoflora from the Eastern Cordillera, Bolivia: implications for the uplift history of the central Andes. Am. J. Bot. 2001;88:1545–1557. doi:10.2307/3558398 [PubMed] [Google Scholar]
- Gregory-Wodzicki K.M. Uplift history of the central and northern Andes: a review. Geol. Soc. Am. Bull. 2000;112:1091–1105. doi:10.1130/0016-7606(2000)112<1091:UHOTCA>2.3.CO;2 [Google Scholar]
- Gregory-Wodzicki K.M. A late Miocene subtropical-dry flora from the northern Altiplano, Bolivia. Palaeogeogr. Palaeocl. 2002;180:331–348. doi:10.1016/S0031-0182(01)00434-5 [Google Scholar]
- Gregory-Wodzicki K.M, McIntosh W.C, Velasquez K. Climatic and tectonic implications of the Late Miocene Jakokkota flora, Bolivian Altiplano. J. S. Am. Earth Sci. 1998;11:533–540. doi:10.1016/S0895-9811(98)00031-5 [Google Scholar]
- Guerrero J. Stratigraphy, sedimentary environments, and the Miocene uplift of the Colombian Andes. In: Kay R.F, Madden R.H, Cifelli R.L, Flynn J.J, editors. Verterbrate paleontology in the neotropics: the Miocene fauna of La Venta, Colombia. Smithsonian Institution Press; Washington, DC: 1997. pp. 15–43. [Google Scholar]
- Haffer J. vol. 14. Nuttall Ornithological Club; Cambridge, UK: 1974. Avian speciation in tropical America. [Google Scholar]
- Hall J.P.W. Montane speciation patterns in Ithomiola butterflies (Lepidoptera: Riodinidae): are they consistently moving up in the world? Proc. R. Soc. B. 2005;272:2457–2466. doi: 10.1098/rspb.2005.3254. doi:10.1098/rspb.2005.3254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooghiemstra H, van der Hammen T. Quaternary Ice-Age dynamics in the Colombian Andes: developing an understanding of our legacy. Phil. Trans. R. Soc. B. 2004;359:173–181. doi: 10.1098/rstb.2003.1420. doi:10.1098/rstb.2003.1420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoorn C, Guerrero J, Sarmiento G.A, Lorente M.A. Andean tectonics as a cause for changing drainage patterns in Miocene northern South America. Geology. 1995;23:237–240. doi:10.1130/0091-7613(1995)023<0237:ATAACF>2.3.CO;2 [Google Scholar]
- Huelsenbeck J.P, Ronquist F. MrBayes: Bayesian inference of phylogeny. Bioinformatics. 2001;17:754–755. doi: 10.1093/bioinformatics/17.8.754. doi:10.1093/bioinformatics/17.8.754 [DOI] [PubMed] [Google Scholar]
- Jansa S.A, Barker F.K, Heaney L.R. The pattern and timing of diversification of Phillipine endemic rodents: evidence from mitochondrial and nuclear gene sequences. Syst. Biol. 2006;55:73–88. doi: 10.1080/10635150500431254. doi:10.1080/10635150500431254 [DOI] [PubMed] [Google Scholar]
- Juniper T, Parr M. Yale University Press; New Haven, CT: 1998. Parrots—a guide to the parrots of the world. [Google Scholar]
- Lovette I.J. Molecular phylogeny and plumage signal evolution in a trans Andean and circum Amazonian avian species complex. Mol. Phylogenet. Evol. 2004;32:512–523. doi: 10.1016/j.ympev.2004.01.007. doi:10.1016/j.ympev.2004.01.007 [DOI] [PubMed] [Google Scholar]
- Lynch J.D. Origins of the high Andean herpetological fauna. In: Vuilleumier F, Monasterio M, editors. High altitude tropical biogeography. Oxford University Press; New York, NY: 1986. pp. 478–499. [Google Scholar]
- Maddison W.P, Maddison D.R. Sinauer Associates; Sunderland, MA: 1999. MacClade: analysis of phylogeny and character evolution. [DOI] [PubMed] [Google Scholar]
- Monasterio M, Vuilleumier F. Introduction: high tropical mountain biota of the world. In: Vuilleumier F, Monasterio M, editors. High altitude tropical biogeography. Oxford University Press; New York, NY: 1986. pp. 3–10. [Google Scholar]
- Nelson G, Platnick N. Columbia University Press; New York, NY: 1981. Systematics and biogeography: cladistics and vicariance. [Google Scholar]
- Pérez-Emán J.L. Molecular phylogenetics and biogeography of the Neotropical redstarts (Myioborus; Aves, Parulinae) Mol. Phylogenet. Evol. 2005;37:511–528. doi: 10.1016/j.ympev.2005.04.013. doi:10.1016/j.ympev.2005.04.013 [DOI] [PubMed] [Google Scholar]
- Randi E. A mitochondrial cytochrome b phylogeny of the Alectoris partridges. Mol. Phylogenet. Evol. 1996;6:214–227. doi: 10.1006/mpev.1996.0072. doi:10.1006/mpev.1996.0072 [DOI] [PubMed] [Google Scholar]
- Reig O.A. Diversity patterns and differentiation of high Andean rodents. In: Vuilleumier F, Monasterio M, editors. High altitude tropical biogeography. Oxford University Press; New York, NY: 1986. pp. 404–440. [Google Scholar]
- Ribas C.C, Gaban-Lima R, Miyaki C.Y, Cracraft J. Historical biogeography and diversification within the Neotropical parrot genus Pionopsitta (Aves: Psittacidae) J. Biogeogr. 2005;32:1409–1427. [Google Scholar]
- Ribas C.C, Joseph L, Miyaki C.Y. Molecular systematics and patterns of diversification of the Pyrrhura parakeets (Aves: Psittacidae) with special reference to the P. picta/P. leucotis complex. Auk. 2006;123:660–680. doi:10.1642/0004-8038(2006)123[660:MSAPOD]2.0.CO;2 [Google Scholar]
- Roelants K, Gower D.J, Wilkinson M, Loader S.P, Biju S.D, Guillaume K, Moriau L, Bossuyt F. Global patterns of diversification in the history of modern amphibians. Proc. Natl Acad. Sci. USA. 2007;104:887–892. doi: 10.1073/pnas.0608378104. doi:10.1073/pnas.0608378104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy M.S, da Silva J.M.C, Arctander P, García-Moreno J, Fjeldså J. The speciation of South American and African birds in montane regions. In: Mindell D.P, editor. Avian molecular evolution and systematics. Academic Press; San Diego, CA: 1997. pp. 325–343. [Google Scholar]
- Sanderson M.J. Estimating absolute rates of molecular evolution and divergence times: a penalized likelihood approach. Mol. Biol. Evol. 2002;19:101–109. doi: 10.1093/oxfordjournals.molbev.a003974. [DOI] [PubMed] [Google Scholar]
- Sanderson M.J. r8s: inferring absolute rates of molecular evolution and divergence times in the absence of a molecular clock. Bioinformatics. 2003;19:301–302. doi: 10.1093/bioinformatics/19.2.301. doi:10.1093/bioinformatics/19.2.301 [DOI] [PubMed] [Google Scholar]
- Steinmann M, Hungerbühler D, Seward D, Winkler W. Neogene tectonic evolution and exhumation of the southern Ecuadorian Andes: a combined stratigraphy and fission-track approach. Tectonophysics. 1999;307:255–276. doi:10.1016/S0040-1951(99)00100-6 [Google Scholar]
- Stotz D.F, Fitzpatrick J.W, Parker T.A, Moskovits D.K. University of Chicago Press; Chicago, IL: 1996. Neotropical birds: ecology and conservation. [Google Scholar]
- Swofford, D. L. 2002 PAUP*: phylogenetic analysis using parsimony (*and other methods). Version 4.0b10. Sunderland, MA: Sinauer.
- Tavares E.S, Yamashita C, Miyaki C.Y. Phylogenetic relationships among some Neotropical parrot genera (Psittacidae; Aves) based on mitochondrial sequences. Auk. 2004;121:230–242. doi:10.1642/0004-8038(2004)121[0230:PRASNP]2.0.CO;2 [Google Scholar]
- Tavares E.S, Baker A.J, Pereira S.L, Miyaki C.Y. Phylogenetic relationships and historical biogeography of Neotropical Parrots (Psittaciformes: Psittacidae: Arini) inferred from mitochondrial and nuclear DNA sequences. Syst. Biol. 2006;55:454–470. doi: 10.1080/10635150600697390. doi:10.1080/10635150600697390 [DOI] [PubMed] [Google Scholar]
- Terborgh J. Distribution on environmental gradients: theory and a preliminary interpretation of distributional patterns in the avifauna of the Cordillera Vilcabamba, Peru. Ecology. 1971;52:23–40. doi:10.2307/1934735 [Google Scholar]
- Thorne J.L, Kishino H. Divergence time and evolutionary rate estimation with multilocus DNA data. Syst. Biol. 2002;51:689–702. doi: 10.1080/10635150290102456. doi:10.1080/10635150290102456 [DOI] [PubMed] [Google Scholar]
- Thorne J.L, Kishino H, Painter I.S. Estimating the rate of evolution of the rate of molecular evolution. Mol. Biol. Evol. 1998;15:1647–1657. doi: 10.1093/oxfordjournals.molbev.a025892. [DOI] [PubMed] [Google Scholar]
- van der Hammen T, Cleef A.M. Development of the high Andean Páramo flora and vegetation. In: Vuilleumier F, Monasterio M, editors. High altitude tropical biogeography. Oxford University Press; New York, NY: 1986. pp. 153–201. [Google Scholar]
- Weir J. Divergent timing and patterns of species accumulation in lowland and highland Neotropical birds. Evolution. 2006;60:842–855. [PubMed] [Google Scholar]
- Weng C, Hooghiemstra H, Duivenvoorden F. Response of pollen diversity to the climate-driven altitudinal shift of vegetation in the Colombian Andes. Phil. Trans. R. Soc. B. 2007;362:253–262. doi: 10.1098/rstb.2006.1985. doi:10.1098/rstb.2006.1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiens J.J, Parra-Olea G, García-París M, Wake D.B. Phylogenetic history underlies elevational biodiversity patterns in tropical salamanders. Proc. R. Soc. B. 2007;274:919–928. doi: 10.1098/rspb.2006.0301. doi:10.1098/rspb.2006.0301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan C.Y, Kroenke L.W. A plate tectonic reconstruction of the southwest Pacific, 0–100 Ma. In: Berger W.H, Kroenke L.W, Mayer L.A, editors. Proc. ODP, Sci. Results, 130. Ocean Drilling Program; College Station, TX: 1993. pp. 697–709. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of diagnosable morphological characters and details on the altitudinal distribution for all basal taxa within Pionus
Details on laboratory protocols and methods of analysis used
Morphological character matrix, taxa sampled, primers used, and divergence dates obtained in the nuclear gene (RAG1 and RAG2) analyses
Chronograms obtained from the Bayesian analysis (Multidivtime) of the RAG1 and RAG2 datasets